Preparation and Characterization of MoB Coating on Mo Substrate
Abstract
:1. Introduction
2. Experimental
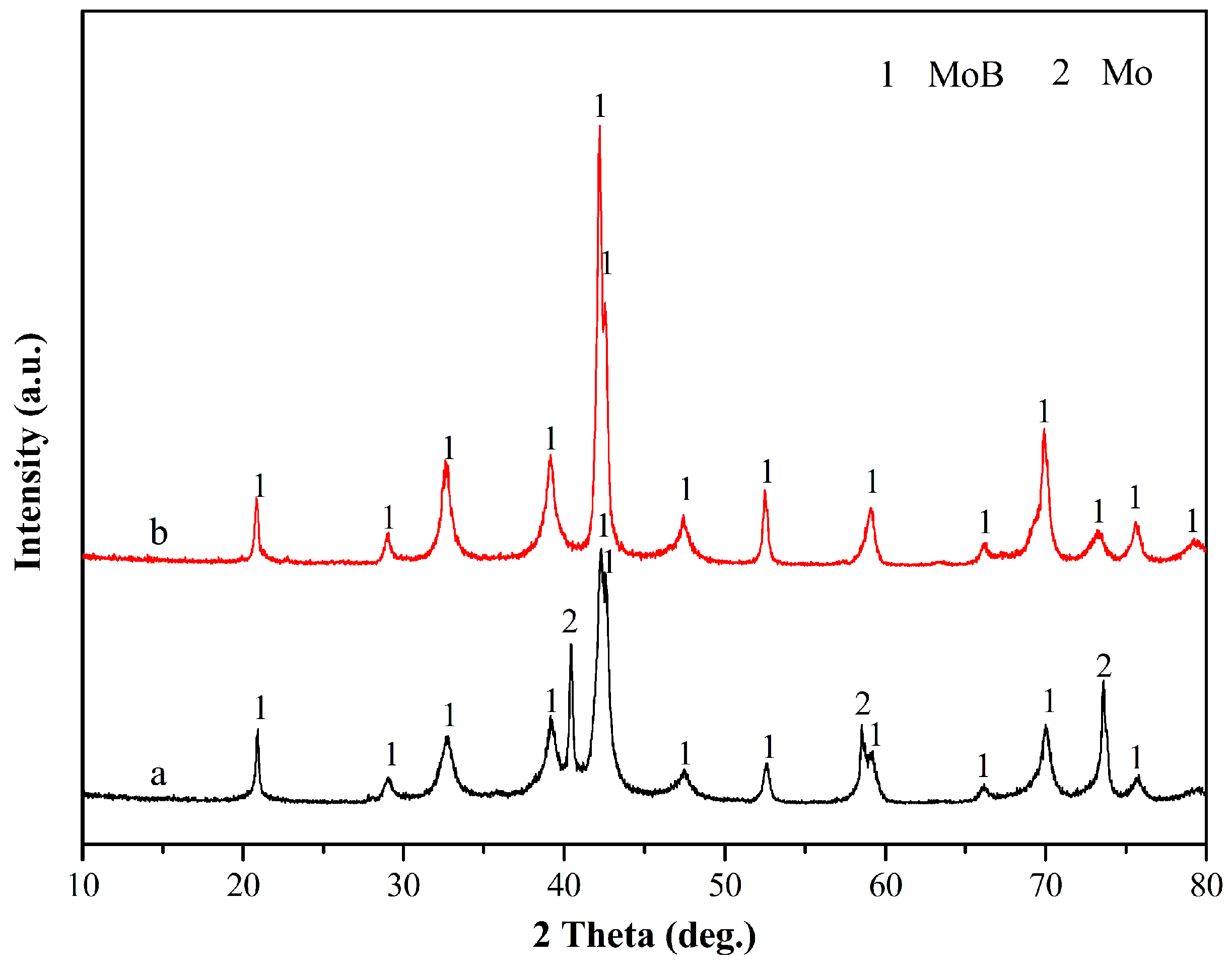
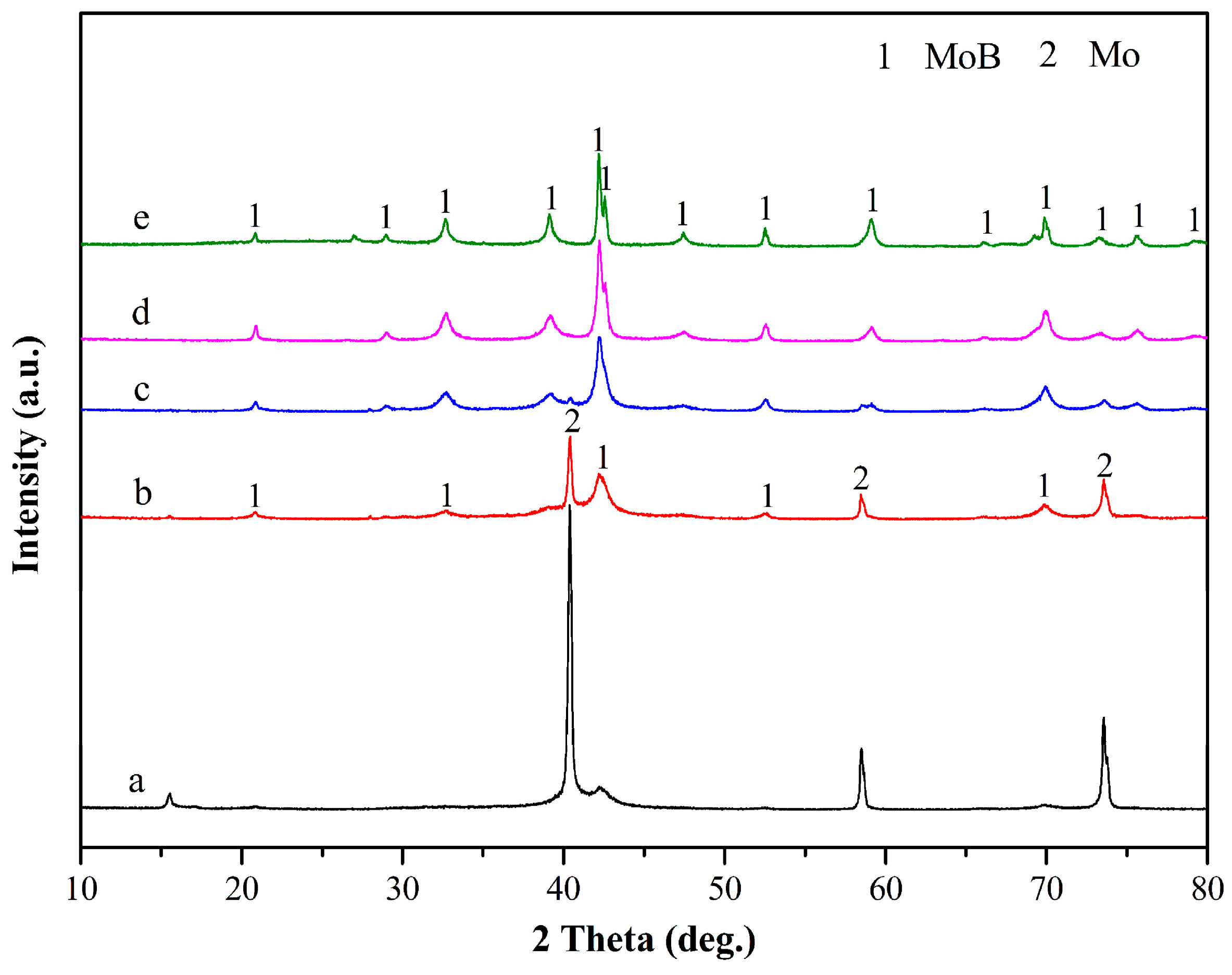
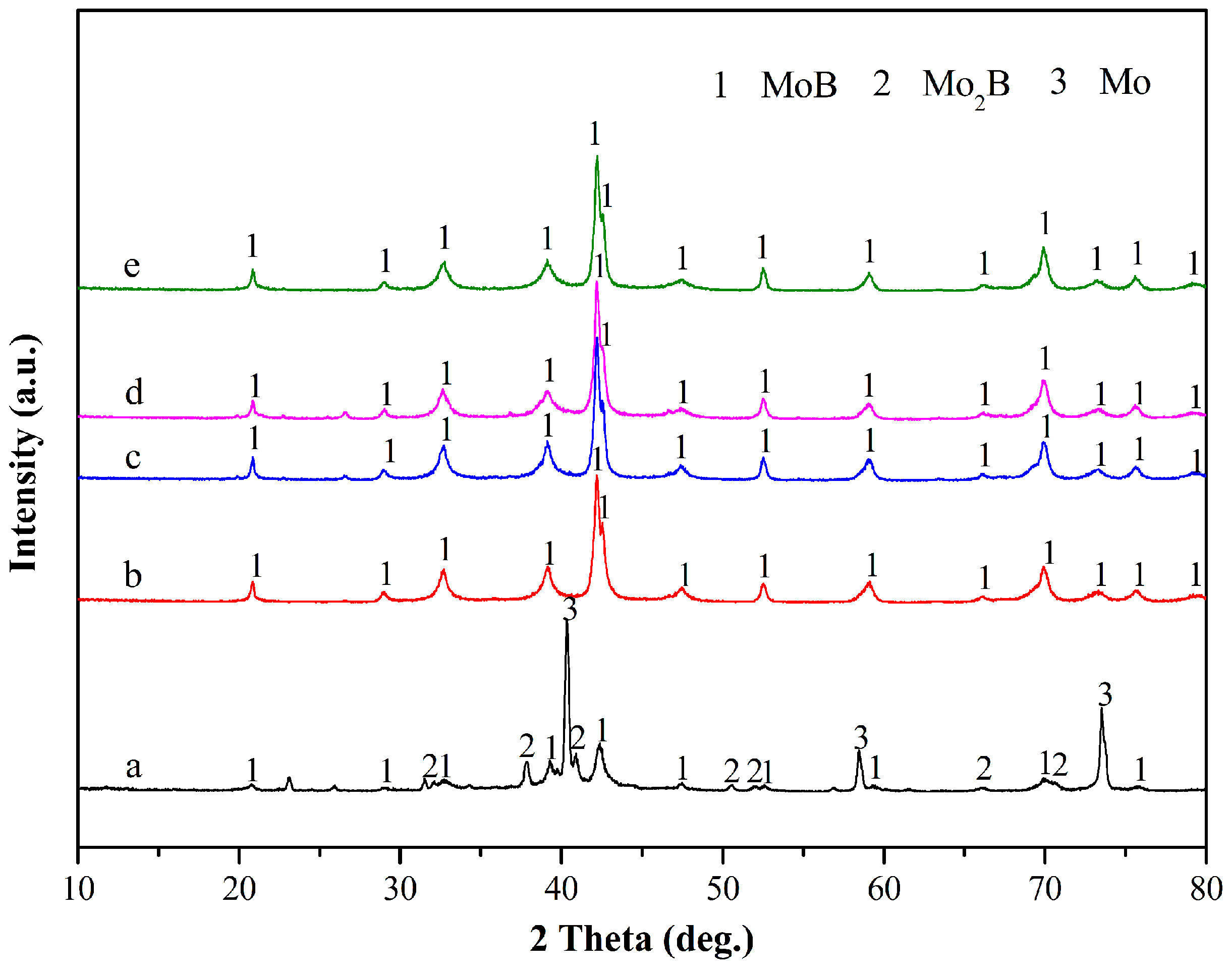
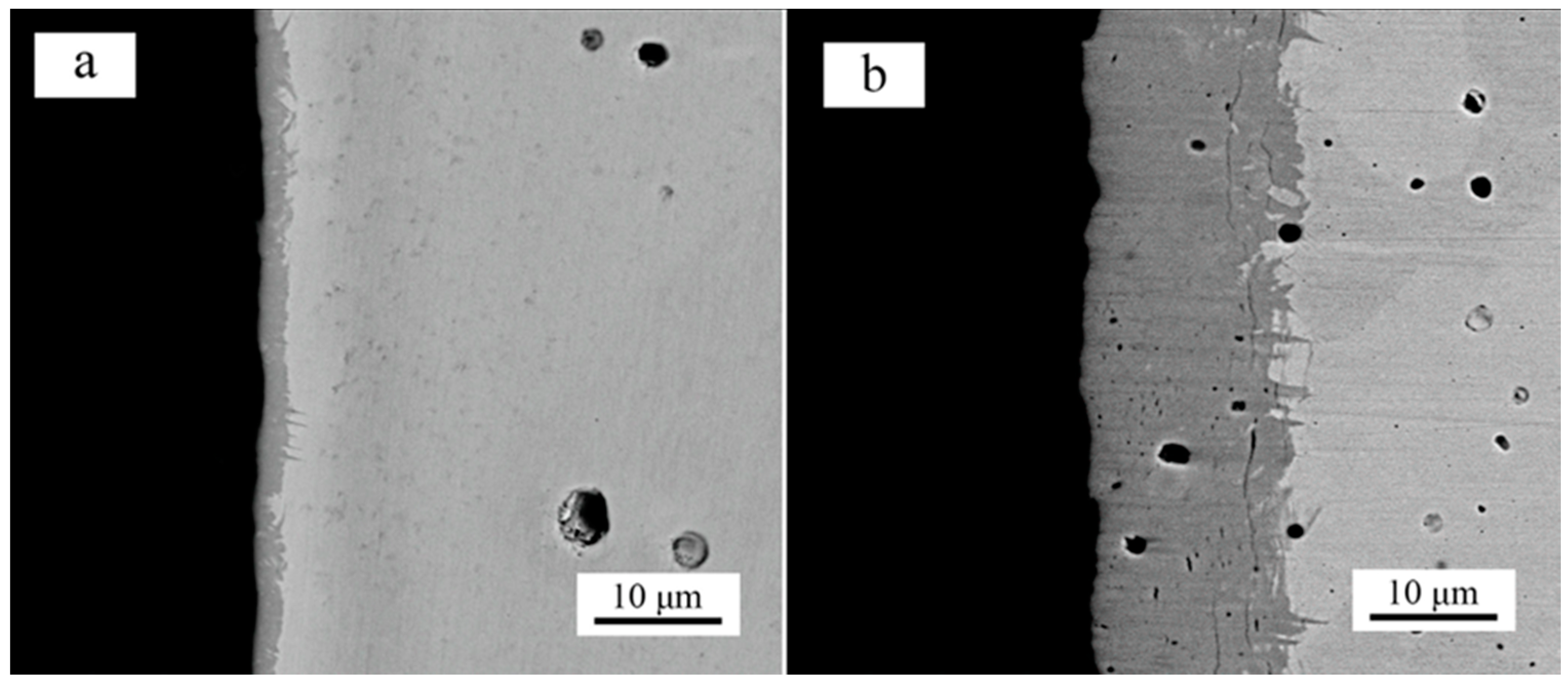
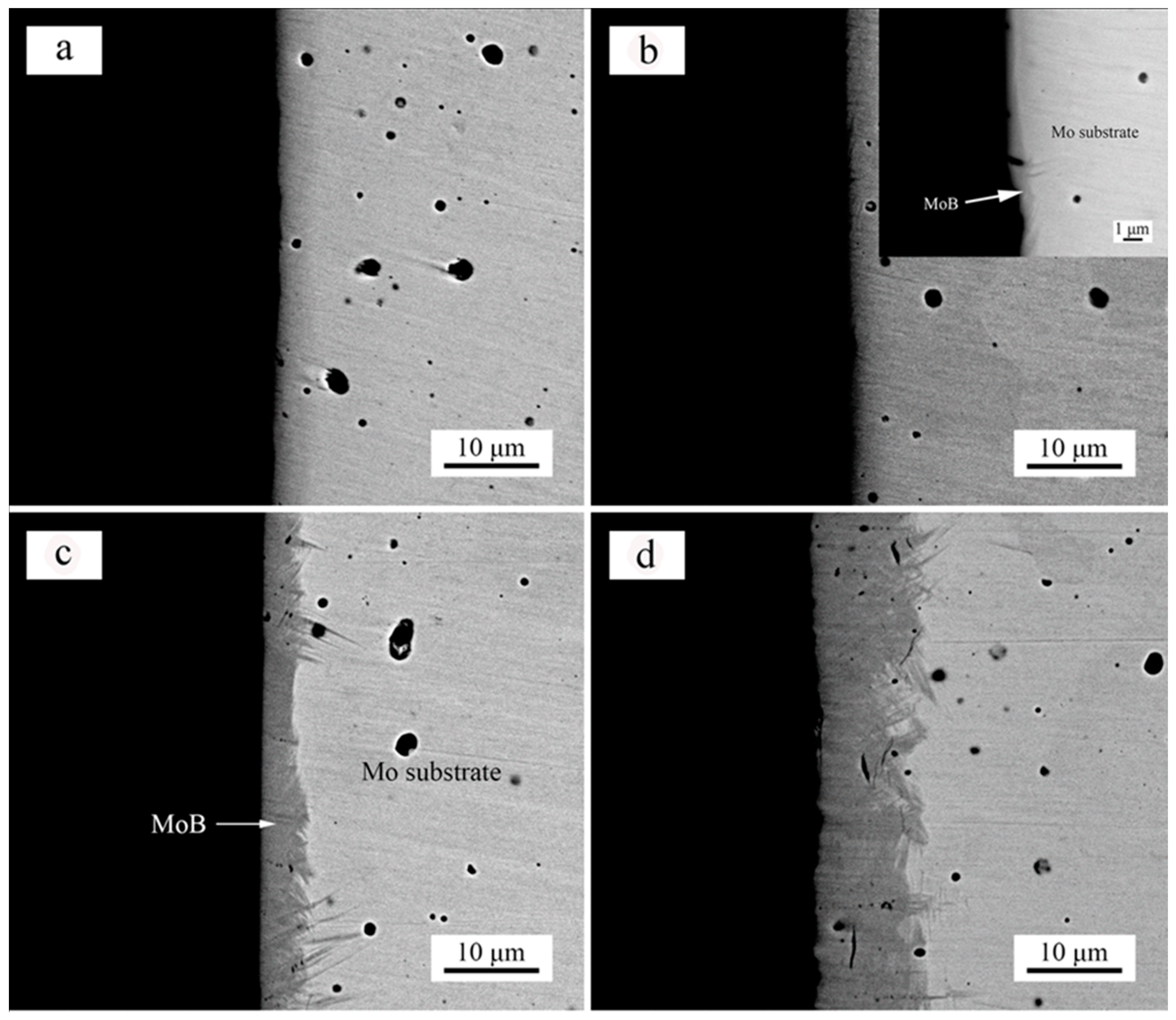
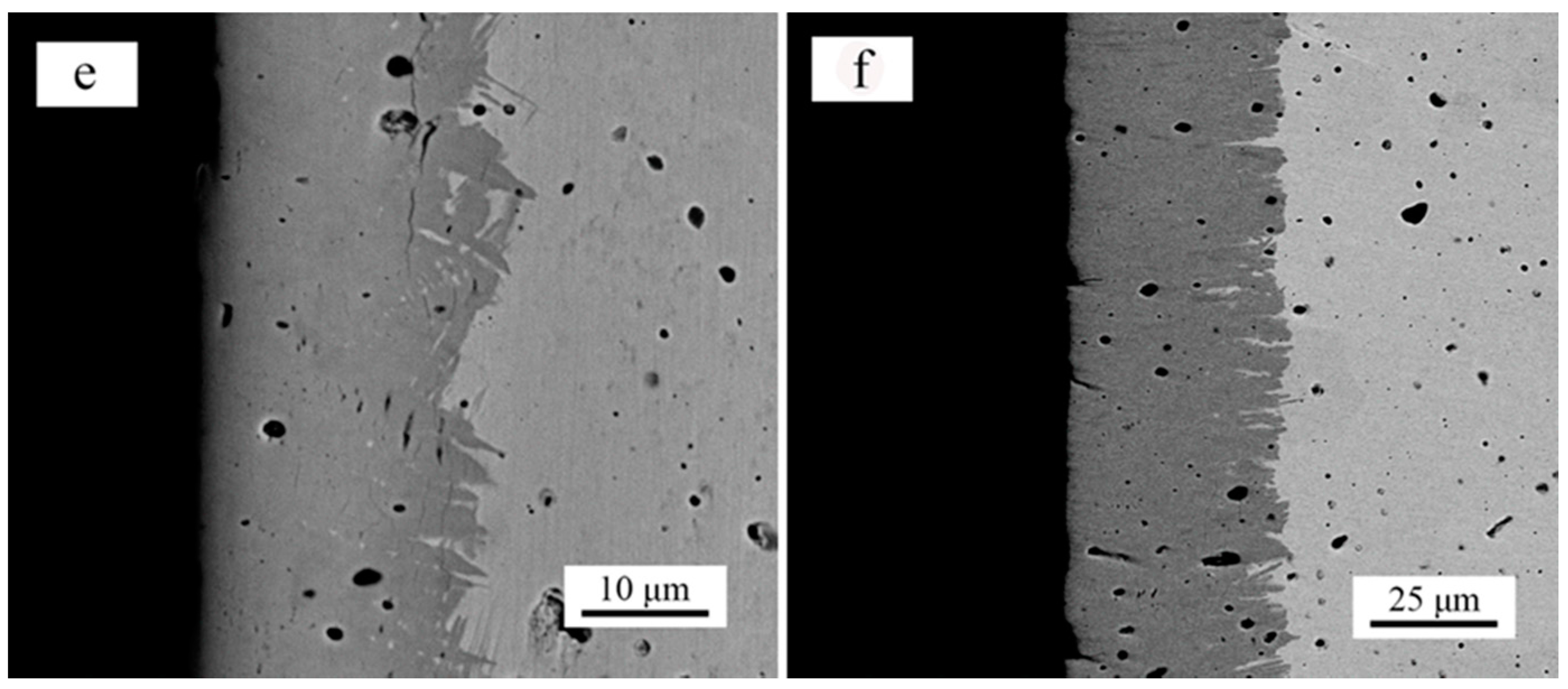
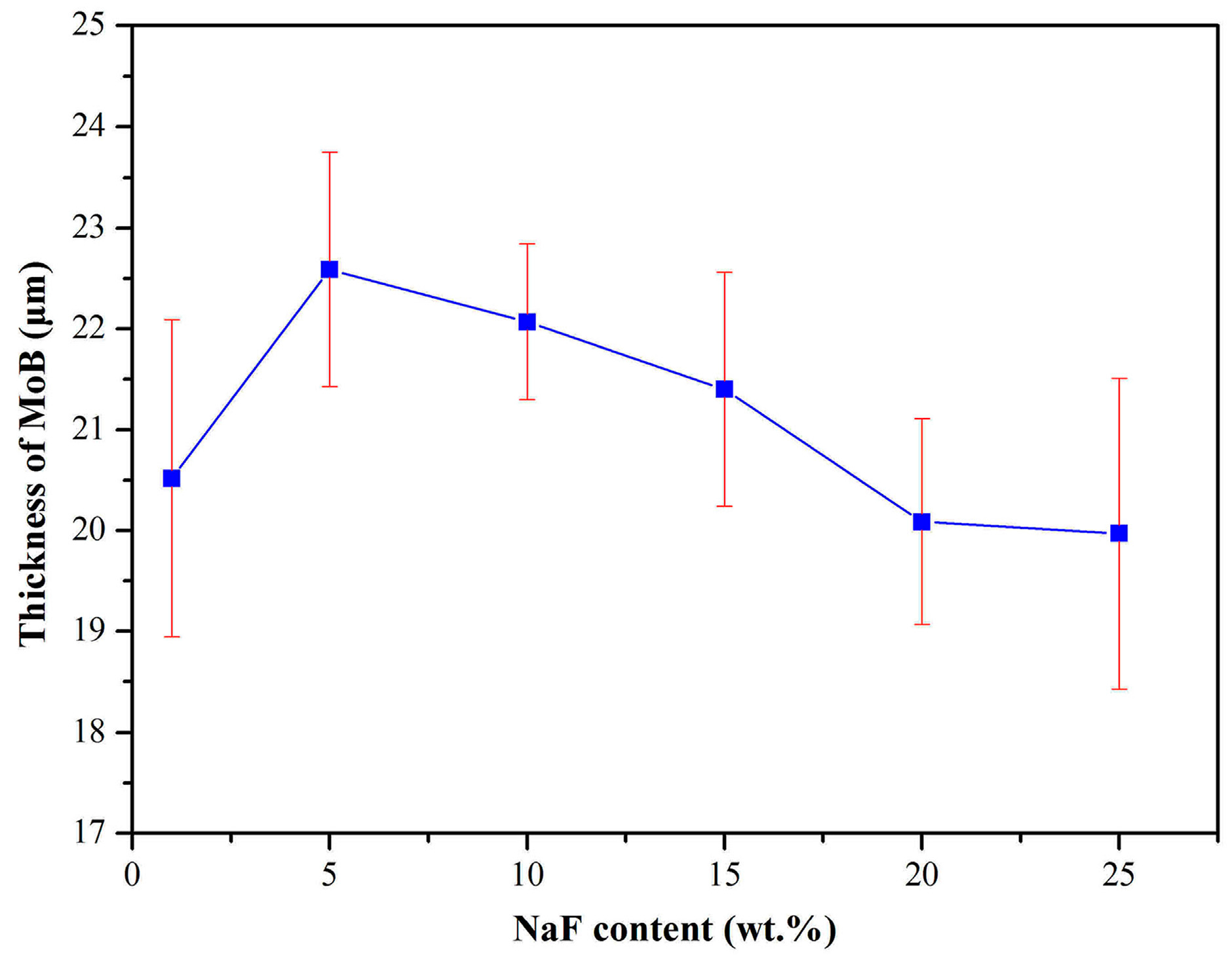
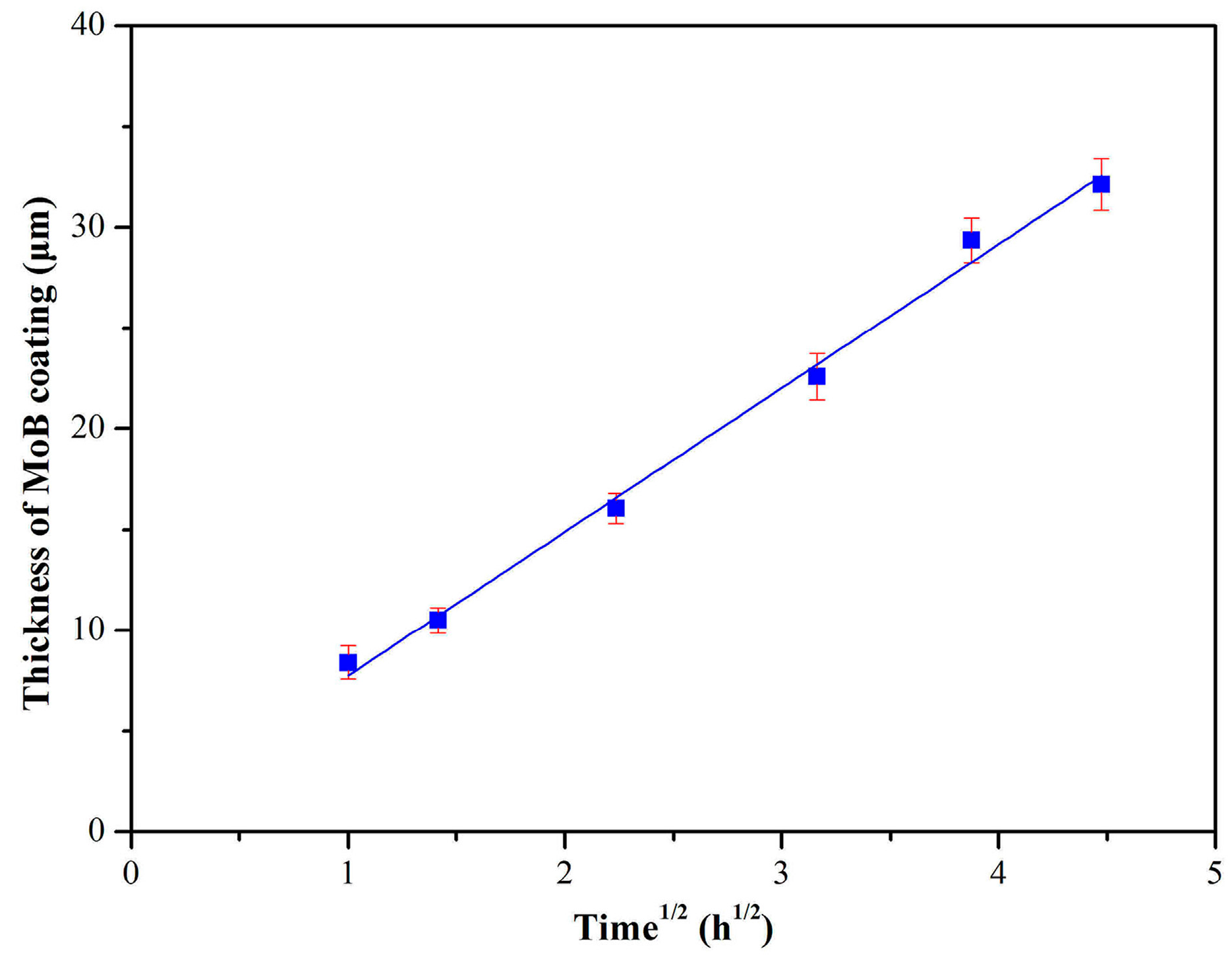
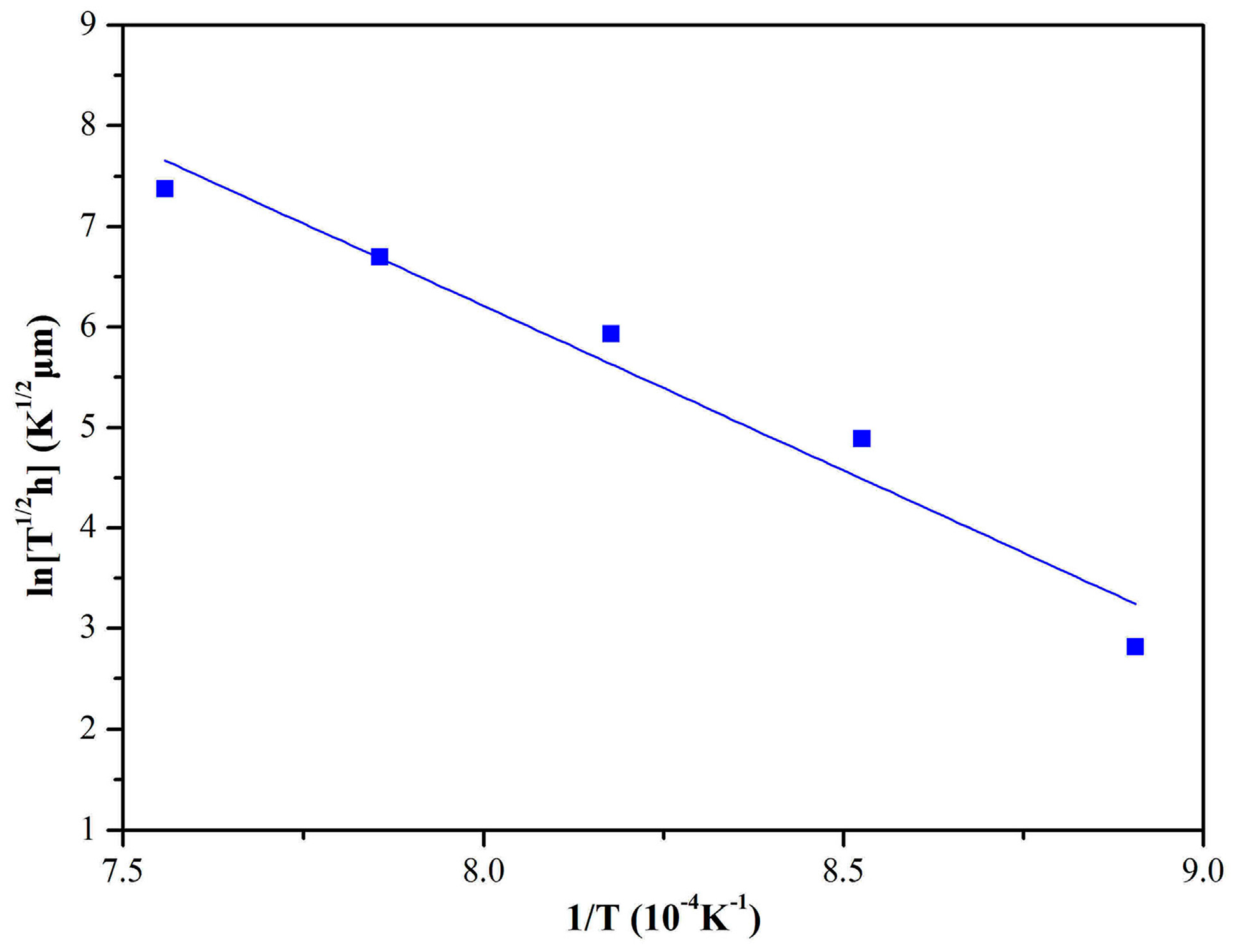
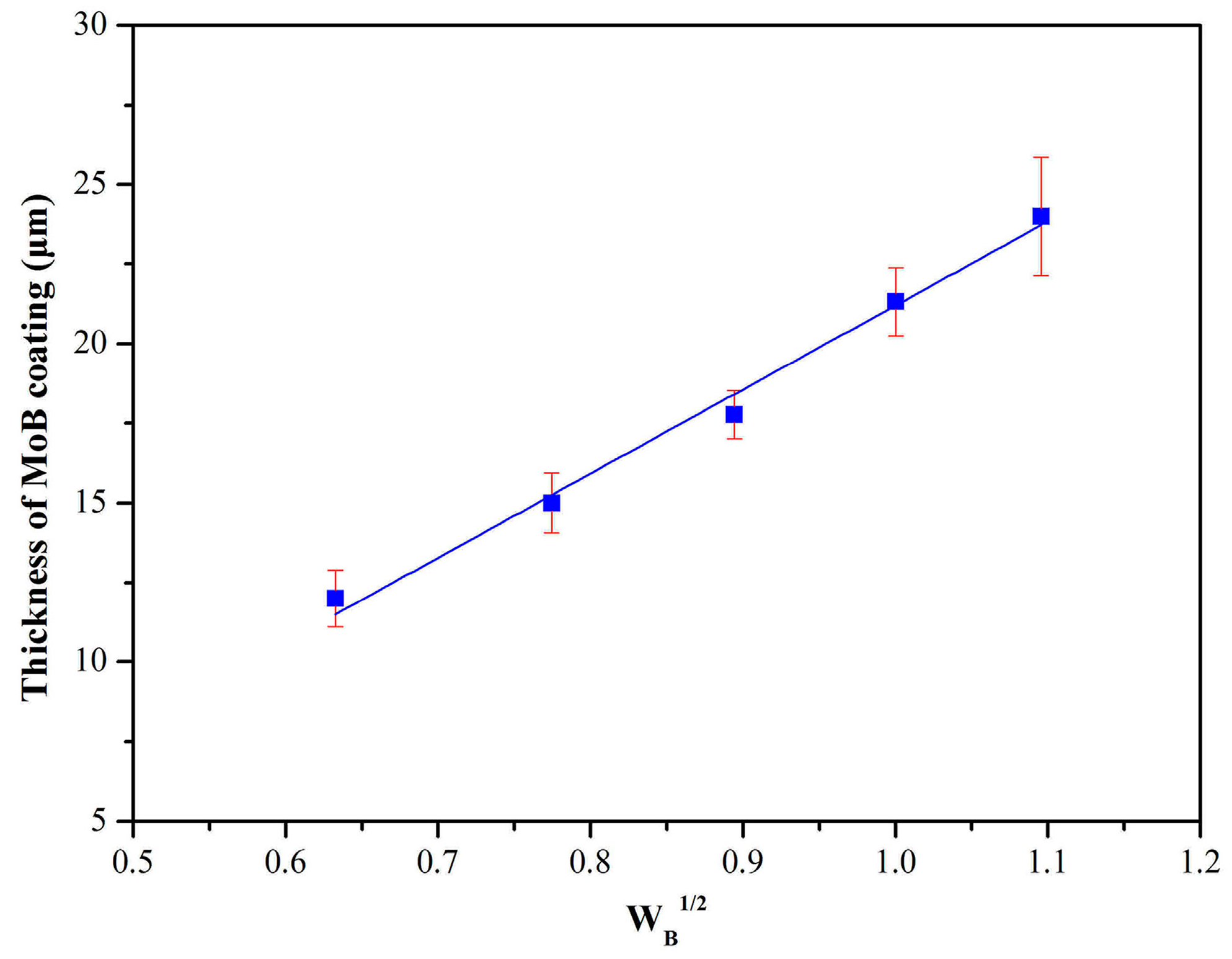
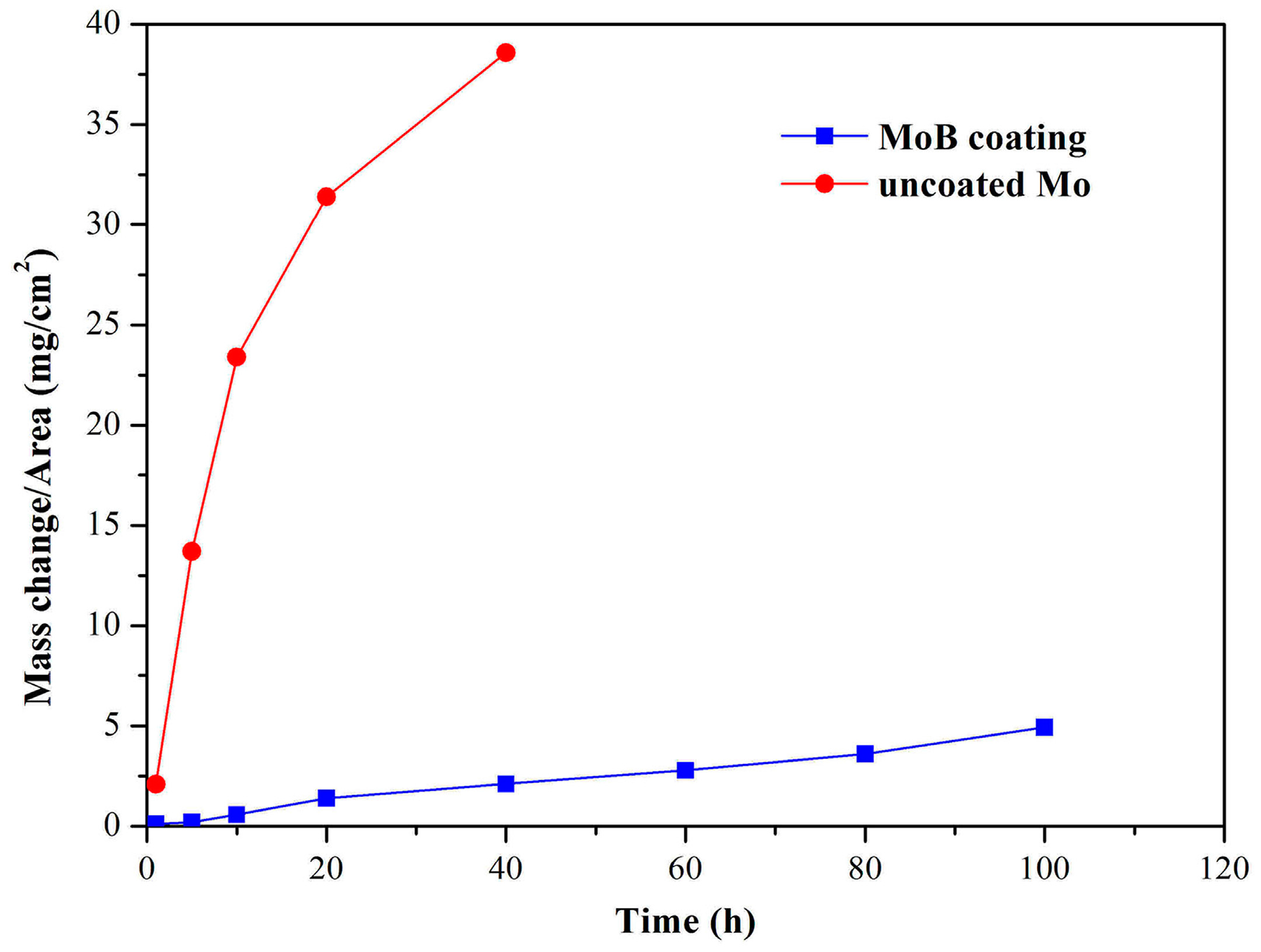
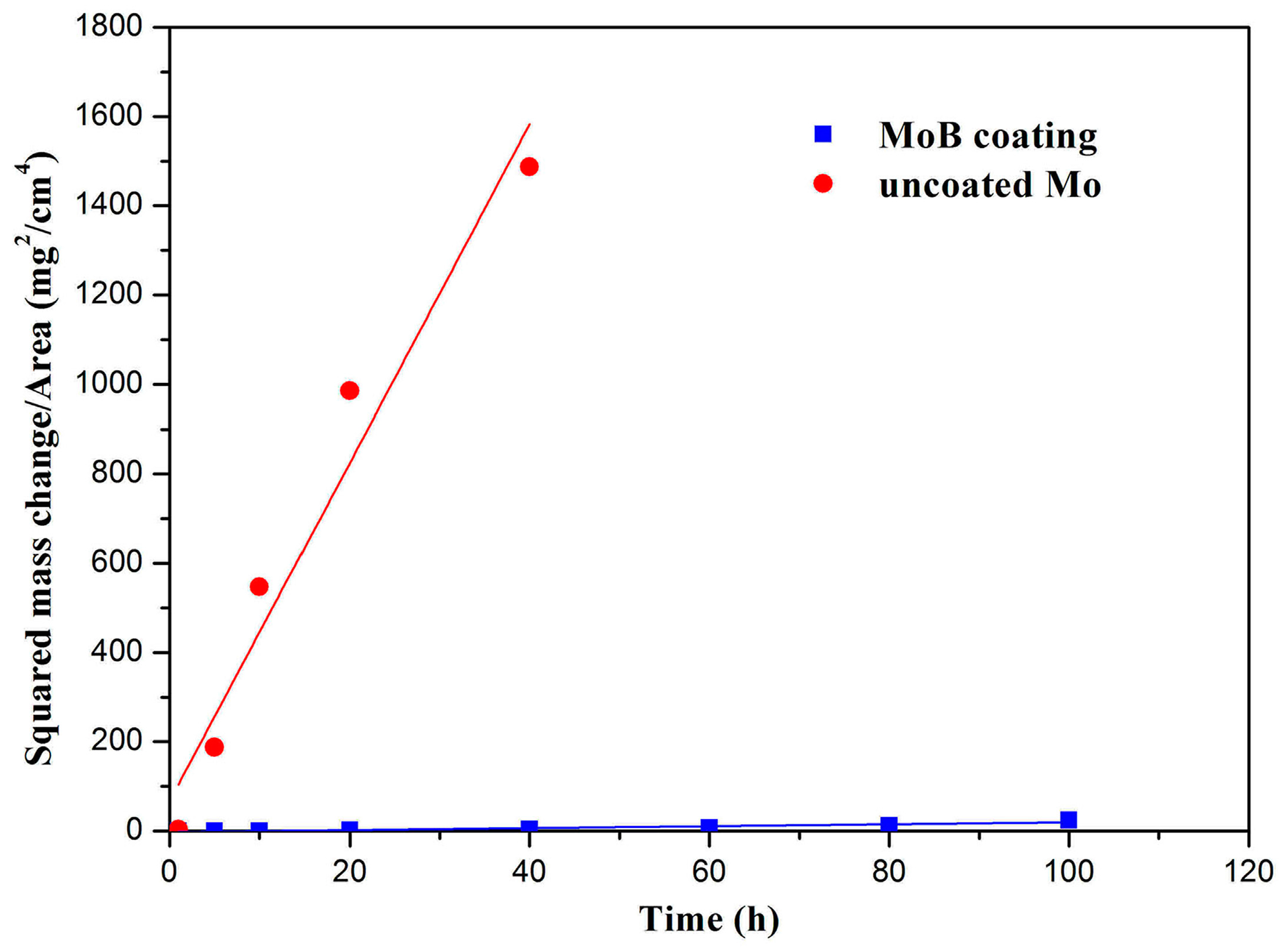
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, L.S.; Duan, Y.H.; Li, P. Microstructure, growth kinetics and some mechanical properties of boride layers produced on pure titanium by molten-salt boriding. J. Mater. Eng. Perform. 2017, 26, 4544–4555. [Google Scholar] [CrossRef]
- Béjar, M.A.; Henríquez, R. Surface hardening of steel by plasma-electrolysis boronizing. Mater. Des. 2009, 30, 1726–1728. [Google Scholar] [CrossRef]
- Aytekin, H.; Akçin, Y. Characterization of borided Incoloy 825 alloy. Mater. Des. 2013, 50, 515–521. [Google Scholar] [CrossRef]
- Majumdar, S.; Sharma, I.; Samajdar, I.; Bhargava, P. Relationship between pack chemistry and growth of silicide coatings on Mo-TZM alloy. J. Electrochem. Soc. 2008, 155, D734–D741. [Google Scholar] [CrossRef]
- Chaia, N.; Portebois, L.; Mathieu, S.; David, N.; Vilasi, M. On the interdiffusion in multilayered silicide coatings for the vanadium-based alloy V-4Cr-4Ti. J. Nucl. Mater. 2017, 484, 148–156. [Google Scholar] [CrossRef]
- Sun, J.; Fu, Q.G.; Guo, L.P.; Wang, L. Silicide coating fabricated by HAPC/SAPS Combination to protect niobium alloy from oxidation. ACS Appl. Mater. Interfaces 2016, 8, 15838–15847. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.D.; Guo, X.P.; Sun, Z.P.; Qu, J.L.; Wang, L.J. Oxidation resistance comparison of MoSi2 and B-modified MoSi2 coatings on pure Mo prepared through pack cementation. Mater. Corros. 2015, 66, 681–687. [Google Scholar] [CrossRef]
- Cheng, J.; Yi, S.; Park, J.S. Simultaneous coating of Si and B on Nb–Si–B alloys by a halide activated pack cementation method and oxidation behaviors of the alloys with coatings at 1100 °C. J. Alloys Compd. 2015, 644, 975–981. [Google Scholar] [CrossRef]
- Tian, X.; Guo, X.; Sun, Z.; Yin, Z.; Wang, L. Formation of B-modified MoSi2 coating on pure Mo prepared through HAPC process. Int. J. Refract. Met. Hard Mater. 2014, 45, 8–14. [Google Scholar] [CrossRef]
- Paul, B.; Prakash, J.; Sarkar, P.S. Formation and characterization of uniform SiC coating on 3-D graphite substrate using halide activated pack cementation method. Surf. Coat. Technol. 2015, 282, 61–67. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Z.; Zhong, J.; Wang, L.; Hou, Z.; Zhang, Y.; Tan, F. Facile preparation of nanocrystalline Fe2B coating by direct electro-spark deposition of coarse-grained Fe2B electrode material. J. Alloys Compd. 2017, 717, 31–40. [Google Scholar] [CrossRef]
- Xie, F.; Wang, X.J.; Pan, J.W. Accelerate pack boriding with reused boriding media by simultaneously employing Al and alternating current field. Vacuum 2017, 141, 166–169. [Google Scholar] [CrossRef]
- Balokhonov, R.R.; Romanova, V.A.; Schmauder, S.; Martynov, S.A.; Kovalevskaya, Z.G. A mesomechanical analysis of plastic strain and fracture localization in a material with a bilayer coating. Compos. Part B Eng. 2014, 66, 276–286. [Google Scholar] [CrossRef]
- Márquez-Herrera, A.; Fernandez-Muñoz, J.L.; Zapata-Torres, M.; Melendez-Lira, M.; Cruz-Alcantar, P. Fe2B coating on ASTM A-36 steel surfaces and its evaluation of hardness and corrosion resistance. Surf. Coat. Technol. 2014, 254, 433–439. [Google Scholar] [CrossRef]
- Tsipas, S.A.; Vázquez-Alcázar, M.R.; Navas, E.M.R.; Gordo, E. Boride coatings obtained by pack cementation deposited on powder metallurgy and wrought Ti and Ti–6Al–4V. Surf. Coat. Technol. 2010, 205, 2340–2347. [Google Scholar] [CrossRef]
- Sarma, B.; Tikekar, N.M.; Ravi Chandran, K.S. Kinetics of growth of superhard boride layers during solid state diffusion of boron into titanium. Ceram. Int. 2012, 38, 6795–6805. [Google Scholar] [CrossRef]
- Dokumaci, E.; Özkan, I.; Özyigit, M.B.; Önay, B. Effect of boronizing on the oxidation of niobium. Int. J. Refract. Met. Hard Mater. 2013, 41, 276–281. [Google Scholar] [CrossRef]
- Ingole, S.; Liang, H.; Usta, M.; Bindal, C.; Ucisik, A.H. Multi-scale wear of a boride coating on tungsten. Wear 2005, 259, 849–860. [Google Scholar] [CrossRef]
- Akca, B.; Calik, A. Characterization of borided pure molybdenum under controlled atmosphere. Prot. Met. Phys. Chem. 2017, 53, 511–517. [Google Scholar] [CrossRef]
- Feridun, O.K.; Sista, V.; Eryilmaz, O.L.; Erdemir, A. Electrochemical boriding of molybdenum in molten borax. Surf. Eng. 2015, 31, 575–580. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Yan, J. Preparation and characterization of MoSi2/MoB composite coating on Mo substrate. J. Alloys Compd. 2014, 589, 384–388. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.; Wang, D. High temperature oxidation and microstructure of MoSi2/MoB composite coating for Mo substrate. Int. J. Refract. Met. Hard Mater. 2017, 68, 60–64. [Google Scholar] [CrossRef]
- Çamurlu, H.E. Preparation of single phase molybdenum boride. J. Alloys Compd. 2011, 509, 5431–5436. [Google Scholar] [CrossRef]
- Yeh, C.L.; Hsu, W.S. Preparation of MoB and MoB–MoSi2 composites by combustion synthesis in SHS mode. J. Alloys Compd. 2007, 440, 193–198. [Google Scholar] [CrossRef]
- Yeh, C.L.; Hsu, W.S. Preparation of molybdenum borides by combustion synthesis involving solid-phase displacement reactions. J. Alloys Compd. 2008, 457, 191–197. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Rebrov, E.V.; Mies, M.J.M.; de Croon, M.H.J.M.; Schouten, J.C. Synthesis of protective Mo-Si-B coatings in molten salts and their oxidation behavior in an air-water mixture. Surf. Coat. Technol. 2006, 201, 971–978. [Google Scholar] [CrossRef]
- Wu, J.; Wang, W.; Zhou, C. Microstructure and oxidation resistance of Mo–Si–B coating on Nb based in situ composites. Corros. Sci. 2014, 87, 421–426. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Kuznetsova, S.V.; Rebrov, E.V.; Mies, M.J.M.; de Croon, M.H.J.M.; Schouten, J.C. Synthesis of molybdenum borides and molybdenum silicides in molten salts and their oxidation behavior in an air-water mixture. Surf. Coat. Technol. 2005, 195, 182–188. [Google Scholar] [CrossRef]


| Coating | HIT (GPa) | Hardness (HV) | EIT (GPa) |
|---|---|---|---|
| MoB | 33.81 | 3130.85 | 519.31 |
| Temperature (°C) | kp (mg2·cm−4·h−1) | |
|---|---|---|
| Pure Mo | MoB | |
| 600 | 37.91 | 0.218 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Shi, H.; Yan, J.; Wang, D. Preparation and Characterization of MoB Coating on Mo Substrate. Metals 2018, 8, 93. https://doi.org/10.3390/met8020093
Wang Y, Shi H, Yan J, Wang D. Preparation and Characterization of MoB Coating on Mo Substrate. Metals. 2018; 8(2):93. https://doi.org/10.3390/met8020093
Chicago/Turabian StyleWang, Yi, Haiyan Shi, Jianhui Yan, and Dezhi Wang. 2018. "Preparation and Characterization of MoB Coating on Mo Substrate" Metals 8, no. 2: 93. https://doi.org/10.3390/met8020093




