Formation of Bimetallic Fe/Au Submicron Particles with Ultrasonic Spray Pyrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiments
- Iron (II) acetate, trace metals basis ≥95%, Molekula (München, Germany)
- Iron (III) chloride hexahydrate, trace metals basis ≥98%, Molekula (München, Germany)
- Iron (III) nitrate nonahydrate, trace metals basis ≥98%, Molekula (München, Germany)
- Gold (III) chloride tetrahydrate, trace metals basis ≥99.9%, Acros Organics (Pittsburgh, PA, USA)
- Gold (III) acetate, trace metals basis ≥99.9%, Alfa Aesar (Haverhill, Massachusetts, USA)
- Gold (III) nitrate, trace metals basis ≥99.9%, American Elements (Los Angeles, California, USA)
2.2. Characterization
3. Results and Discussion
3.1. FeCl-AuCl
3.2. FeCl-2AuCl
3.3. FeCl-AuAc
3.4. FeCl-AuN
3.5. DLS and Zeta Potential Measurements
3.6. Summary of the Experiments
4. Conclusions
- The final form of the synthesized particles with USP depends greatly on the precursor used. Fe acetate forms meshes of Fe oxide nanoparticles; Fe nitrate forms spherical Fe oxide particles; and Fe chloride forms irregular Fe oxide particles. The salt type does not affect the sizes of Fe oxide particles.
- With a one-step USP synthesis of Fe/Au particles, the AuNPs form on Fe oxide particles. The AuNPs become finer and are dispersed more evenly with higher concentrations.
- Using different combinations of precursor salts produces particle shapes that correspond to the given salt. Knowing a salt’s effect on final particle morphology, predictions can be made. Intermediate products are also formed. Acetate will produce more mesh-like structures; nitrate will produce more spherical shapes. Chloride promotes anisotropic, irregular shapes.
- For more uniform Fe/Au particle production with USP, combinations of Fe chloride with Au chloride and Fe chloride with Au nitrate can be investigated further. The Fe chloride with Au acetate combination is not favorable for this endeavor.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ban, Z.; Barnakov, Y.A.; Li, F.; Golub, V.O.; O’Connor, C.J. The synthesis of core–shell iron@gold nanoparticles and their characterization. J. Mater. Chem. 2005, 15, 4660–4662. [Google Scholar] [CrossRef]
- Calvo, F.; Combe, N.; Morillo, J.; Benoit, M. Modeling Iron–Gold Nanoparticles Using a Dedicated Semi-Empirical Potential: Application to the Stability of Core–Shell Structures. J. Phys. Chem. C 2017, 121, 4680–4691. [Google Scholar] [CrossRef]
- Hoskins, C.; Min, Y.; Gueorguieva, M.; McDougall, C.; Volovick, A.; Prentice, P.; Wang, Z.; Melzer, A.; Cuschieri, A.; Wang, L. Hybrid gold-iron oxide nanoparticles as a multifunctional platform for biomedical application. J. Nanobiotechnol. 2012, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Kayal, S.; Ramanujan, R.V. Anti-Cancer Drug Loaded Iron–Gold Core–Shell Nanoparticles (Fe@Au) for Magnetic Drug Targeting. J. Nanosci. Nanotechnol. 2010, 10, 5527–5539. [Google Scholar] [CrossRef] [PubMed]
- Stafford, S.; Serrano Garcia, R.; Gun’ko, Y.K. Multimodal Magnetic-Plasmonic Nanoparticles for Biomedical Applications. Appl. Sci. 2018, 8, 97. [Google Scholar] [CrossRef]
- Kwizera, E.A.; Chaffin, E.; Wang, Y.; Huang, X. Synthesis and properties of magnetic-optical core–shell nanoparticles. RSC Adv. 2017, 7, 17137–17153. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.; Pradhan, D.; Kang, J.-S.; Leung, K.T. Nanoscale architecture of bimetallic hybrid Fe–Au nanostructures with and without 1,4-phenylene diisocyanide pre-functionalization. RSC Adv. 2015, 5, 31472–31478. [Google Scholar] [CrossRef]
- Swiatkowska-Warkocka, Z.; Koga, K.; Kawaguchi, K.; Wang, H.; Pyatenko, A.; Koshizaki, N. Pulsed laser irradiation of colloidal nanoparticles: A new synthesis route for the production of non-equilibrium bimetallic alloy submicrometer spheres. RSC Adv. 2012, 3, 79–83. [Google Scholar] [CrossRef]
- Swiatkowska-Warkocka, Z.; Pyatenko, A.; Koga, K.; Kawaguchi, K.; Wang, H.; Koshizaki, N. Various Morphologies/Phases of Gold-Based Nanocomposite Particles Produced by Pulsed Laser Irradiation in Liquid Media: Insight in Physical Processes Involved in Particles Formation. J. Phys. Chem. C 2017, 121, 8177–8187. [Google Scholar] [CrossRef]
- Majerič, P.; Jenko, D.; Friedrich, B.; Rudolf, R. Formation mechanisms for gold nanoparticles in a redesigned Ultrasonic Spray Pyrolysis. Adv. Powder Technol. 2017, 28, 876–883. [Google Scholar] [CrossRef]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.C.; Song, Y.L.; Tsai, C.S.; Yang, C.C.; Chiu, W.Y.; Lin, H.M. Ultrasonic spray pyrolysis for nanoparticles synthesis. J. Mater. Sci. 2004, 39, 3647–3657. [Google Scholar] [CrossRef]
- Santoyo Salazar, J.; Perez, L.; de Abril, O.; Truong Phuoc, L.; Ihiawakrim, D.; Vazquez, M.; Greneche, J.-M.; Begin-Colin, S.; Pourroy, G. Magnetic Iron Oxide Nanoparticles in 10−40 nm Range: Composition in Terms of Magnetite/Maghemite Ratio and Effect on the Magnetic Properties. Chem. Mater. 2011, 23, 1379–1386. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- González-Carreño, T.; Morales, M.P.; Gracia, M.; Serna, C.J. Preparation of uniform γ-Fe2O3 particles with nanometer size by spray pyrolysis. Mater. Lett. 1993, 18, 151–155. [Google Scholar] [CrossRef]
- Wu, Y.-N.; Wu, P.-C.; Yang, L.-X.; Ratinac, K.R.; Thordarson, P.; Jahn, K.A.; Chen, D.-H.; Shieh, D.-B.; Braet, F. The anticancer properties of iron core–gold shell nanoparticles in colorectal cancer cells. Int. J. Nanomed. 2013, 8, 3321–3331. [Google Scholar] [CrossRef] [PubMed]
- Shariq, M.; Majerič, P.; Friedrich, B.; Budic, B.; Jenko, D.; Dixit, A.R.; Rudolf, R. Application of Gold(III) Acetate as a New Precursor for the Synthesis of Gold Nanoparticles in PEG Through Ultrasonic Spray Pyrolysis. J. Clust. Sci. 2017, 28, 1647–1665. [Google Scholar] [CrossRef]
- Bogović, J.; Stopić, S.; Friedrich, B. Nanosized metallic oxide produced by Ultrasonic Spray Pyrolysis. In Proceedings of the European Metallurgical Conference, Düsseldorf, Germany, 26–29 June 2011. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Kodas, T.T.; Hampden-Smith, M.J. Aerosol Processing of Materials, 1st ed.; Wiley-VCH: New York, NY, USA, 1998; ISBN 978-0-471-24669-5. [Google Scholar]
- Jung, D.S.; Park, S.B.; Kang, Y.C. Design of particles by spray pyrolysis and recent progress in its application. Korean J. Chem. Eng. 2010, 27, 1621–1645. [Google Scholar] [CrossRef]
- Alkan, G.; Rudolf, R.; Bogovic, J.; Jenko, D.; Friedrich, B. Structure and Formation Model of Ag/TiO2 and Au/TiO2 Nanoparticles Synthesized through Ultrasonic Spray Pyrolysis. Metals 2017, 7, 389. [Google Scholar] [CrossRef]
- Pingali, K.C.; Rockstraw, D.A.; Deng, S. Silver Nanoparticles from Ultrasonic Spray Pyrolysis of Aqueous Silver Nitrate. Aerosol Sci. Technol. 2005, 39, 1010–1014. [Google Scholar] [CrossRef]
- Lee, S.D.; Nam, S.-H.; Kim, M.-H.; Kim, Y.D.; Boo, J.-H. Synthesis of ZnO nanoparticles by spray-pyrolysis method and their photocatalytic effect. In Proceedings of the 2010 3rd International Nanoelectronics Conference (INEC), HongKong, China, 3–8 January 2010; pp. 572–573. [Google Scholar]
- Yu, J.-K.; Suh, S.-K.; Choi, J.-H.; Sohn, J.-G. Manufacture of Ultra Fine CuO Powder from Waste Copper Chloride Solution by Spray Pyrolysis Process. Geosyst. Eng. 2002, 5, 13–19. [Google Scholar] [CrossRef]
- Bogovic, J.; Rudolf, R.; Friedrich, B. The Controlled Single-Step Synthesis of Ag/TiO2 and Au/TiO2 by Ultrasonic Spray Pyrolysis (USP). JOM J. Miner. Met. Amp Mater. Soc. 2015, 68, 330–335. [Google Scholar] [CrossRef]
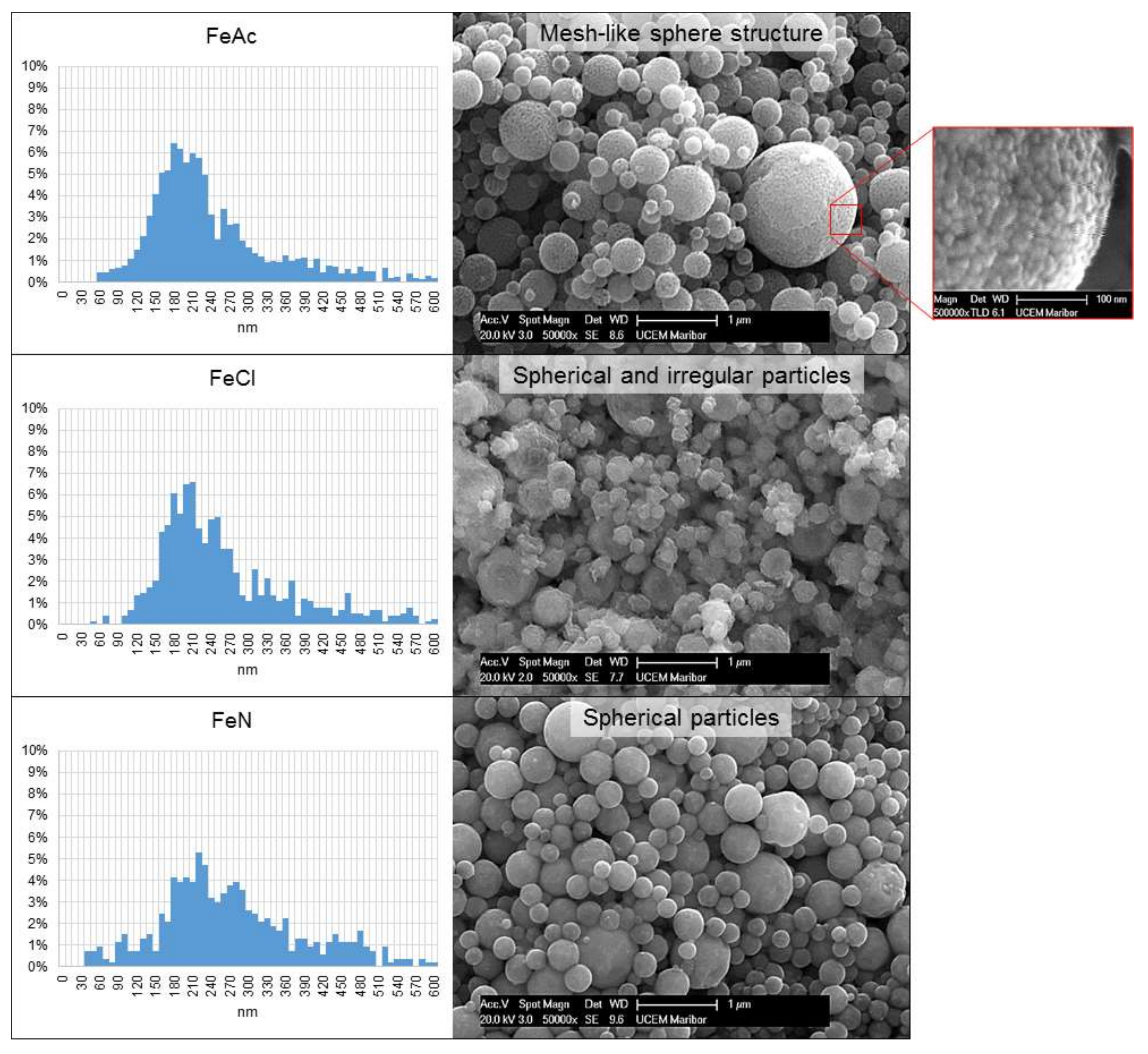

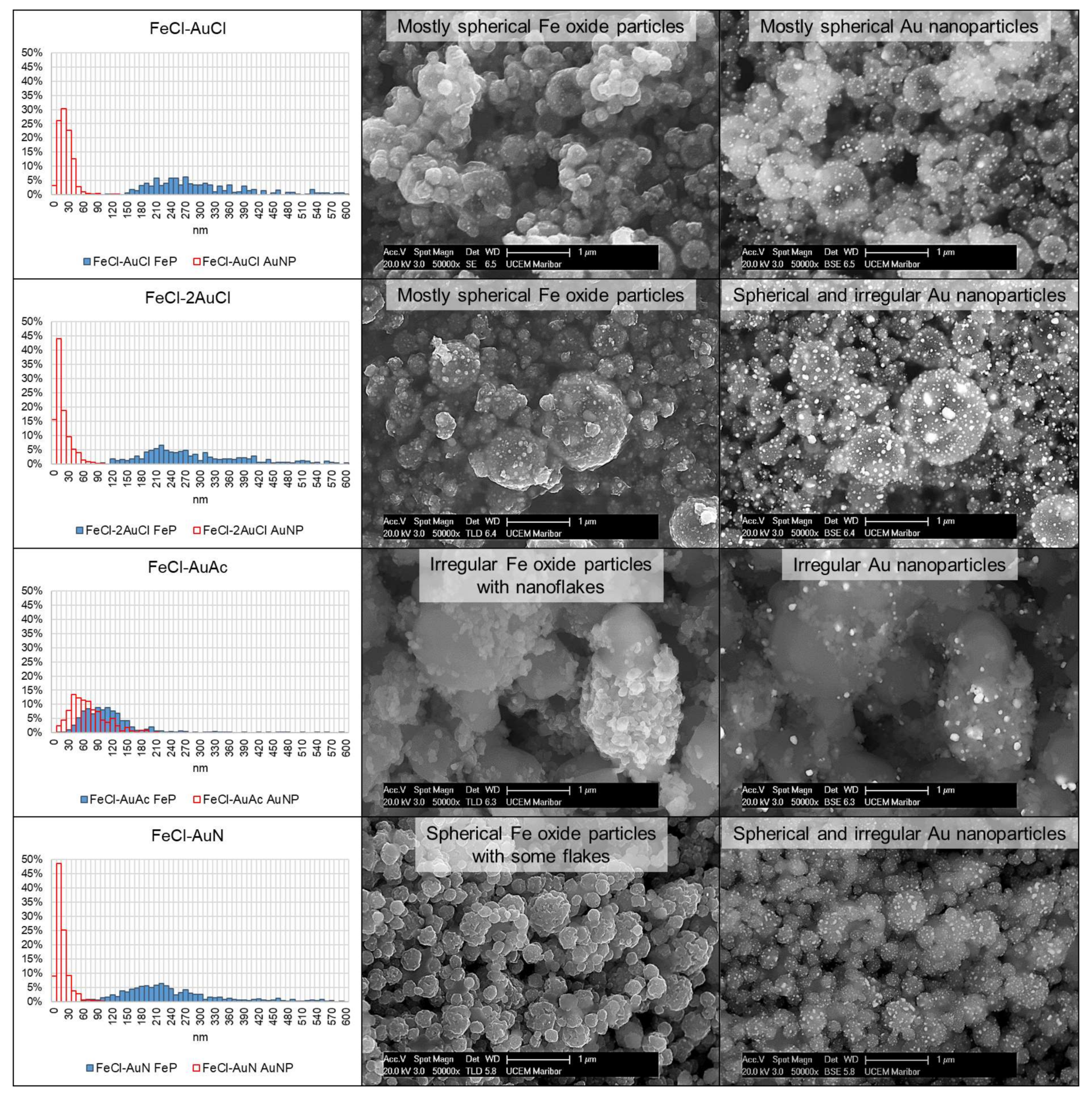
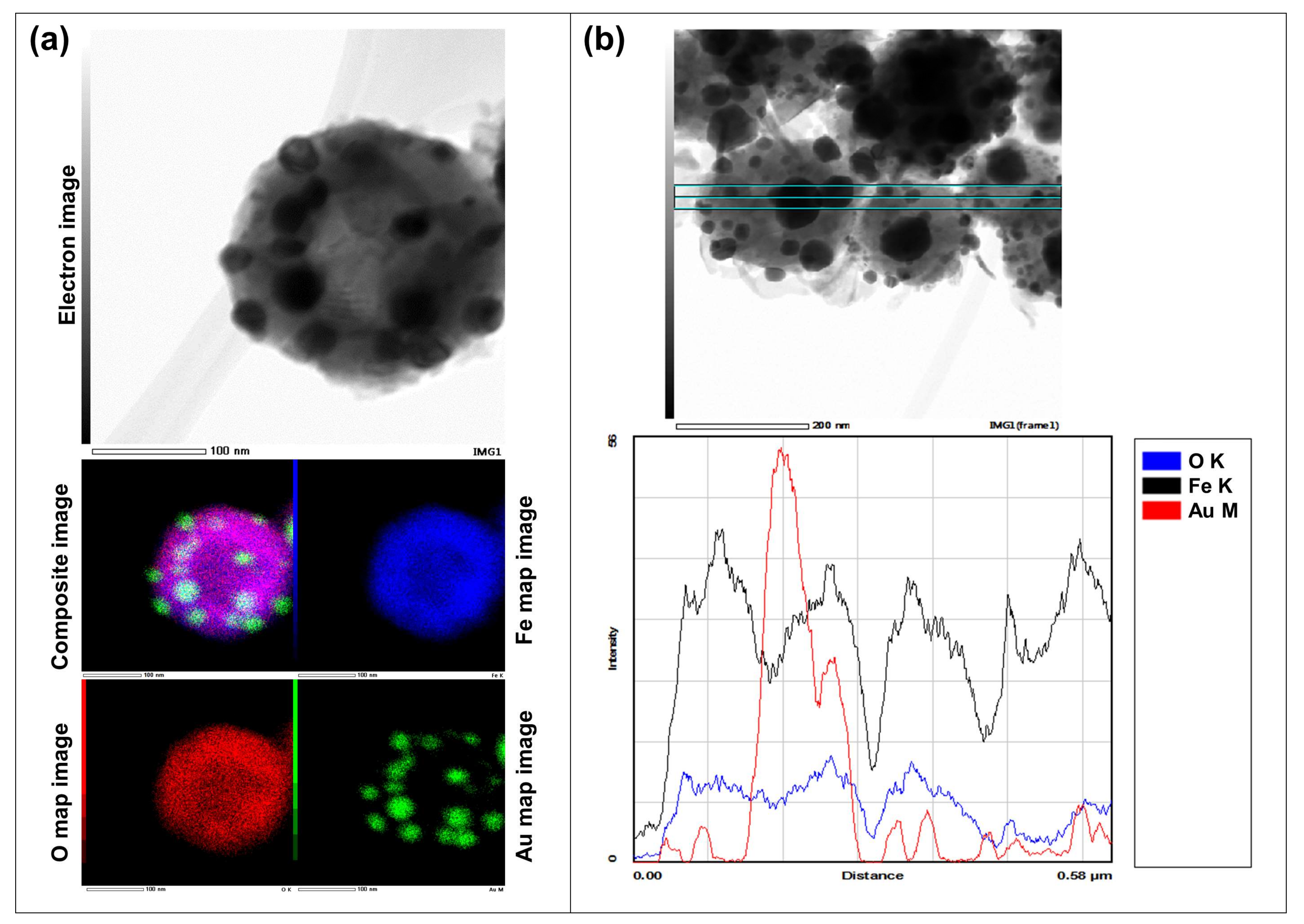
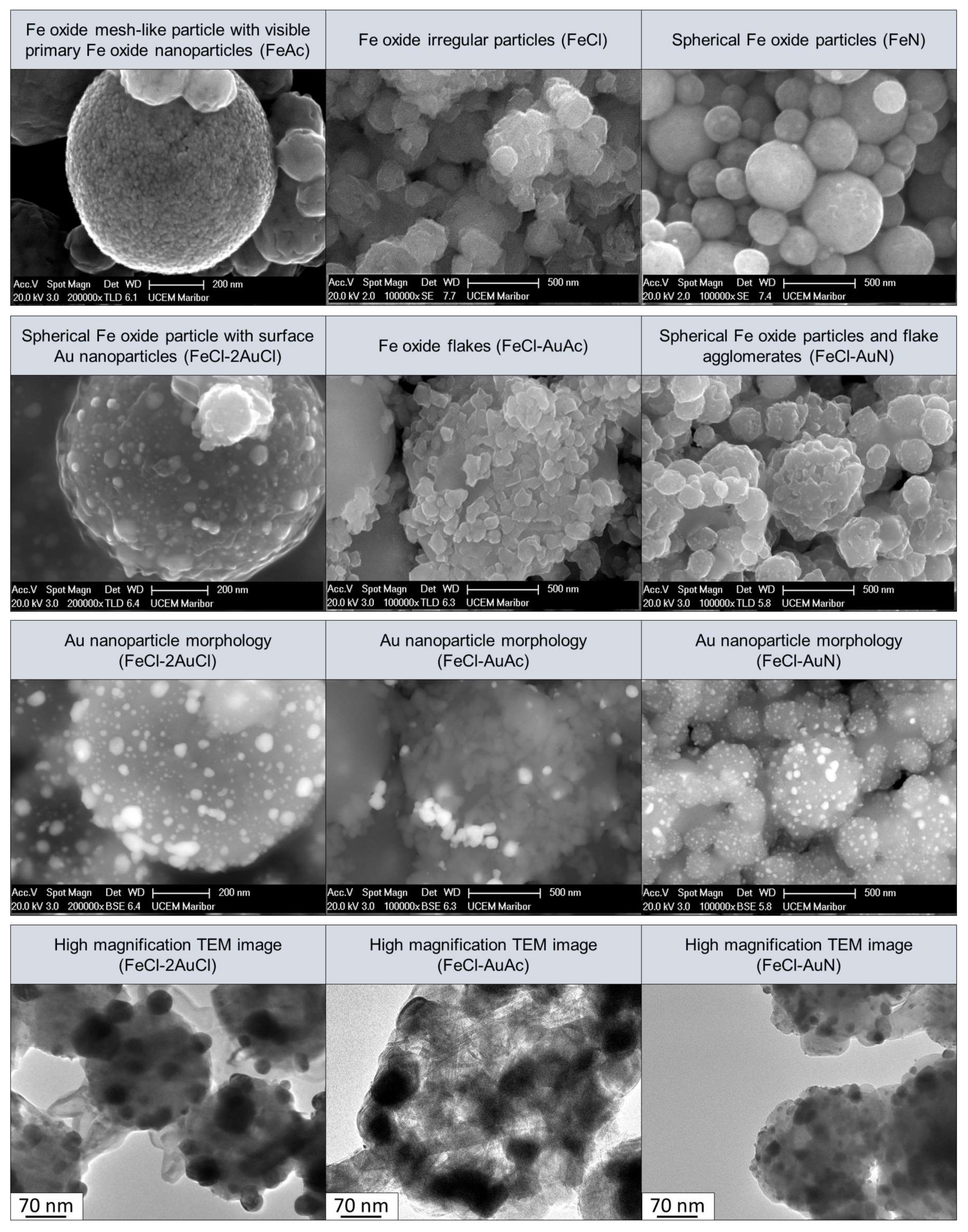
| Experiment | Precursor 1 | Concentration | Reaction Temperature | Gas Flow | Synthesis Time |
|---|---|---|---|---|---|
| FeAc | Iron (II) acetate | 1 g/L Fe | 600 °C (preheating zone), 600 °C (reaction zone), 300 °C (cooling zone) | 4 L/min N2 + 2 L/min H2 | 4 h |
| FeCl | Iron (III) chloride hexahydrate | 1 g/L Fe | |||
| FeN | Iron (III) nitrate nonahydrate | 1 g/L Fe | |||
| FeCl-AuCl | Iron (III) chloride hexahydrate + Gold (III) chloride tetrahydrate | 1 g/L Fe + 0.25 g/L Au | |||
| FeCl-2AuCl | Iron (III) chloride hexahydrate + Gold (III) chloride tetrahydrate | 1 g/L Fe + 0.5 g/L Au | |||
| FeCl-AuAc | Iron (III) chloride hexahydrate + Gold (III) acetate (reflux boiling) | 1 g/L Fe + 0.25 g/L Au | |||
| FeCl-AuN | Iron (III) chloride hexahydrate + Gold (III) nitrate (reflux boiling) | 1 g/L Fe + 0.25 g/L Au |
| Fe Oxide Particle Sizes from Fe Precursor Solutions (nm) | |||
|---|---|---|---|
| Experiment | FeAc | FeCl | FeN |
| MEAN | 261 | 283 | 296 |
| SD | 139 | 145 | 144 |
| MAX | 1537 | 1245 | 918 |
| MIN | 55 | 59 | 42 |
| n | 1492 | 742 | 532 |
| Fe Oxide Particle Sizes from Fe/Au Precursor Solutions (nm) | ||||
| Experiment | FeCl-AuCl FeP | FeCl-2AuCl FeP | FeCl-AuAc FeP | FeCl-AuN FeP |
| MEAN | 323 | 314 | 160 | 258 |
| SD | 144 | 160 | 195 | 124 |
| MAX | 1327 | 1499 | 1814 | 863 |
| MIN | 115 | 108 | 14 | 70 |
| n | 372 | 338 | 669 | 622 |
| Au nanoparticle sizes from Fe/Au precursor solutions (nm) | ||||
| Experiment | FeCl-AuCl AuNP | FeCl-2AuCl AuNP | FeCl-AuAc AuNP | FeCl-AuN AuNP |
| MEAN | 28 | 24 | 75 | 22 |
| SD | 14 | 18 | 37 | 13 |
| MAX | 132 | 212 | 211 | 104 |
| MIN | 6 | 2 | 13 | 5 |
| n | 833 | 1090 | 332 | 898 |
| Sample | Initial Zeta Potential (mV) | Zeta Potential after 6 Months (mV) |
|---|---|---|
| FeAc | 19.7 | 1.4 |
| FeCl | 32.8 | 19.0 |
| FeN | 16.1 | 3.9 |
| FeCl-AuCl | 23.7 | 15.6 |
| FeCl-2AuCl | 26.2 | 11.1 |
| FeCl-AuAc | 23.1 | 17.8 |
| FeCl-AuN | 22.0 | 14.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majerič, P.; Jenko, D.; Friedrich, B.; Rudolf, R. Formation of Bimetallic Fe/Au Submicron Particles with Ultrasonic Spray Pyrolysis. Metals 2018, 8, 278. https://doi.org/10.3390/met8040278
Majerič P, Jenko D, Friedrich B, Rudolf R. Formation of Bimetallic Fe/Au Submicron Particles with Ultrasonic Spray Pyrolysis. Metals. 2018; 8(4):278. https://doi.org/10.3390/met8040278
Chicago/Turabian StyleMajerič, Peter, Darja Jenko, Bernd Friedrich, and Rebeka Rudolf. 2018. "Formation of Bimetallic Fe/Au Submicron Particles with Ultrasonic Spray Pyrolysis" Metals 8, no. 4: 278. https://doi.org/10.3390/met8040278







