Microstructural Characteristics and m23c6 Precipitate Behavior of the Course-Grained Heat-Affected Zone of T23 Steel without Post-Weld Heat Treatment
Abstract
:1. Introduction
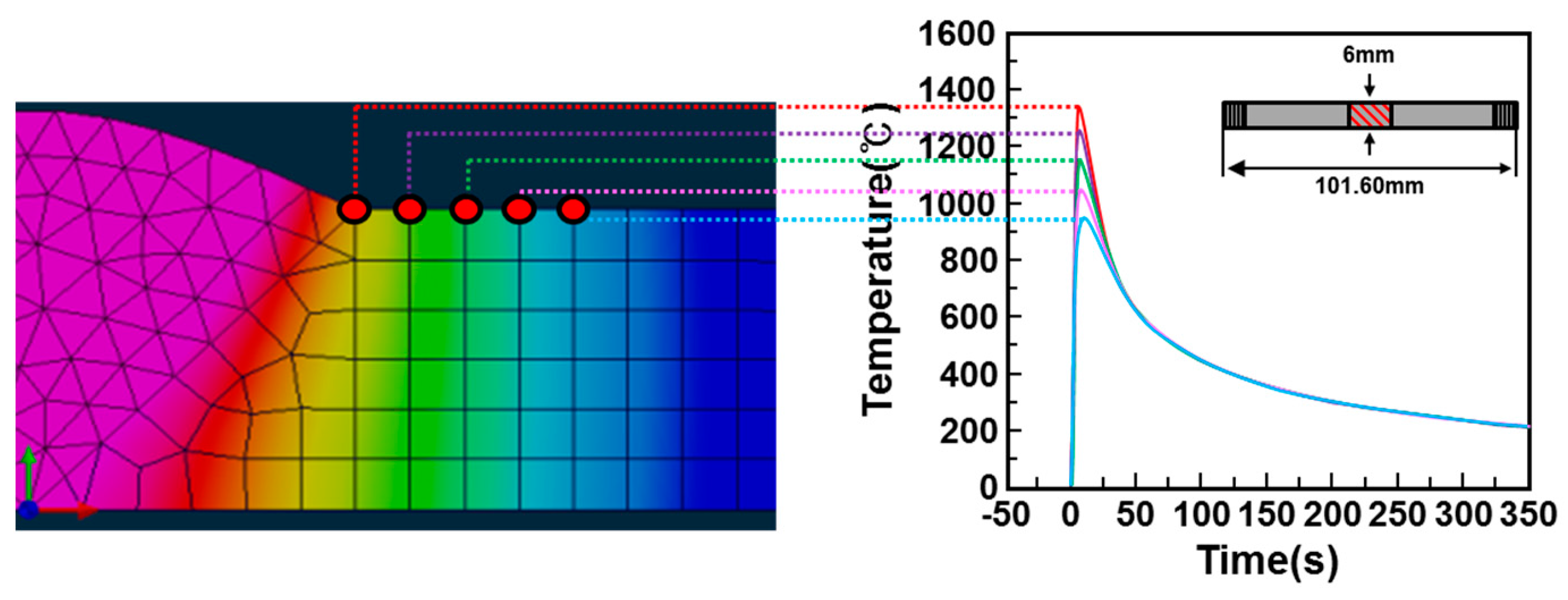
2. Materials and Methods
3. Results
3.1. Microstructure Evolution of the Base Metal
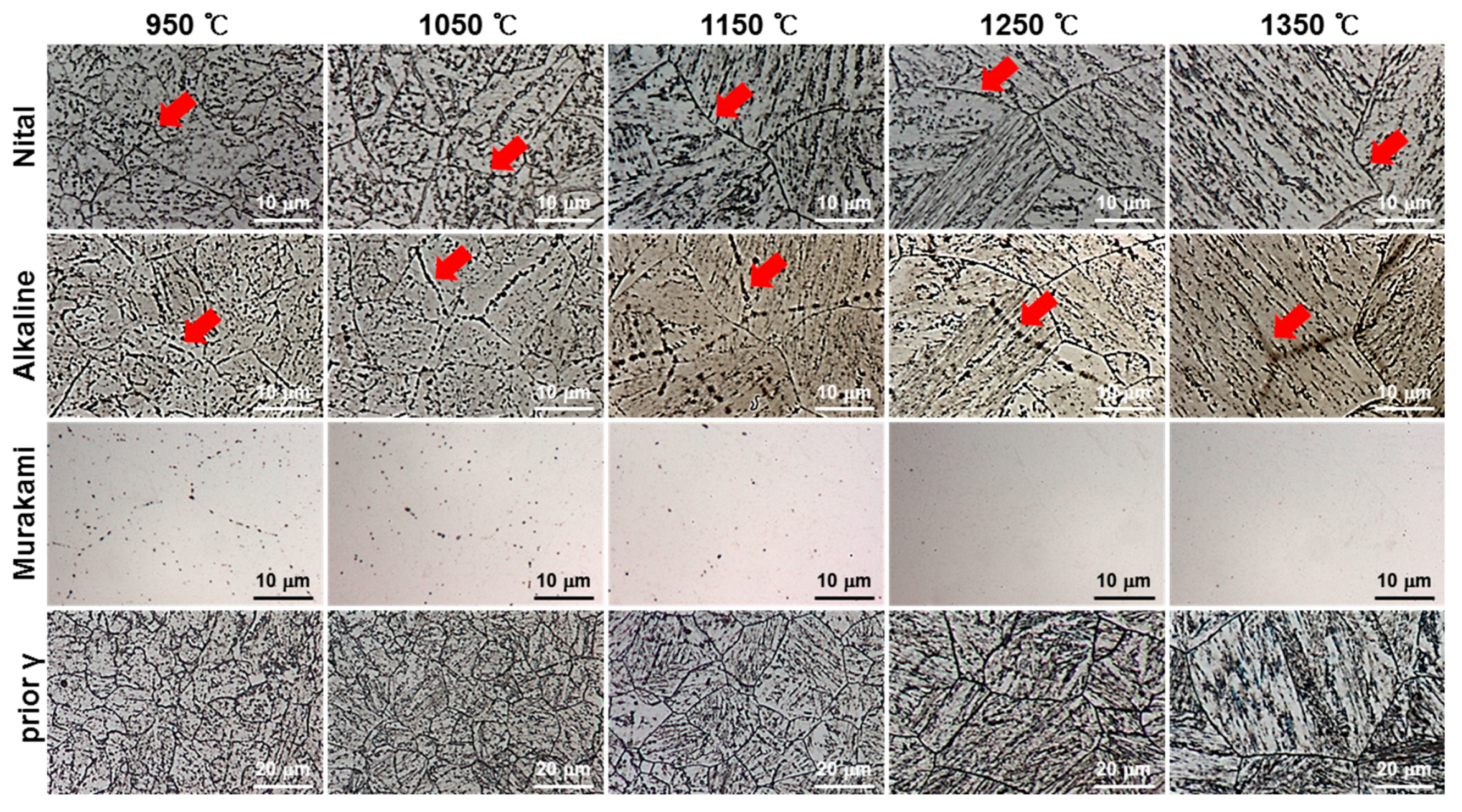
3.2. Microstructure Evolution of the Simulated HAZ at Various Peak Temperatures
4. Discussion
4.1. Correlation between Peak Temperature and Grain Size
4.2. Correlation between Carbide Dissolution and Austenite Formation
5. Conclusions
- (1)
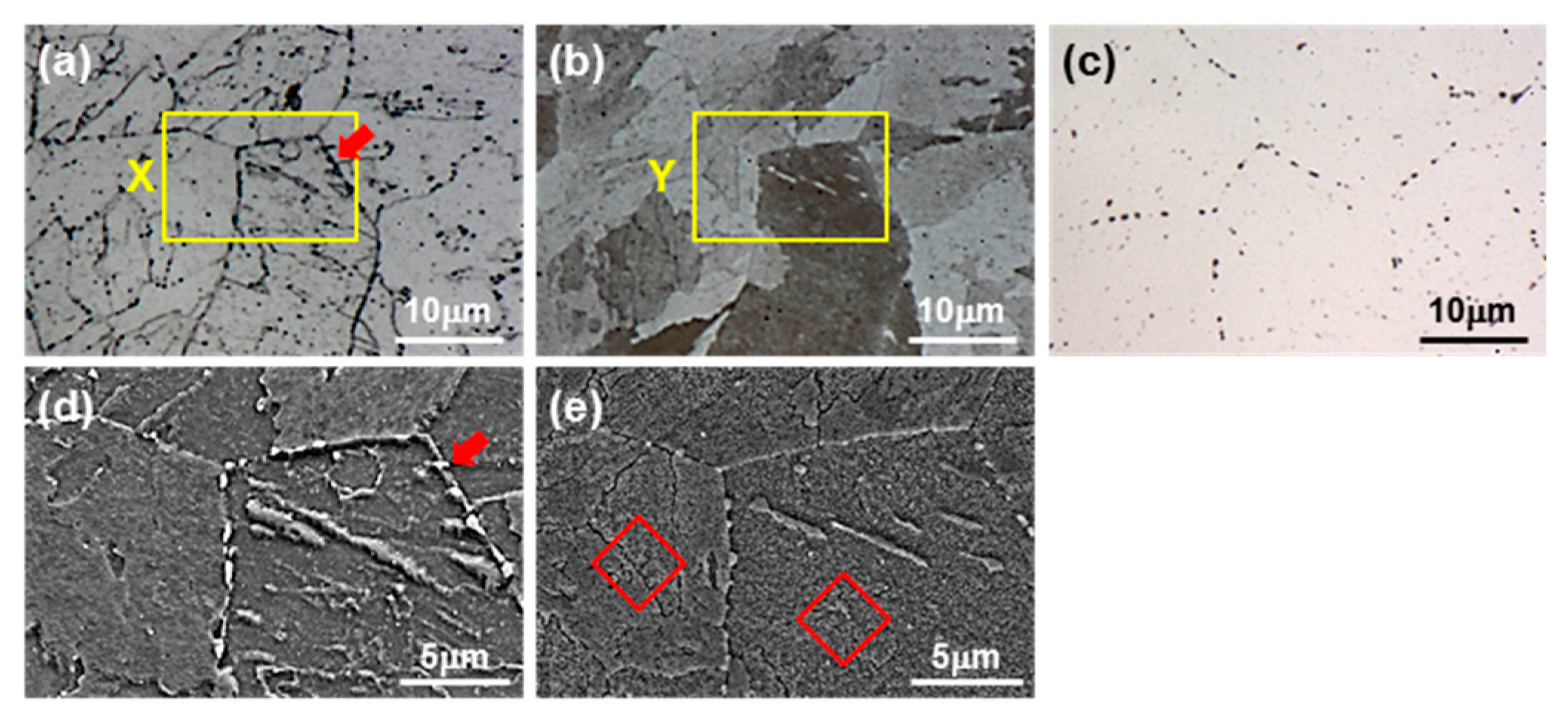
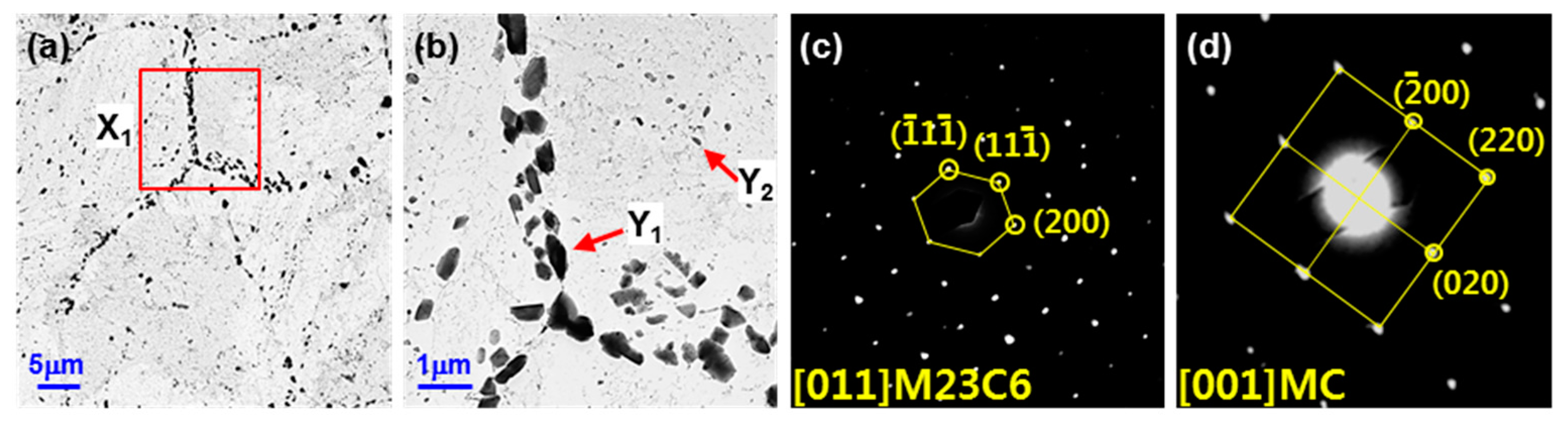
- When the base metal of T23 was etched with alkaline sodium picrate, the ivory color obtained could be identified as tempered martensite and the brown color obtained was identified as bainite. In the case of Murakami etching, carbide was observed, and the grain boundary precipitate was M23C6 and the transgranular precipitate was MC.
- (2)
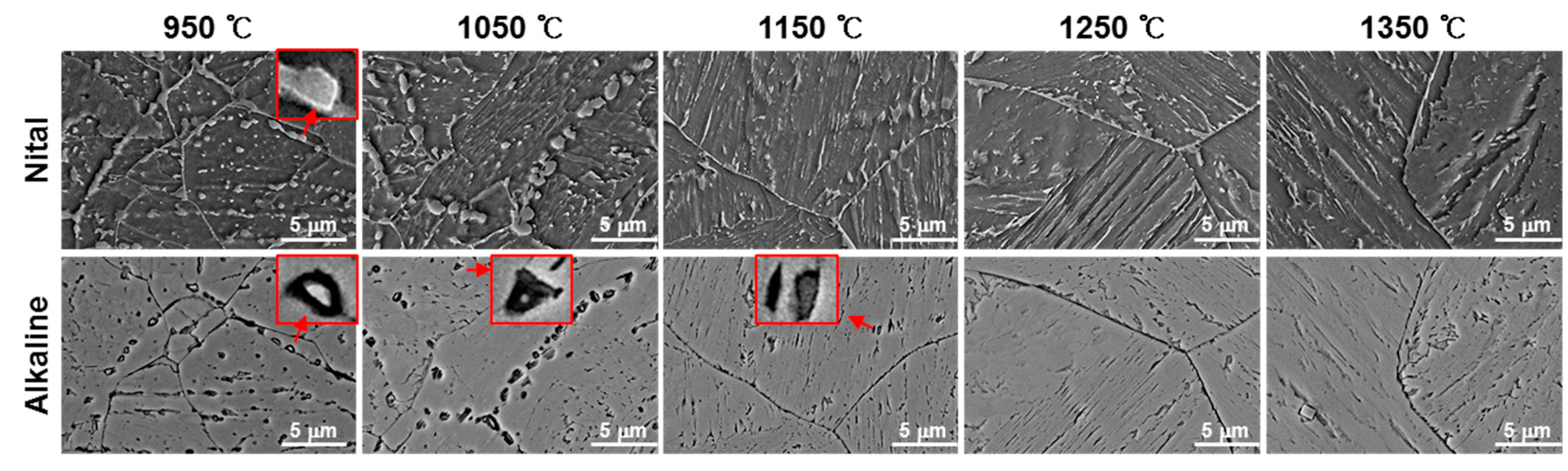
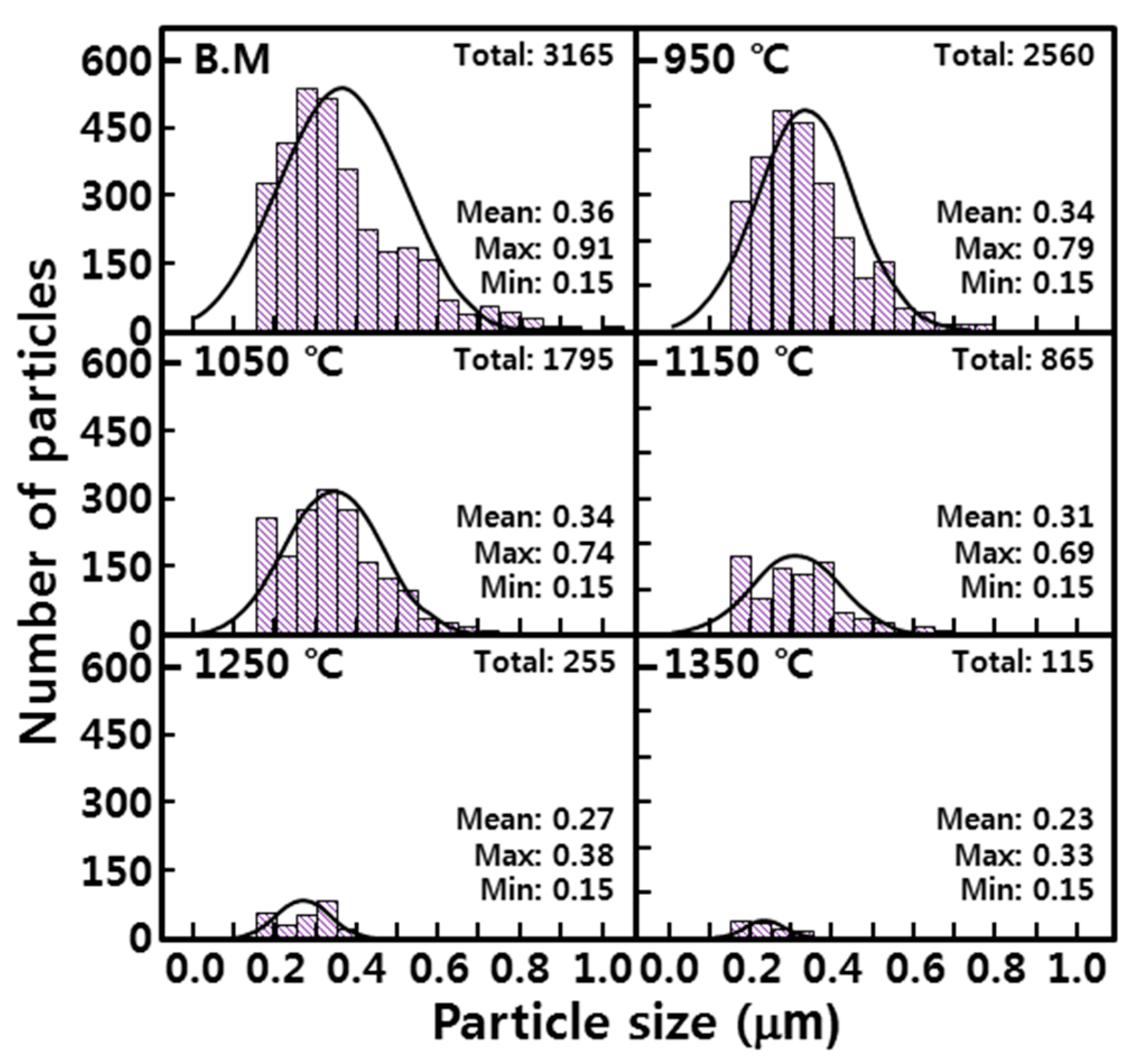
- In order to investigate the microstructure formation process of CGHAZ, the microstructure of the simulated HAZ at various peak temperatures (950–1350 °C) was observed by etching separately with alkaline sodium picrate and nital. Alkaline-sodium-picrate-etched microstructures exhibited a black dot or band, which was not observed in the nital-etched microstructure. The black dot or band had a transgranular distribution, regardless of the nature of the prior austenite grain boundary. As the temperature increased from 950 to 1350 °C, the black dot became wider and thicker and turned into black bands.
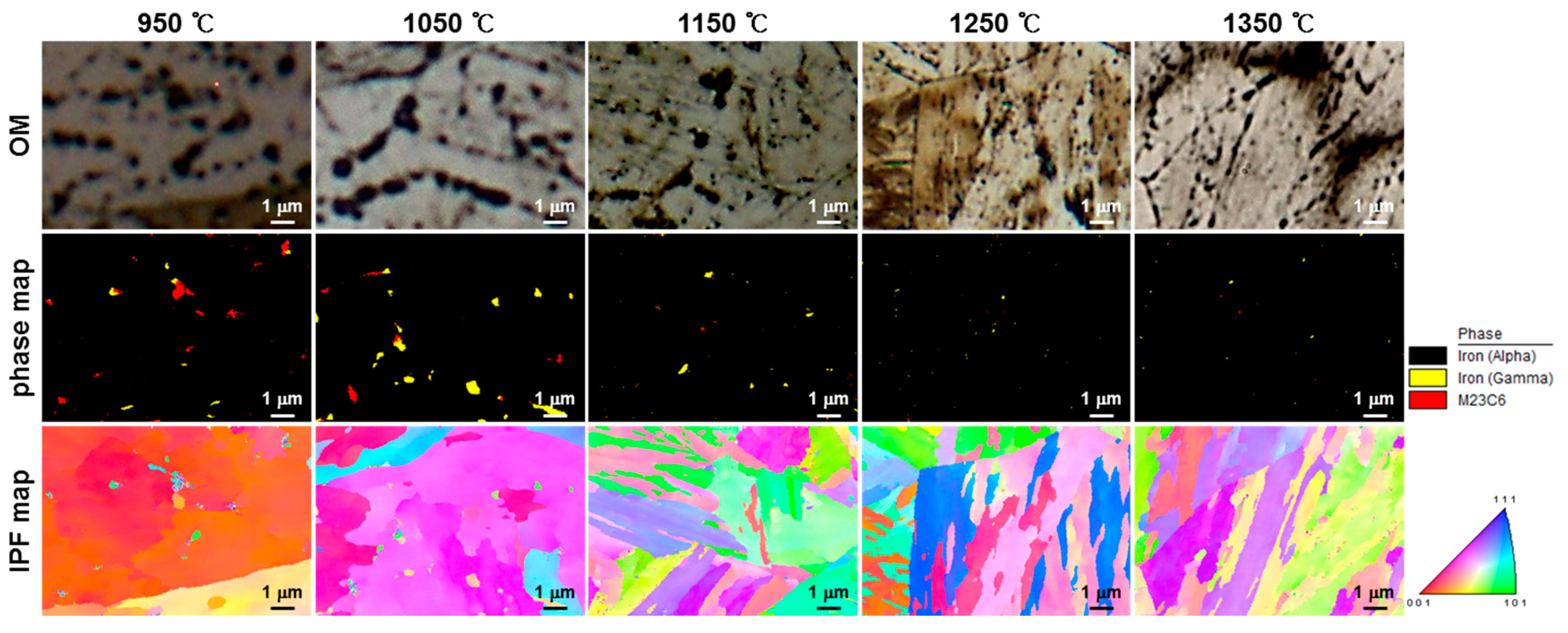
- (3)
- Rectangular M23C6 precipitate was present at the center of the black dot with the size of a few micrometers at the peak temperatures of 950 and 1050 °C, and austenite was present around the precipitates. As the peak temperature increased (1150–1350 °C), the M23C6 and austenite decreased, and the martensite phase, which is the same as the matrix, increased in the black band region.
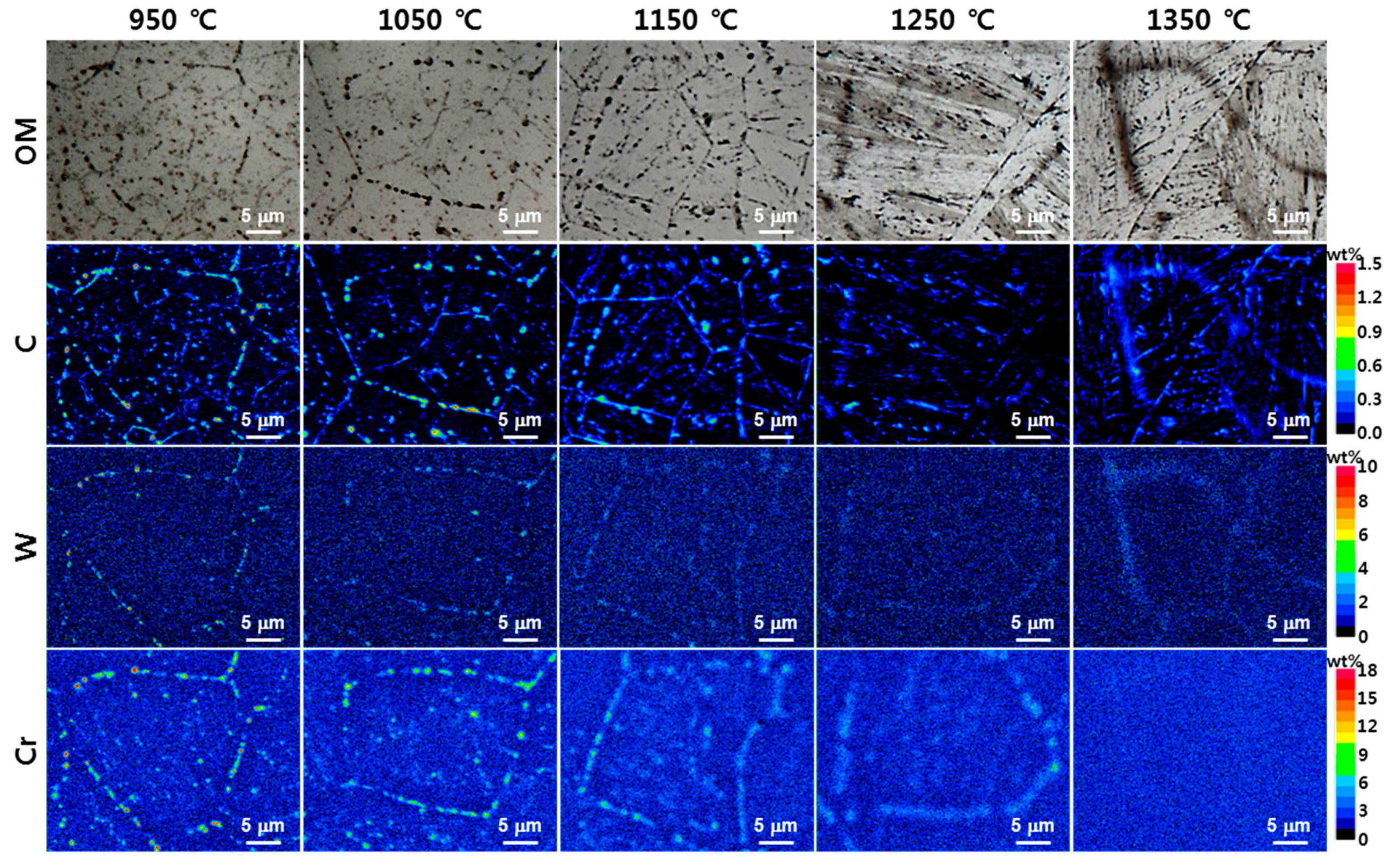
- (4)
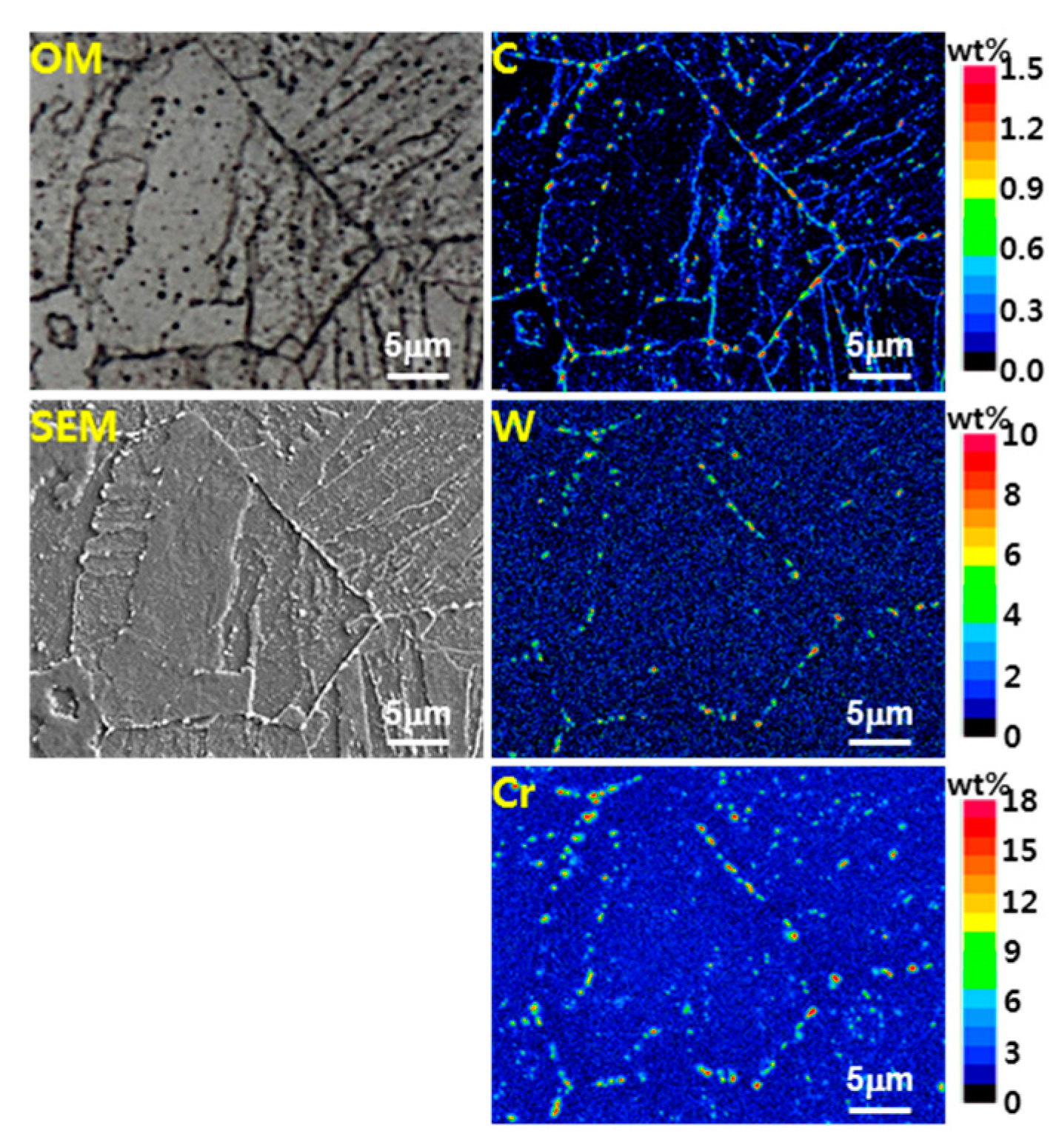
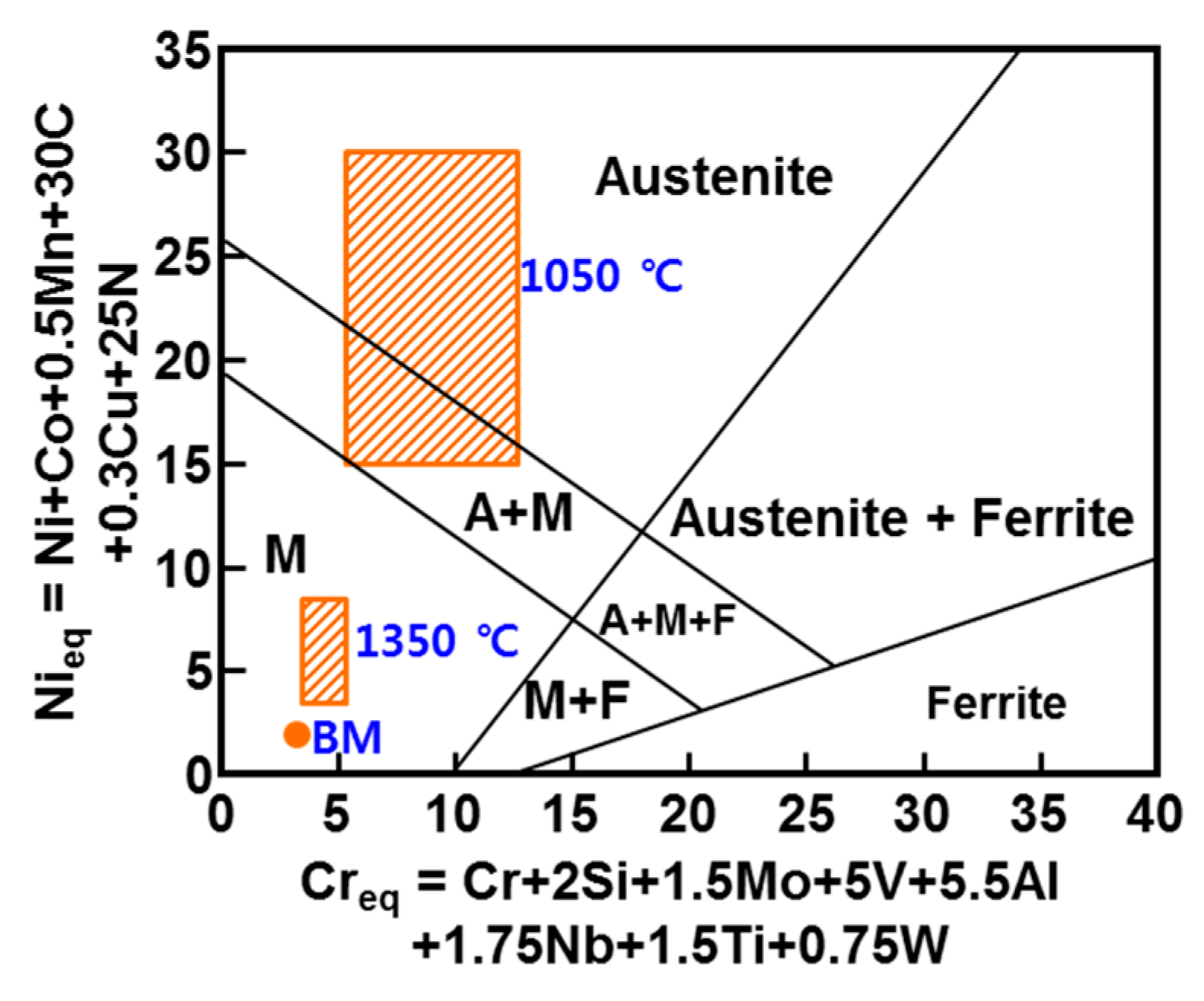
- In the EPMA analysis, the M23C6 (C-, W-, and Cr-rich phases) precipitate of the prior austenite grain boundary in the base metal gradually dissolved with the increase in peak temperature (950–1350 °C). Therefore, it was observed that C, W, and Cr, which are the main alloying elements of M23C6, gradually diffused into the matrix. Therefore, the M23C6 with a size less than 1 µm in the base metal changed to a black dot that was coarser than the M23C6 in the base material when the peak temperature increased to 950 and 1050 °C. As the peak temperature increased from 1050 to 1350 °C, the M23C6 carbide changed into a thick black band because M23C6 (C, W-, and Cr-rich phases) gradually dissolved and diffused into the matrix. According to the amount of M23C6 diffusion, the Schaeffler diagram showed that the alloying component of the black dot (C: 0.6–1.0%; W: 3–4%; Cr: 7–12%) at the peak temperature of 1050 °C was the austenite region and that the alloy component of the black band (C: 0.2–0.3%; W: 2–3%; Cr: 2–3%) at the peak temperature of 1350 °C was the martensite region.
- (5)
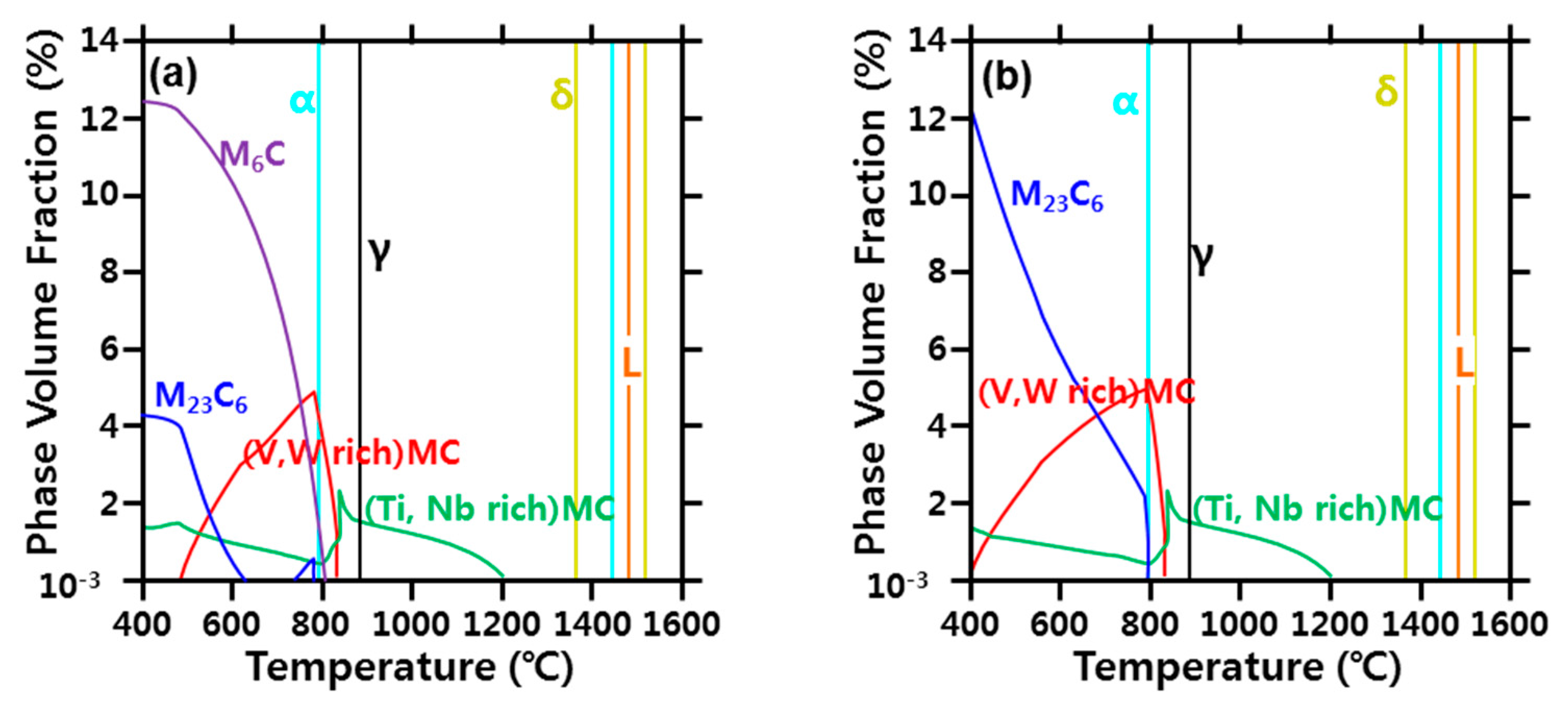
- In the calculation using Thermo-Calc, the M23C6 carbide (C-, W-, and Cr-rich phases) was stable at 800 °C or less. M23C6 dissolved above 800 °C, and the size and volume fraction of the M23C6 carbide decreased rapidly as the peak temperature increased (950–1350 °C). Therefore, it was concluded that the austenite grain size increased because the precipitates that interfered with grain boundary migration decreased.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klueh, R.; Nelson, A. Ferritic/martensitic steels for next-generation reactors. J. Nucl. Mater. 2007, 371, 37–52. [Google Scholar] [CrossRef]
- Bendick, W.; Gabrel, J.; Hahn, B.; Vandenberghe, B. New low alloy heat resistant ferritic steels T/P23 and T/P24 for power plant application. Int. J. Press. Vessels Pip. 2007, 84, 13–20. [Google Scholar] [CrossRef]
- Viswanathan, R.; Bakker, W. Materials for Ultra Supercritical Fossil Power Plants; EPRI Report No. TR-114750; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Dhooge, A.; Vekeman, J. New generation 21/4Cr steels T/P 23 and T/P 24 weldability and high temperature properties. Weld. World 2005, 49, 75–93. [Google Scholar] [CrossRef]
- Evans, G. The effect of heat input on the microstructure and properties of C-Mn all-weld-metal deposits. Weld. J. 1982, 61, 125–132. [Google Scholar]
- Dixon, B.; Hakansson, K. Effects of welding parameters on weld zone toughness and hardness in 690 MPa steel. Weld. J. 1995, 74, 122S–132S. [Google Scholar]
- Haarmann, K.; Kottmann, G.; Vaillant, J. The T23/24 Book; Vallourec & Mannesmann Tubes: Houston, TX, USA, 2000. [Google Scholar]
- Nawrocki, J.; Dupont, J.; Robino, C.V.; Marder, A. The Stress-Relief Cracking Susceptibility of a New Ferritic Steel-Part I: Single-Pass Heat-Affected Zone Simulations; Sandia National Labs.: Albuquerque, NM, USA; Livermore, CA, USA, 1999. [Google Scholar]
- Nawrocki, J.; Dupont, J.; Robino, C.; Puskar, J.; Marder, A. The mechanism of stress-relief cracking in a ferritic alloy steel. Weld. J. N. Y. 2003, 82, 25S–35S. [Google Scholar]
- Masuyama, F.; Yokoyama, T.; Sawaragi, Y.; Iseda, A. Service Exposure and Reliability Improvement: Nuclear, Fossil, and Petrochemical Plants; The American Society of Mechanical Engineers (ASME): New York, NY, USA, 1994. [Google Scholar]
- Jiménez, J.A.; Carsí, M.; Ruano, O.A. Effect of rhenium on the microstructure and mechanical behavior of Fe–2.25 Cr–1.6 W–0.25 V-0.1 C bainitic steels. J. Mater. Sci. Technol. 2017, 33, 1487–1493. [Google Scholar] [CrossRef]
- Di Gianfrancesco, A. Materials for Ultra-Supercritical and Advanced Ultra-Supercritical Power Plants; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Wang, B.; Xue, X.; Feng, Z.Y.; An, F.Q.; Zheng, R.L.; Bo, Z.; Yong, Q.J.; Xiao, L. Research on water wall tubes and welded joints of 1000 MW USC tower boiler. In Proceedings of the 7th International Conference on Advances in Materials Technology for Fossil Power Plants, Waikoloa, HI, USA, 22–25 October 2013. [Google Scholar]
- Viswanathan, R. Advances in materials technology for fossil power plants. In Proceedings of the Fifth International Conference, Marco Island, FL, USA, 3–5 October 2007. [Google Scholar]
- Heo, N.; Chang, J.; Kim, S.-J. Elevated temperature intergranular cracking in heat-resistant steels. Mater. Sci. Eng. A 2013, 559, 665–677. [Google Scholar] [CrossRef]
- Park, K.; Kim, S.; Chang, J.; Lee, C. Post-weld heat treatment cracking susceptibility of T23 weld metals for fossil fuel applications. Mater. Des. 2012, 34, 699–706. [Google Scholar] [CrossRef]
- Chang, J.; Kim, B.; Heo, N. Stress relief cracking on the weld of T/P 23 steel. Procedia Eng. 2011, 10, 734–739. [Google Scholar] [CrossRef]
- Heo, N.; Chang, J.; Yoo, K.; Lee, J.; Kim, J. The mechanism of elevated temperature intergranular cracking in heat-resistant alloys. Mater. Sci. Eng. A 2011, 528, 2678–2685. [Google Scholar] [CrossRef]
- Chang, J.; Heo, N.; Lee, C. Intergranular cracking susceptibility of 2.25 Cr1. 3W and 9Cr1MoVNb weld metals at elevated temperatures. Met. Mater. Int. 2010, 16, 981–985. [Google Scholar] [CrossRef]
- Chang, J.; Heo, N.; Lee, C. Effects of mo addition on intergranular cracking behavior of 2.25 CrW (P23) weld metal at elevated temperatures. Met. Mater. Int. 2011, 17, 131–135. [Google Scholar] [CrossRef]
- Bang, H.-S.; Kim, J.-M. Proposed specimen for reheating cracking susceptibility and mechanical behaviour assessment in 2.25 Cr–1Mo steel pressure vessel weld joint. Sci. Technol. Weld. Join. 2001. [Google Scholar] [CrossRef]
- TAMAKI koreaki. Reheat cracking of Cr-Mo steels. J. Jpn. Weld. Soc. 2003, 72, 193–200. [Google Scholar]
- Lundin, C.; Kelley, S.; Menon, R.; Kruse, B. Stress rupture behavior of postweld heat treated 2. 25 Cr-1 Mo steel weld metal. Weld. Res. Counc. Bull. 1986, 315, 1–66. [Google Scholar]
- Hippsley, C.; Knott, J.; Edwards, B. A study of stress relief cracking in 214 Cr 1 mo steel—II. The effects of multi-component segregation. Acta Metall. 1982, 30, 641–654. [Google Scholar] [CrossRef]
- Shin, J.; McMahon, C. Mechanisms of stress relief cracking in a ferritic steel. Acta Metall. 1984, 32, 1535–1552. [Google Scholar] [CrossRef]
- Hippsley, C.; Knott, J.; Edwards, B. A study of stress relief cracking in 214Cr 1 Mo steel—I. The effects of P segregation. Acta Metall. 1980, 28, 869–885. [Google Scholar] [CrossRef]
- Balaguer, J.; Wang, Z.; Nippes, E. Stress-relief cracking of a copper-containing HSLA steel. Weld. J. 1989, 68, 121–131. [Google Scholar]
- Vaillant, J.; Vandenberghe, B.; Hahn, B.; Heuser, H.; Jochum, C. T/P23, 24, 911 and 92: New grades for advanced coal-fired power plants—properties and experience. Int. J. Press. Vessels Pip. 2008, 85, 38–46. [Google Scholar] [CrossRef]
- Zieliński, A.; Golański, G.; Sroka, M.; Skupień, P. Microstructure and mechanical properties of the T23 steel after long-term ageing at elevated temperature. Mater. High Temp. 2016, 33, 154–163. [Google Scholar] [CrossRef]
- Orr, J.; Robertson, D.G. Low alloy steels–the foundation of the power generation industry. In Proceedings of the 2nd International ECCC Conference, Creep & Fracture in High Temperature Components-Design & Life Assessment, Zurich, Switzerland, 21–23 April 2009; pp. 585–598. [Google Scholar]
- Wang, X.; Li, Y.; Li, H.; Lin, S.; Ren, Y. Effect of long-term aging on the microstructure and mechanical properties of T23 steel weld metal without post-weld heat treatment. J. Mater. Process. Technol. 2018, 252, 618–627. [Google Scholar] [CrossRef]
- Manohar, P.A.; Ferry, M.; Chandra, T. Five decades of the zener equation. ISIJ Int. 1998, 38, 913–924. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, M.; Zheng, Y.; Liu, S.; Wang, W.; Zhang, Z. Finite-element thermal analysis and grain growth behavior of HAZ on argon tungsten-Arc welding of 443 stainless steel. Metals 2016, 6, 77. [Google Scholar] [CrossRef]
- Du, Z.; Xiao, S.; Liu, J.; Lv, S.; Xu, L.; Kong, F.; Chen, Y. Hot deformation behavior of Ti-3.5 Al-5Mo-6V-3Cr-2Sn-0.5 Fe alloy in α + β field. Metals 2015, 5, 216–227. [Google Scholar] [CrossRef]
- Onoro, J. Martensite microstructure of 9–12% Cr steels weld metals. J. Mater. Process. Technol. 2006, 180, 137–142. [Google Scholar] [CrossRef]



| Material | Chemical Composition (atom %) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | Ni | Cr | Mo | V | Nb | Al | Ti | B | W | N | Fe | |
| SA213-T23 | 0.07 | 0.24 | 0.50 | 0.11 | 2.09 | 0.15 | 0.22 | 0.028 | 0.018 | 0.042 | 0.0017 | 1.66 | 0.0095 | Bal |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-H.; Na, H.-S.; Lee, K.-W.; Choe, Y.; Kang, C.Y. Microstructural Characteristics and m23c6 Precipitate Behavior of the Course-Grained Heat-Affected Zone of T23 Steel without Post-Weld Heat Treatment. Metals 2018, 8, 170. https://doi.org/10.3390/met8030170
Lee S-H, Na H-S, Lee K-W, Choe Y, Kang CY. Microstructural Characteristics and m23c6 Precipitate Behavior of the Course-Grained Heat-Affected Zone of T23 Steel without Post-Weld Heat Treatment. Metals. 2018; 8(3):170. https://doi.org/10.3390/met8030170
Chicago/Turabian StyleLee, Seong-Hyeong, Hye-Sung Na, Kyong-Woon Lee, Youngson Choe, and Chung Yun Kang. 2018. "Microstructural Characteristics and m23c6 Precipitate Behavior of the Course-Grained Heat-Affected Zone of T23 Steel without Post-Weld Heat Treatment" Metals 8, no. 3: 170. https://doi.org/10.3390/met8030170





