In Vitro Corrosion Assessment of Additively Manufactured Porous NiTi Structures for Bone Fixation Applications
Abstract
:1. Introduction
2. Materials and Methods
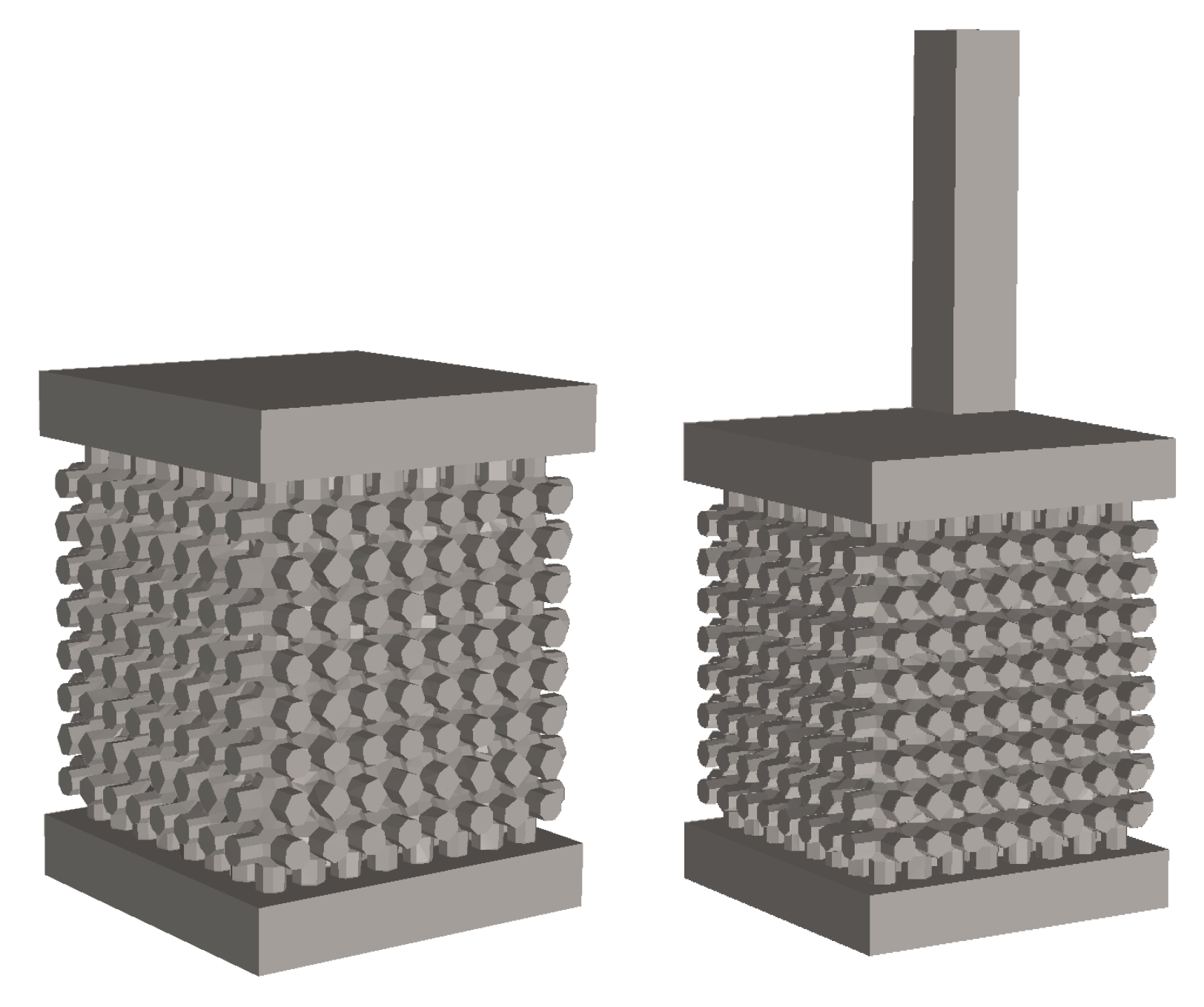
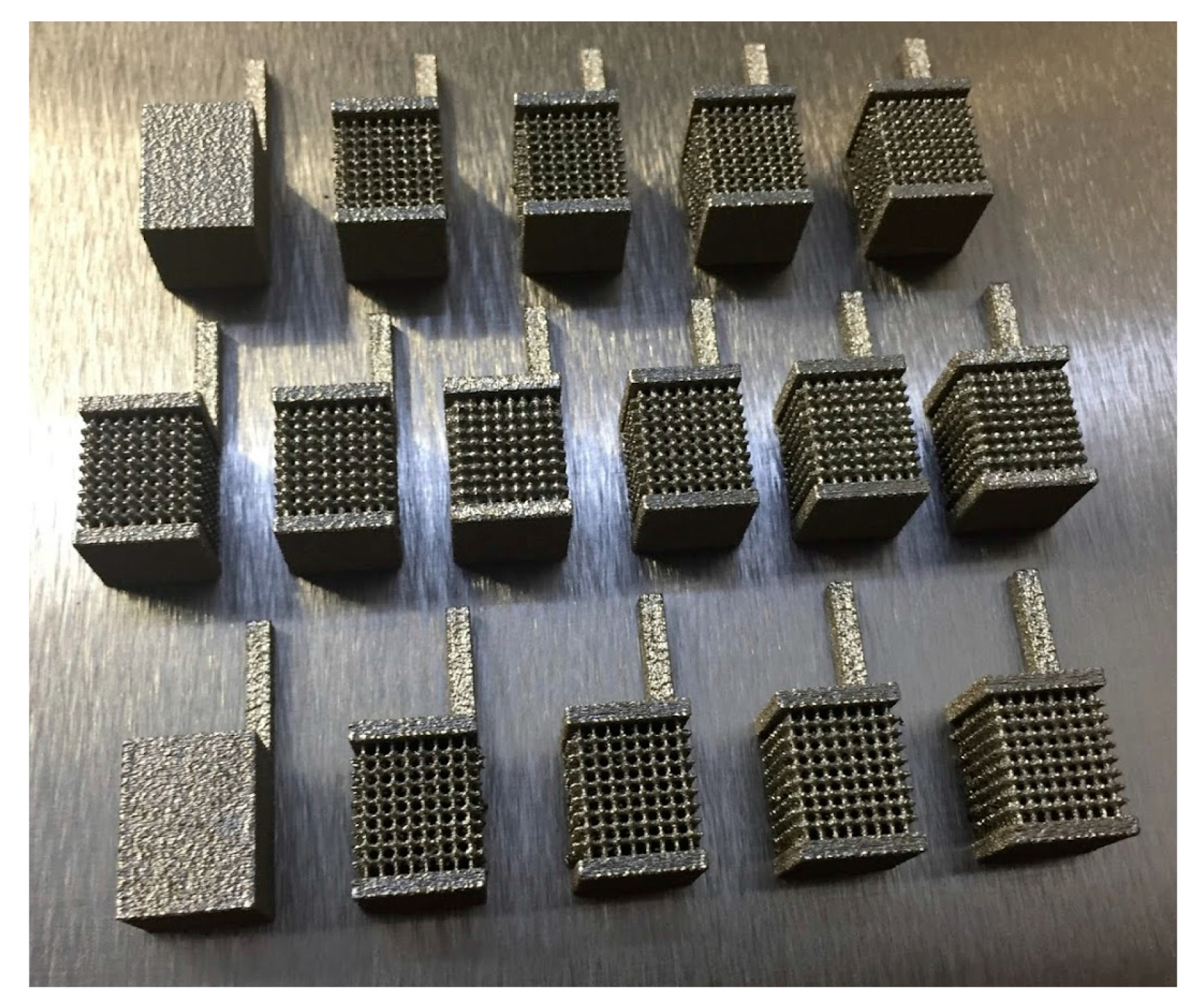
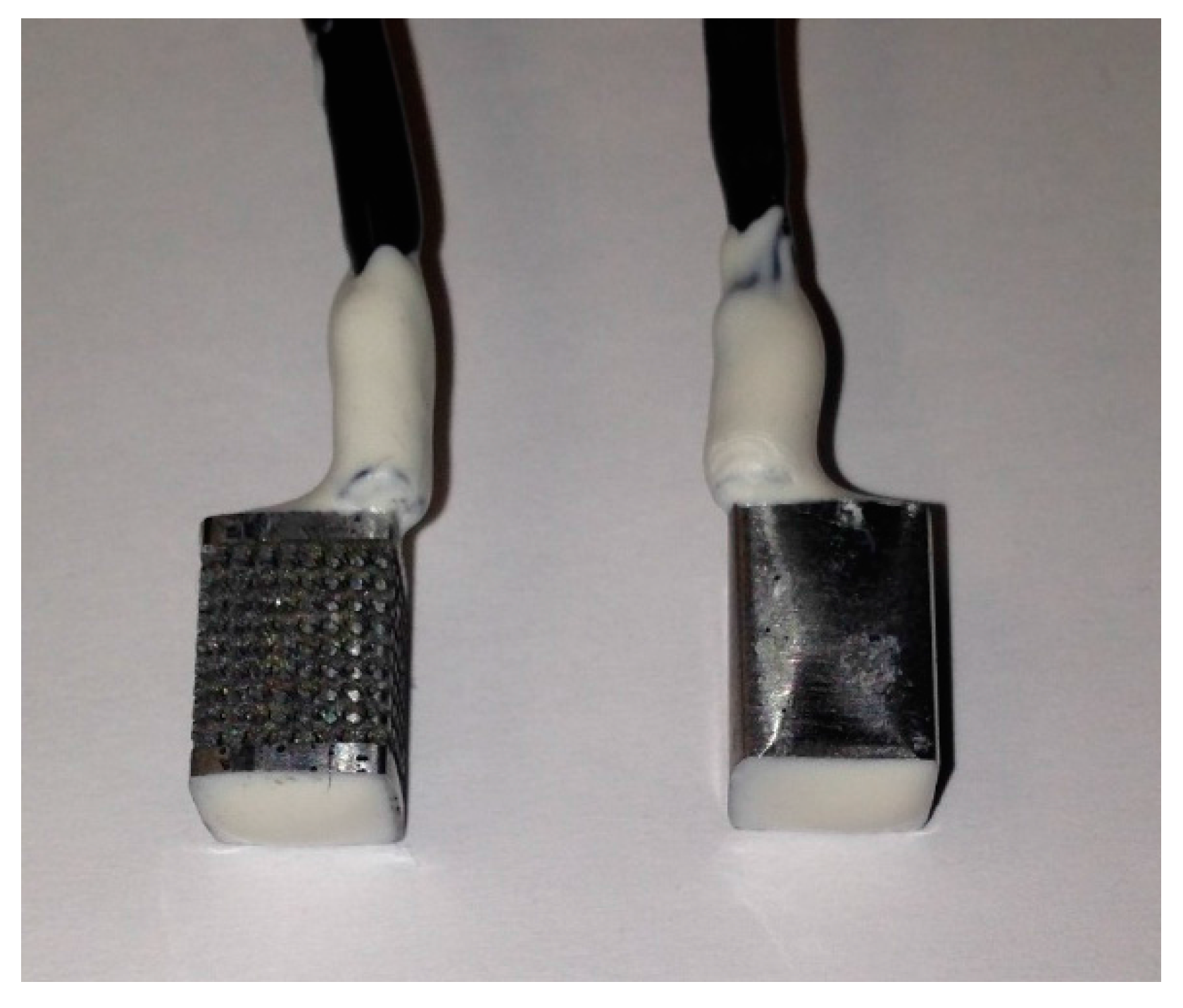
2.1. Design and Fabrication of the Samples
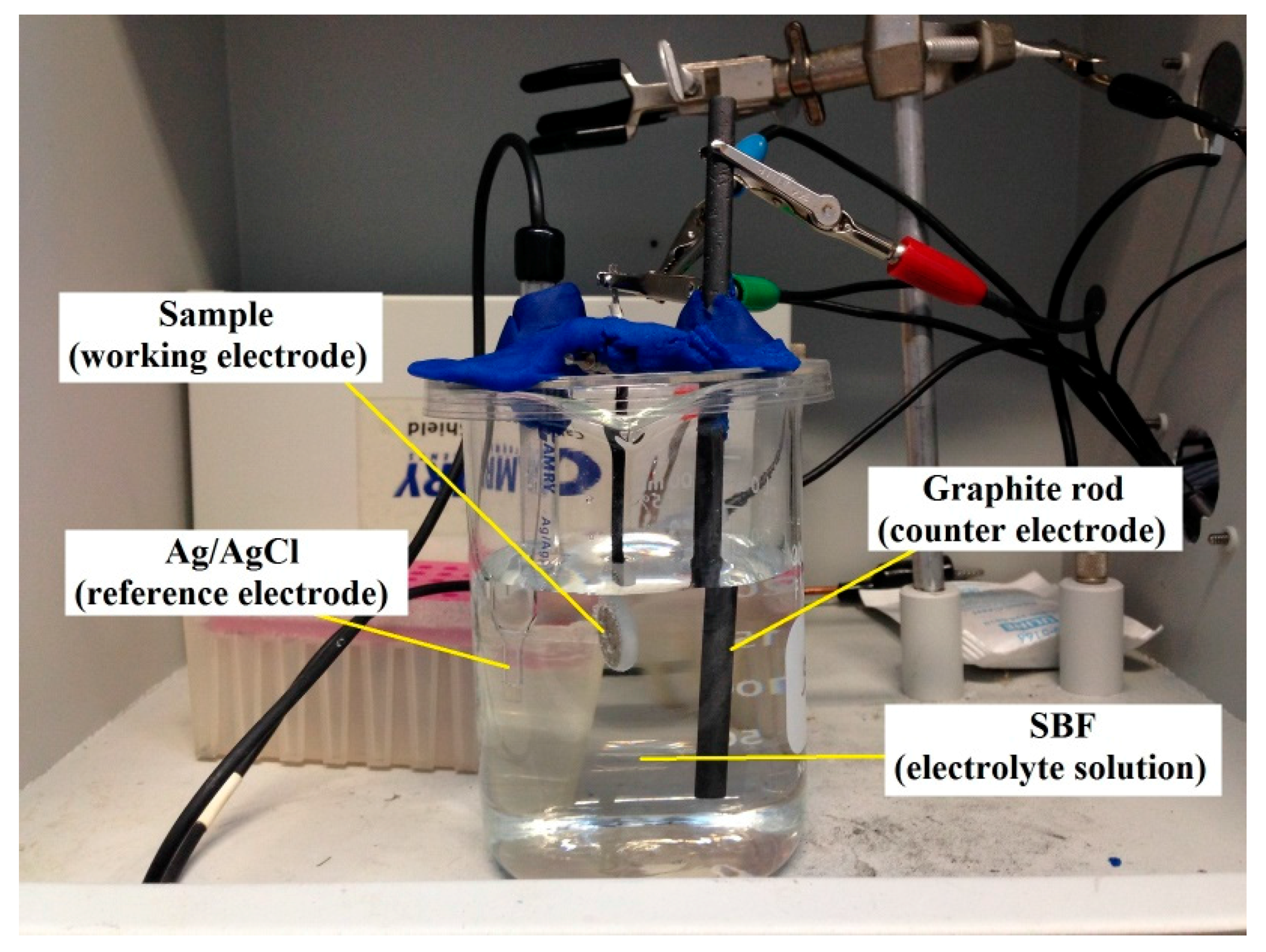
2.2. Electrochemical Corrosion Test
2.3. Surface Morphology
2.4. X-ray Diffraction (XRD)
2.5. Ni Ion Release (Immersion Test)
3. Results and Discussion
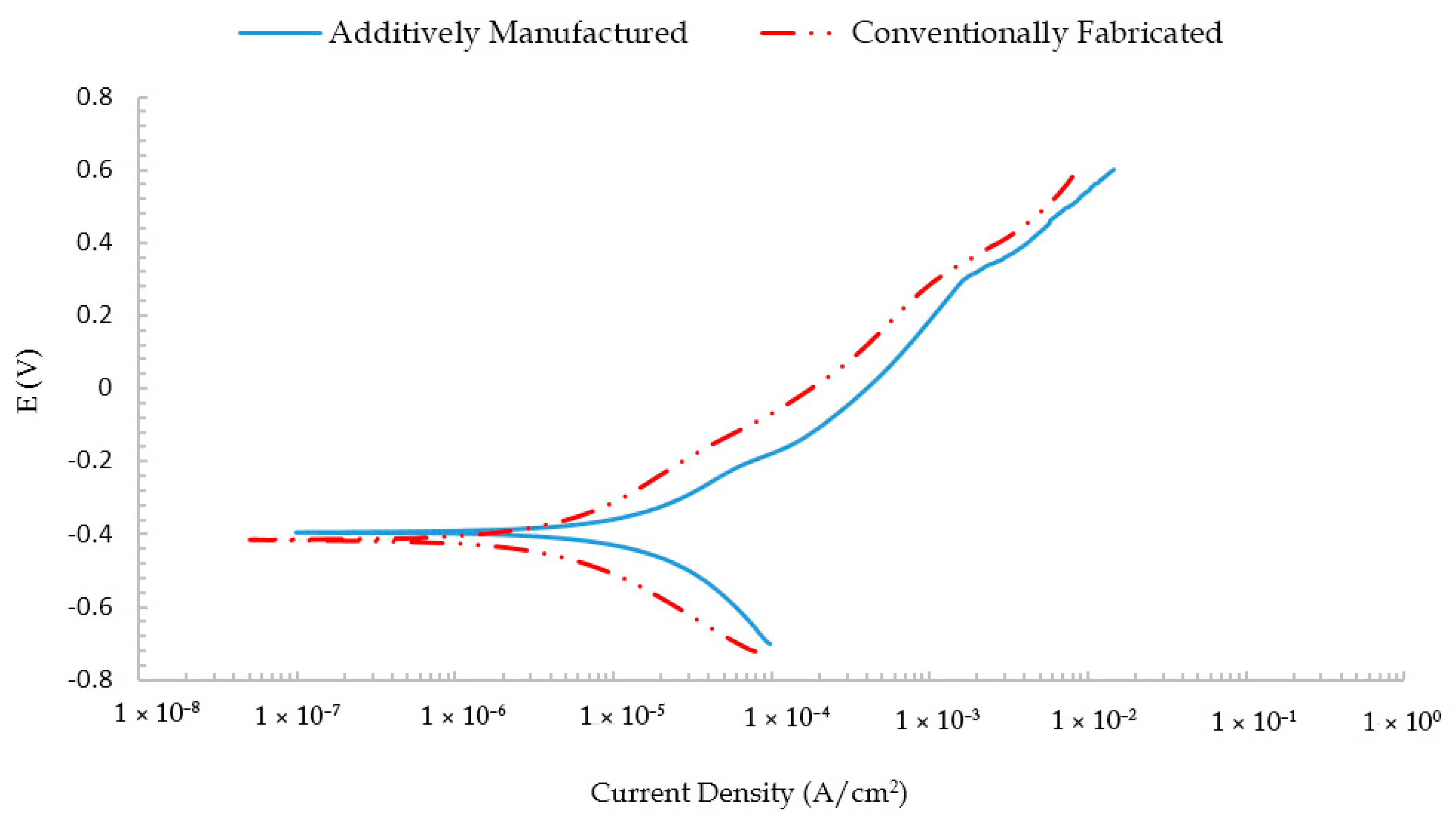
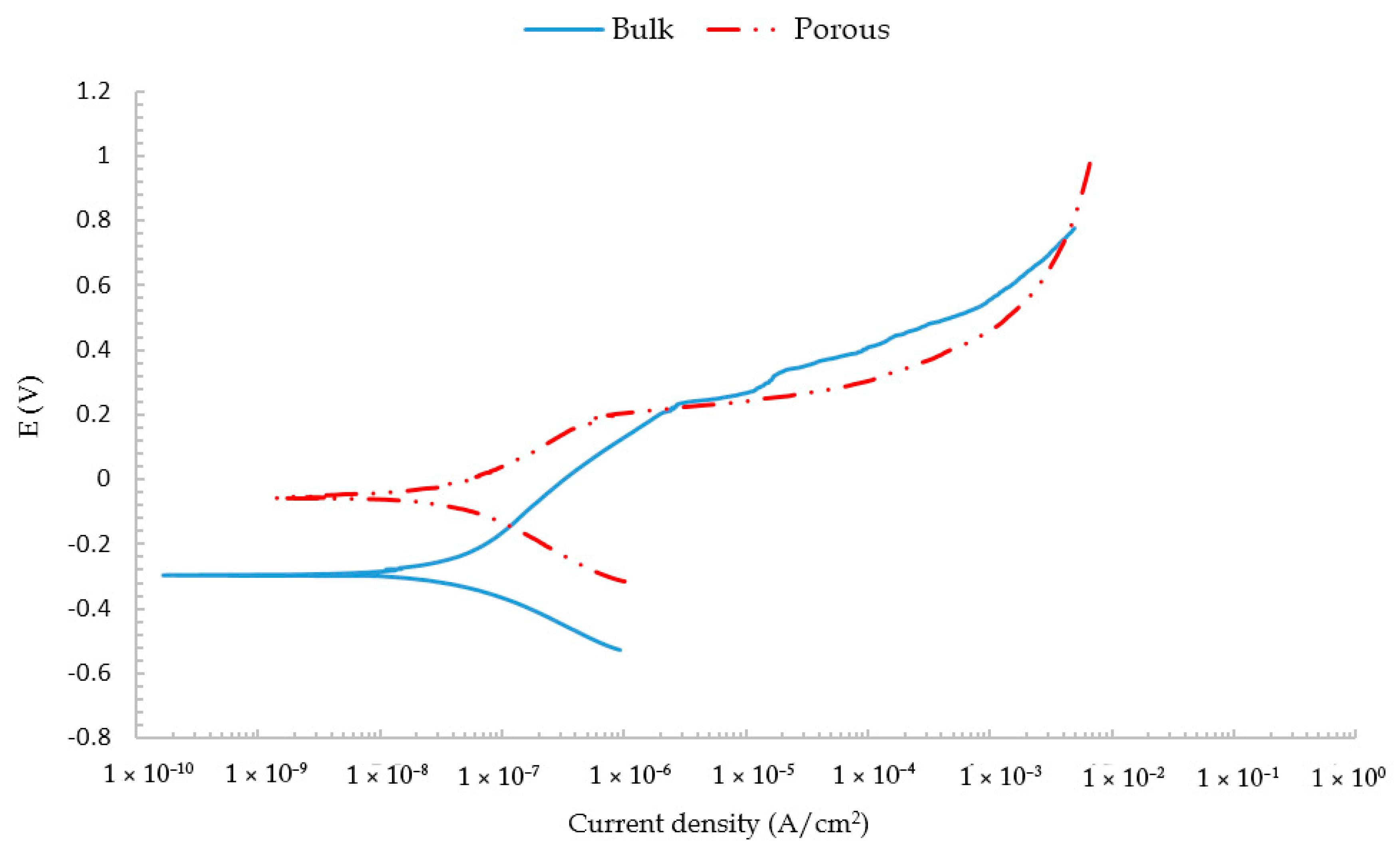
3.1. Electrochemical Corrosion Test
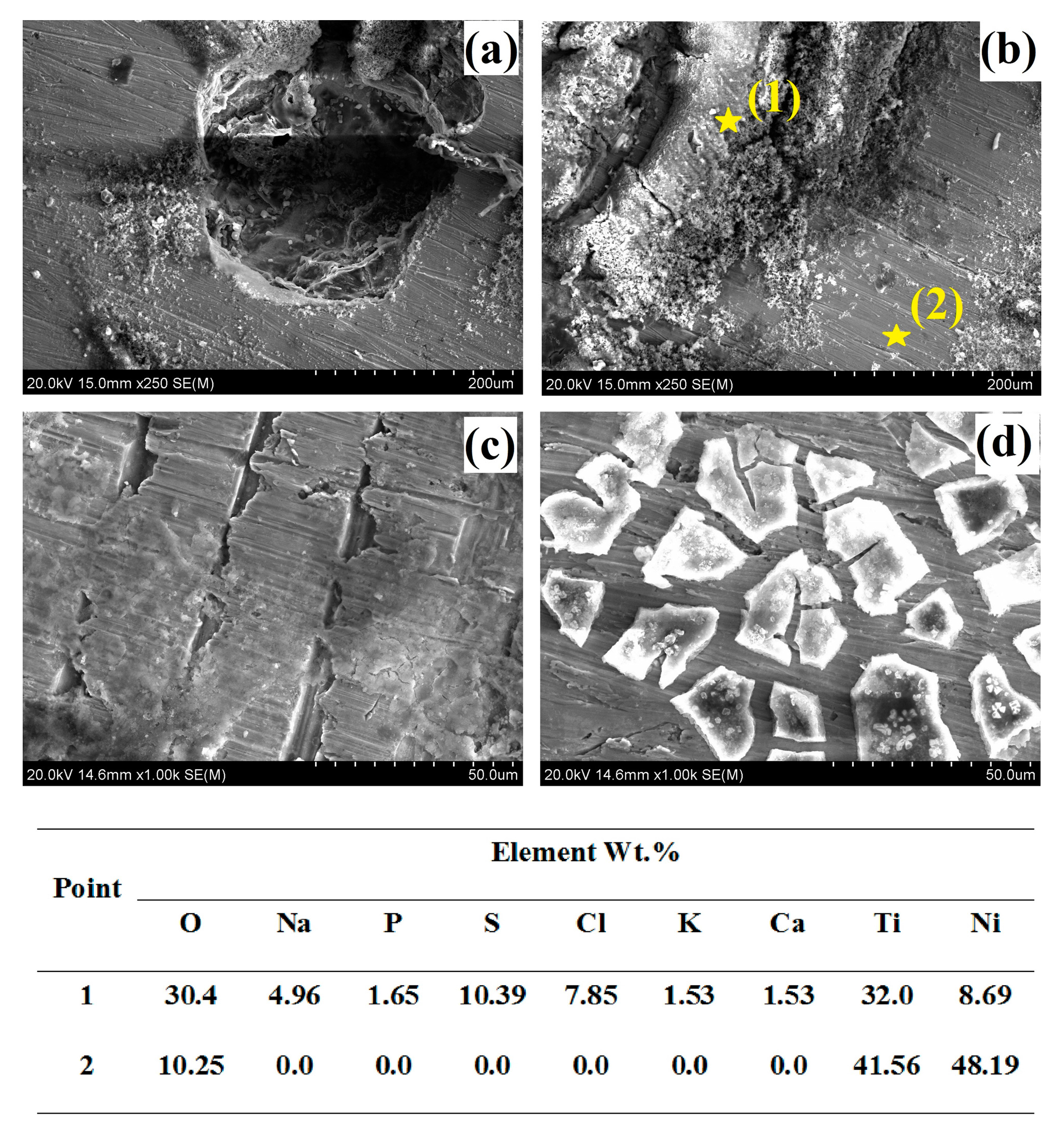
3.2. Surface Morphology
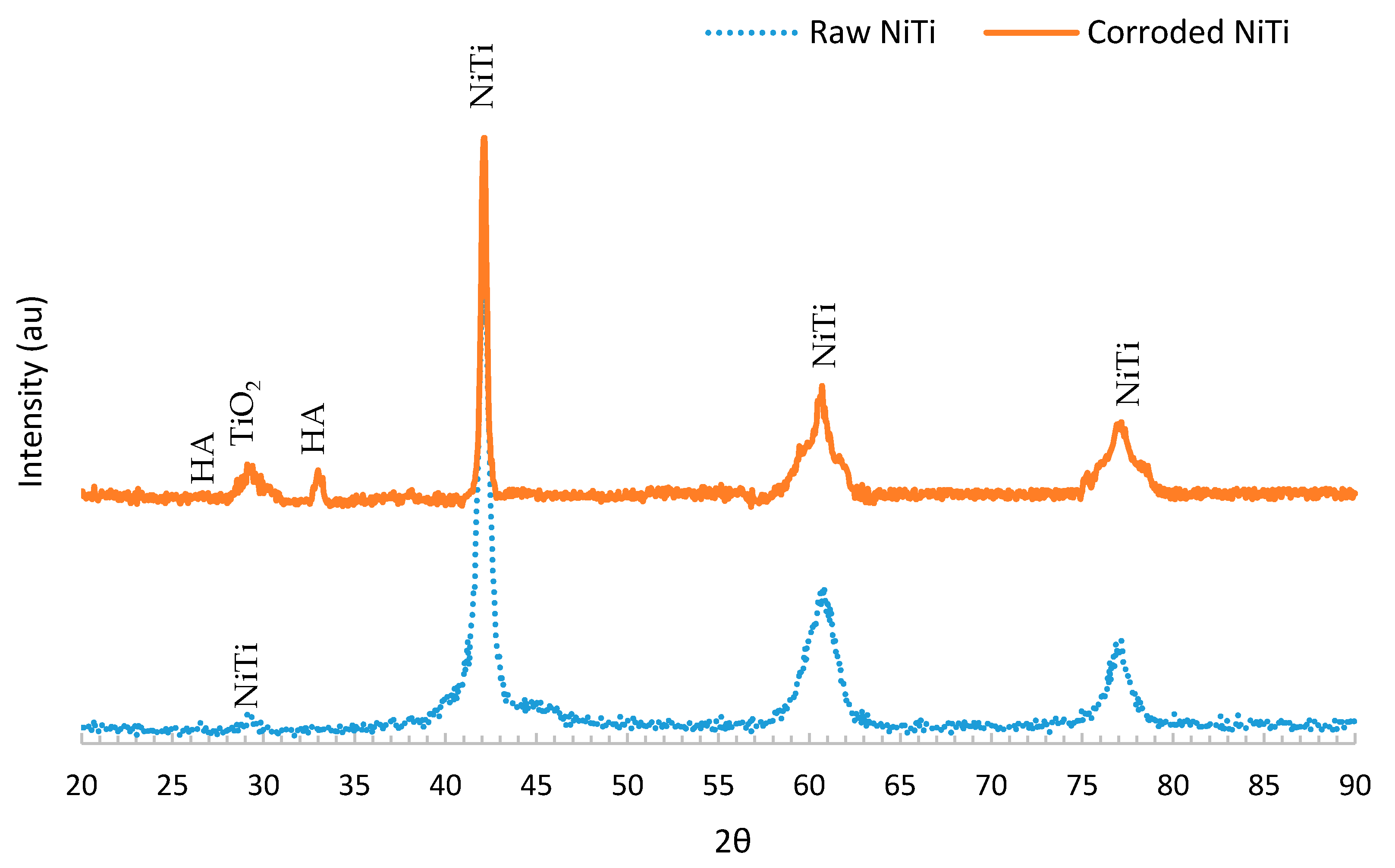
3.3. X-ray Diffraction (XRD)
3.4. Ni Ion Release (Immersion Test)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Esenwein, S.; Bogdanski, D.; Köller, M.; Krone, L.; Epple, M.; Muhr, G. Clinical applications of shape memory alloys based on niti as implant materials—Possibilities in trauma and orthopaedic surgery. SMST Proc. 2006. [Google Scholar] [CrossRef]
- Duerig, T.; Pelton, A.; Stöckel, D. An overview of nitinol medical applications. Mater. Sci. Eng. A 1999, 273, 149–160. [Google Scholar] [CrossRef]
- Peitsch, T.; Klocke, A.; Kahl-Nieke, B.; Prymak, O.; Epple, M. The release of nickel from orthodontic niti wires is increased by dynamic mechanical loading but not constrained by surface nitridation. J. Biomed. Mater. Res. Part A 2007, 82, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Lima de Miranda, R.; Zamponi, C.; Quandt, E. Micropatterned freestanding superelastic tini films. Adv. Eng. Mater. 2013, 15, 66–69. [Google Scholar] [CrossRef]
- Elahinia, M.H.; Hashemi, M.; Tabesh, M.; Bhaduri, S.B. Manufacturing and processing of niti implants: A review. Prog. Mater. Sci. 2012, 57, 911–946. [Google Scholar] [CrossRef]
- Shabalovskaya, S.A. On the nature of the biocompatibility and on medical applications of niti shape memory and superelastic alloys. Bio-Med. Mater. Eng. 1996, 6, 267–289. [Google Scholar]
- Rahmanian, R.; Moghaddam, N.S.; Haberland, C.; Dean, D.; Miller, M.; Elahinia, M. Load bearing and stiffness tailored niti implants produced by additive manufacturing: A simulation study. In SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring: International Society for Optics and Photonics; SPIE: Bellingham, WA, USA, 2014; pp. 905814–905818. [Google Scholar]
- Stergioudi, F.; Vogiatzis, C.; Pavlidou, E.; Skolianos, S.; Michailidis, N. Corrosion resistance of porous niti biomedical alloy in simulated body fluids. Smart Mater. Struct. 2016, 25, 095024. [Google Scholar] [CrossRef]
- Hu, T.; Chu, C.; Xin, Y.; Wu, S.; Yeung, K.W.; Chu, P.K. Corrosion products and mechanism on niti shape memory alloy in physiological environment. J. Mater. Res. 2010, 25, 350–358. [Google Scholar] [CrossRef]
- Hu, T.; Xin, Y.; Wu, S.; Chu, C.; Lu, J.; Guan, L.; Chen, H.; Hung, T.F.; Yeung, K.; Chu, P.K. Corrosion behavior on orthopedic niti alloy with nanocrystalline/amorphous surface. Mater. Chem. Phys. 2011, 126, 102–107. [Google Scholar] [CrossRef]
- Figueira, N.; Silva, T.; Carmezim, M.; Fernandes, J. Corrosion behaviour of niti alloy. Electrochim. Acta 2009, 54, 921–926. [Google Scholar] [CrossRef]
- Tan, L.; Dodd, R.; Crone, W. Corrosion and wear-corrosion behavior of niti modified by plasma source ion implantation. Biomaterials 2003, 24, 3931–3939. [Google Scholar] [CrossRef]
- Xu, J.; Jin, X.; Luo, J.; Zhong, Z. Fabrication and properties of porous niti alloys by microwave sintering for biomedical applications. Mater. Lett. 2014, 124, 110–112. [Google Scholar] [CrossRef]
- Cheng, F.; Shi, P.; Man, H. A preliminary study of TiO2 deposition on niti by a hydrothermal method. Surf. Coat. Technol. 2004, 187, 26–32. [Google Scholar] [CrossRef]
- Rongo, R.; Ametrano, G.; Gloria, A.; Spagnuolo, G.; Galeotti, A.; Paduano, S.; Valletta, R.; D’Antò, V. Effects of intraoral aging on surface properties of coated nickel-titanium archwires. Angle Orthod. 2013, 84, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Wever, D.; Veldhuizen, A.; De Vries, J.; Busscher, H.; Uges, D.; Van Horn, J. Electrochemical and surface characterization of a nickel-titanium alloy. Biomaterials 1998, 19, 761–769. [Google Scholar] [CrossRef]
- Ma, C.; Andani, M.T.; Qin, H.; Moghaddam, N.S.; Ibrahim, H.; Jahadakbar, A.; Amerinatanzi, A.; Ren, Z.; Zhang, H.; Doll, G.L. Improving surface finish and wear resistance of additive manufactured nickel-titanium by ultrasonic nano-crystal surface modification. J. Mater. Process. Technol. 2017, 249, 433–440. [Google Scholar] [CrossRef]
- Wu, H.; Wang, T.; Liu, X.; Lin, N.; Liu, X.; He, Z.; Wang, Z. Fabrication and corrosion resistance of the ti-rich alloyed layer on the surface of niti alloys. Int. J. Electrochem. Sci. 2017, 12, 2376–2388. [Google Scholar] [CrossRef]
- Bolat, G.; Mareci, D.; Iacoban, S.; Cimpoesu, N.; Munteanu, C. The estimation of corrosion behavior of niti and nitinb alloys using dynamic electrochemical impedance spectroscopy. J. Spectrosc. 2012, 2013, 714920. [Google Scholar] [CrossRef]
- Ryhänen, J.; Niemi, E.; Serlo, W.; Niemelä, E.; Sandvik, P.; Pernu, H.; Salo, T. Biocompatibility of nickel-titanium shape memory metal and its corrosion behavior in human cell cultures. J. Biomed. Mater. Res. Part A 1997, 35, 451–457. [Google Scholar] [CrossRef]
- El Medawar, L.; Rocher, P.; Hornez, J.-C.; Traisnel, M.; Breme, J.; Hildebrand, H. Electrochemical and cytocompatibility assessment of nitinol memory shape alloy for orthodontic use. Biomol. Eng. 2002, 19, 153–160. [Google Scholar] [CrossRef]
- Bernard, S.A.; Balla, V.K.; Davies, N.M.; Bose, S.; Bandyopadhyay, A. Bone cell-materials interactions and ni ion release of anodized equiatomic niti alloy. Acta Biomater. 2011, 7, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Andani, M.T.; Moghaddam, N.S.; Haberland, C.; Dean, D.; Miller, M.J.; Elahinia, M. Metals for bone implants. Part 1. Powder metallurgy and implant rendering. Acta Biomater. 2014, 10, 4058–4070. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Esfahani, S.N.; Poorganji, B.; Dean, D.; Elahinia, M. Resorbable bone fixation alloys, forming, and post-fabrication treatments. Mater. Sci. Eng. C 2017, 70, 870–888. [Google Scholar] [CrossRef] [PubMed]
- Amerinatanzi, A.; Moghaddam, N.S.; Ibrahim, H.; Elahinia, M. The effect of porosity type on the mechanical performance of porous niti bone implants. In Proceedings of the ASME 2016 Conference on Smart Materials, Adaptive Structures and Intelligent Systems, San Antonio, TX, USA, 10–12 September 2016; American Society of Mechanical Engineers: New York, NY, USA, 2016; p. V002T006A018. [Google Scholar]
- Jahadakbar, A.; Shayesteh Moghaddam, N.; Amerinatanzi, A.; Dean, D.; Karaca, H.E.; Elahinia, M. Finite element simulation and additive manufacturing of stiffness-matched niti fixation hardware for mandibular reconstruction surgery. Bioengineering 2016, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, N.S.; Skoracki, R.; Miller, M.; Elahinia, M.; Dean, D. Three dimensional printing of stiffness-tuned, nitinol skeletal fixation hardware with an example of mandibular segmental defect repair. Procedia CIRP 2016, 49, 45–50. [Google Scholar] [CrossRef]
- Youssef, A.; Hollister, S.J.; Dalton, P.D. Additive manufacturing of polymer melts for implantable medical devices and scaffolds. Biofabrication 2017, 9, 012002. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Mehanny, S.; Darwish, L.; Farag, M. A comparative study on the mechanical and biodegradation characteristics of starch-based composites reinforced with different lignocellulosic fibers. J. Polym. Environ. 2017, 1–14. [Google Scholar] [CrossRef]
- Ibrahim, H.; Klarner, A.D.; Poorganji, B.; Dean, D.; Luo, A.A.; Elahinia, M. Microstructural, mechanical and corrosion characteristics of heat-treated Mg-1.2 Zn-0.5 ca (wt %) alloy for use as resorbable bone fixation material. J. Mech. Behav. Biomed. Mater. 2017, 69, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Martorelli, M.; Maietta, S.; Gloria, A.; De Santis, R.; Pei, E.; Lanzotti, A. Design and analysis of 3d customized models of a human mandible. Procedia CIRP 2016, 49, 199–202. [Google Scholar] [CrossRef]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. Part A 2003, 65, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.W.; Führ, L.T.; Antonini, L.M.; Villarinho, D.J.; Marino, C.E.B.; Malfatti, C.D.F. The electrochemical behavior of the niti alloy in different simulated body fluids. Mater. Res. 2015, 18, 184–190. [Google Scholar] [CrossRef]
- Ramazanzadeh, B.A.; Ahrari, F.; Sabzevari, B.; Habibi, S. Nickel ion release from three types of nickel-titanium-based orthodontic archwires in the as-received state and after oral simulation. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 71. [Google Scholar]
- Azizi, A.; Jamilian, A.; Nucci, F.; Kamali, Z.; Hosseinikhoo, N.; Perillo, L. Release of metal ions from round and rectangular niti wires. Prog. Orthod. 2016, 17, 10. [Google Scholar] [CrossRef] [PubMed]
| Effective Laser Power (W) | Layer Thickness (μm) | Scanning Velocity (m/s) | Hatch Distance (μm) | Energy Input (J/mm3) |
|---|---|---|---|---|
| 250 | 30 | 1.25 | 120 | 55.5 |
| Reagent | Amount |
|---|---|
| NaCl | 5.403 g |
| NaHCO3 | 0.504 g |
| Na2CO3 | 0.426 g |
| KCl | 0.225 g |
| K2HPO4·3H2O | 0.23 g |
| MgCl2·6H2O | 0.311 g |
| 0.2 mol L−1 NaOH | 100 mL |
| HEPES | 17.892 g |
| CaCl2 | 0.293 g |
| Na2SO4 | 0.072 g |
| 1 mol L−1 NaOH | 15 mL |
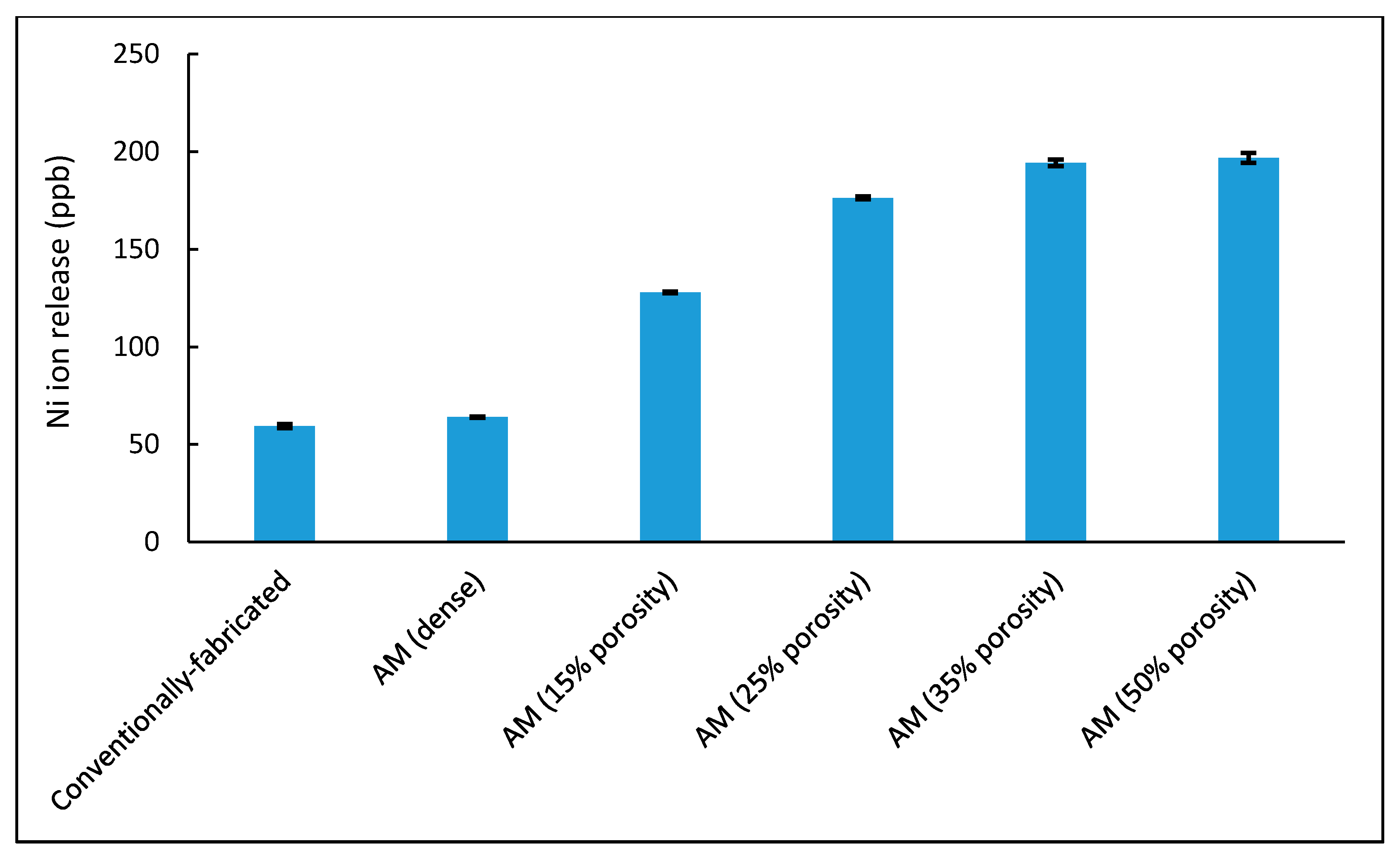
| Sample Type | Surface Area (mm2) | Ni Ions (ppb) (Diluted) | Ni Ions (ppb) |
|---|---|---|---|
| Conventionally-fabricated | 590 | 5.945 | 59.45 |
| AM (dense) | 594 | 6.4 | 64 |
| AM (15% porosity) | 1620.3 | 12.79 | 127.94 |
| AM (25% porosity) | 1997.5 | 17.64 | 176.35 |
| AM (35% porosity) | 2106.6 | 19.43 | 194.3 |
| AM (50% porosity) | 2116.3 | 19.68 | 196.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, H.; Jahadakbar, A.; Dehghan, A.; Moghaddam, N.S.; Amerinatanzi, A.; Elahinia, M. In Vitro Corrosion Assessment of Additively Manufactured Porous NiTi Structures for Bone Fixation Applications. Metals 2018, 8, 164. https://doi.org/10.3390/met8030164
Ibrahim H, Jahadakbar A, Dehghan A, Moghaddam NS, Amerinatanzi A, Elahinia M. In Vitro Corrosion Assessment of Additively Manufactured Porous NiTi Structures for Bone Fixation Applications. Metals. 2018; 8(3):164. https://doi.org/10.3390/met8030164
Chicago/Turabian StyleIbrahim, Hamdy, AhmadReza Jahadakbar, Amir Dehghan, Narges Shayesteh Moghaddam, Amirhesam Amerinatanzi, and Mohammad Elahinia. 2018. "In Vitro Corrosion Assessment of Additively Manufactured Porous NiTi Structures for Bone Fixation Applications" Metals 8, no. 3: 164. https://doi.org/10.3390/met8030164





