Parametric Effects of Mechanical Alloying on Carbon Nanofiber Catalyst Production in the Ni-Cu System
Abstract
:1. Introduction
2. Experiments
2.1. Material Processing
2.2. Characterization
3. Results
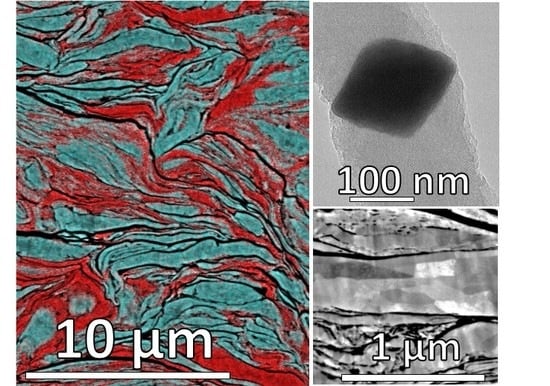
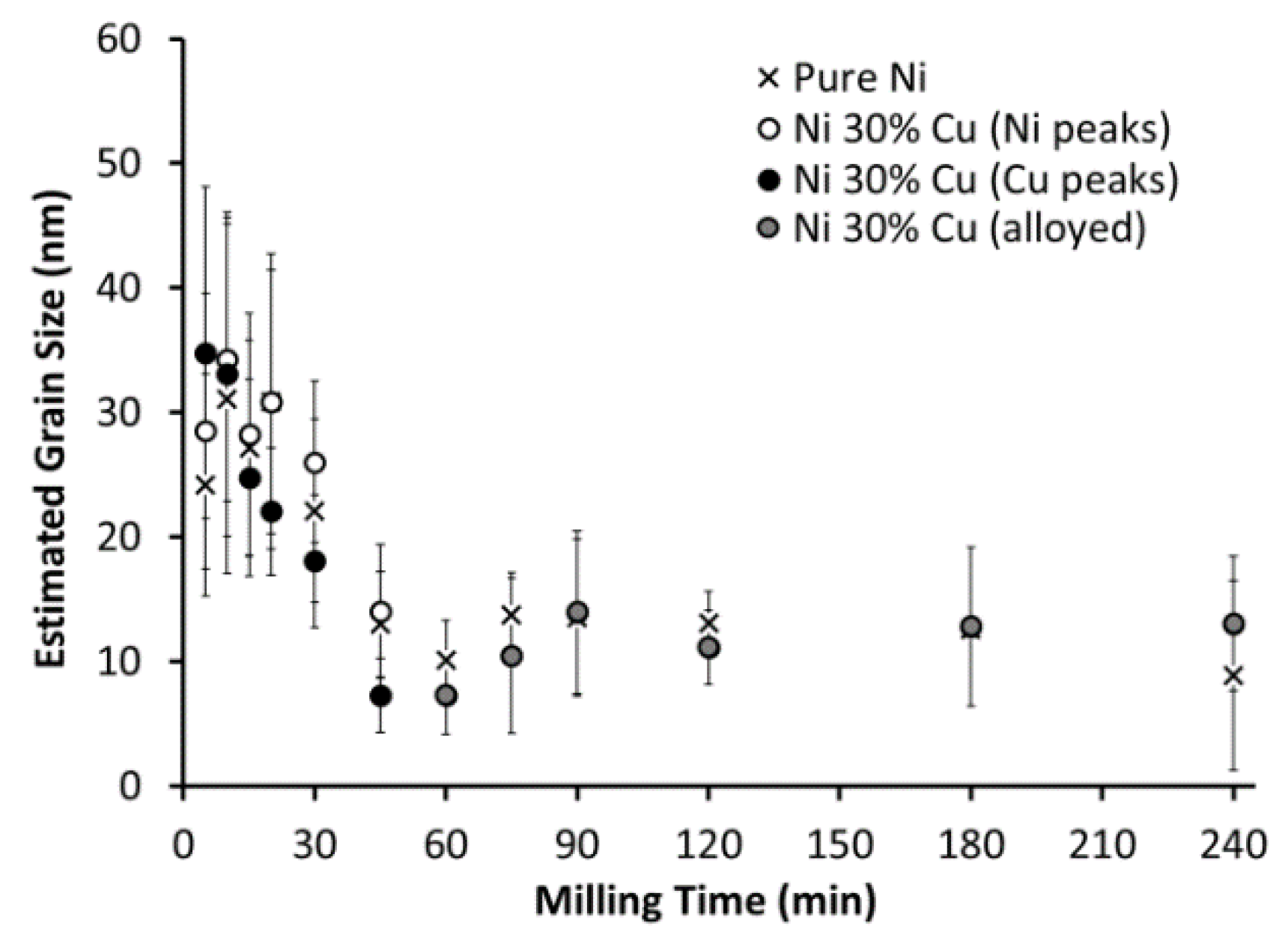
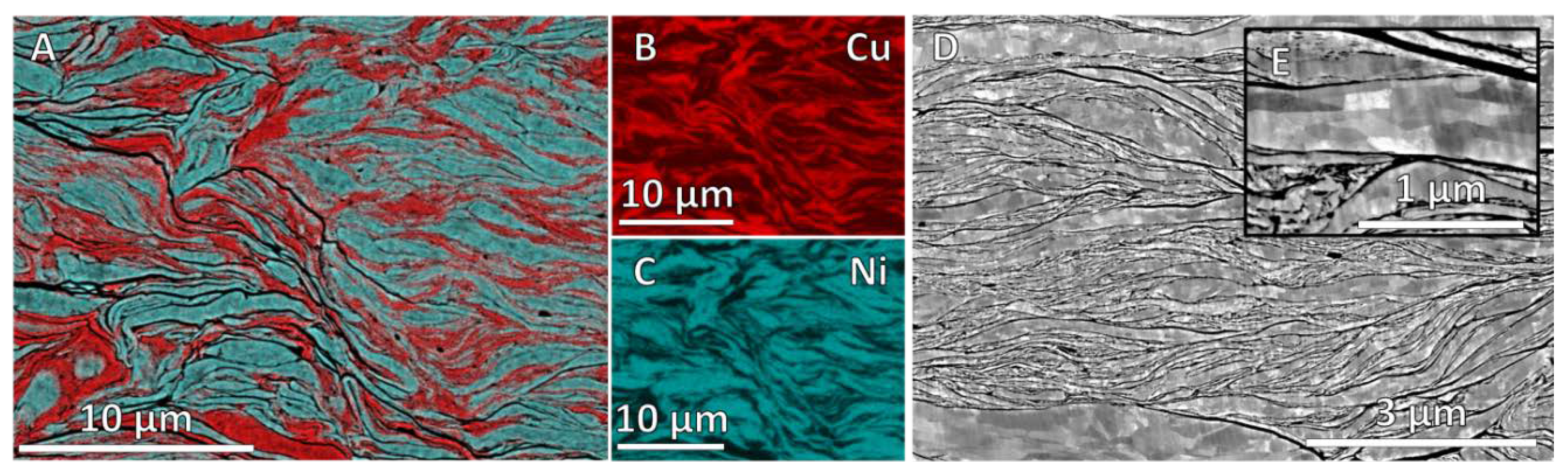
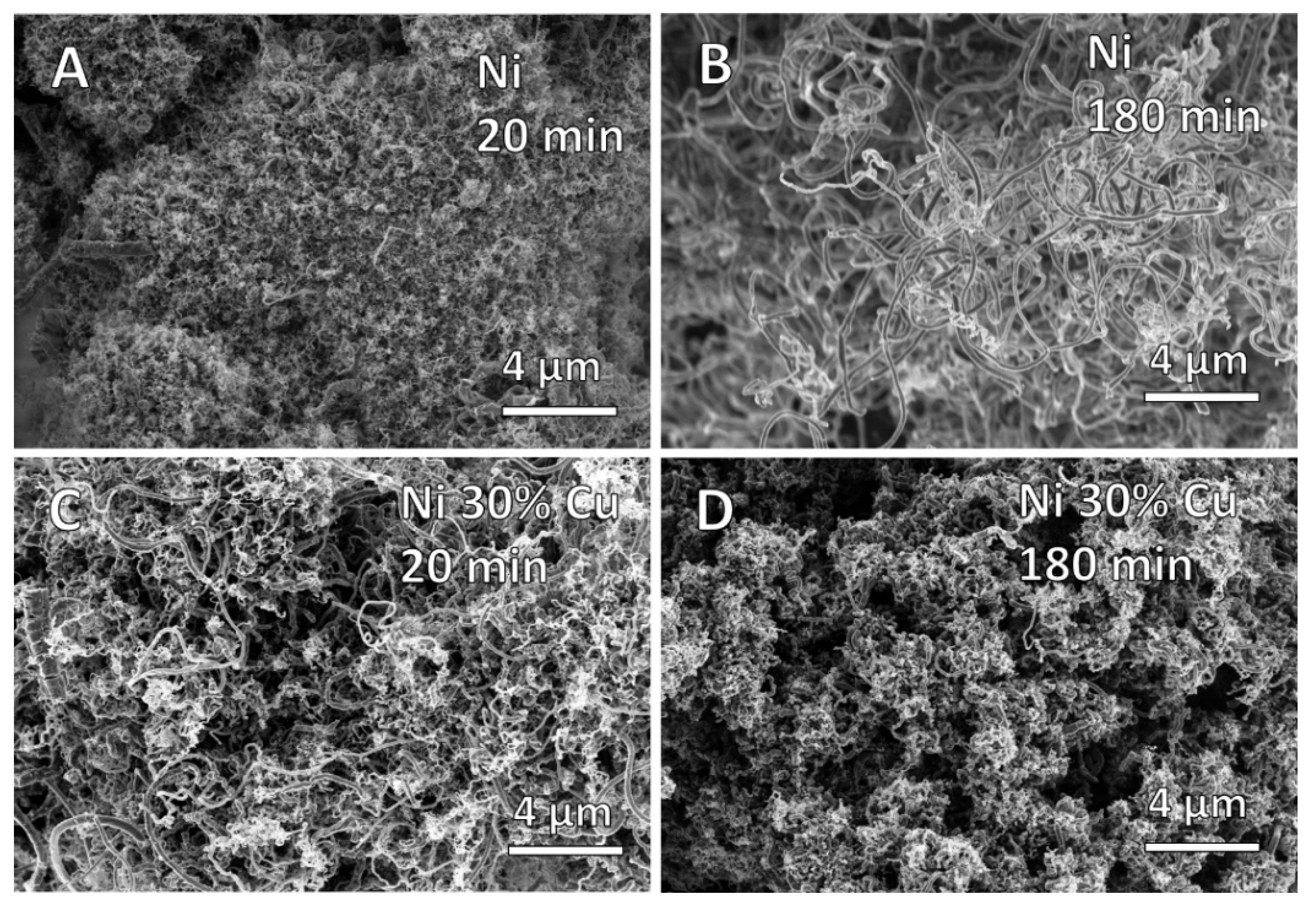
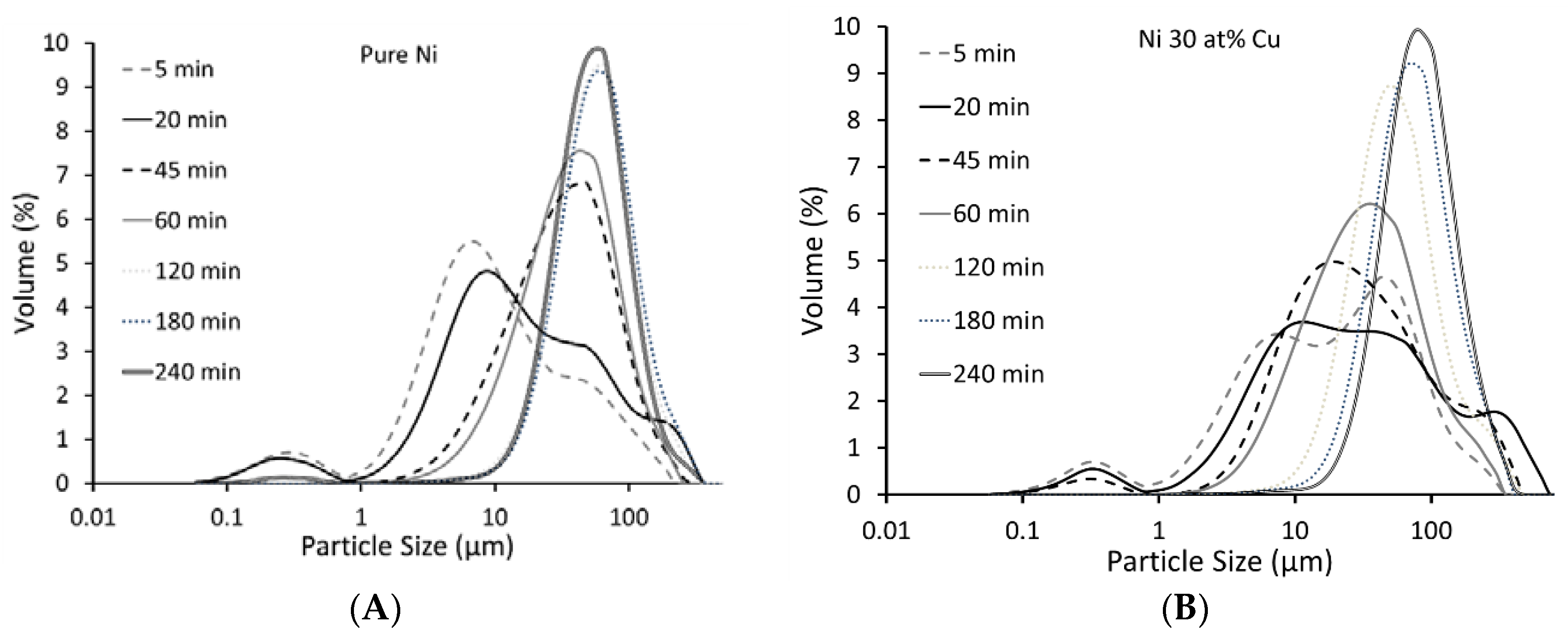
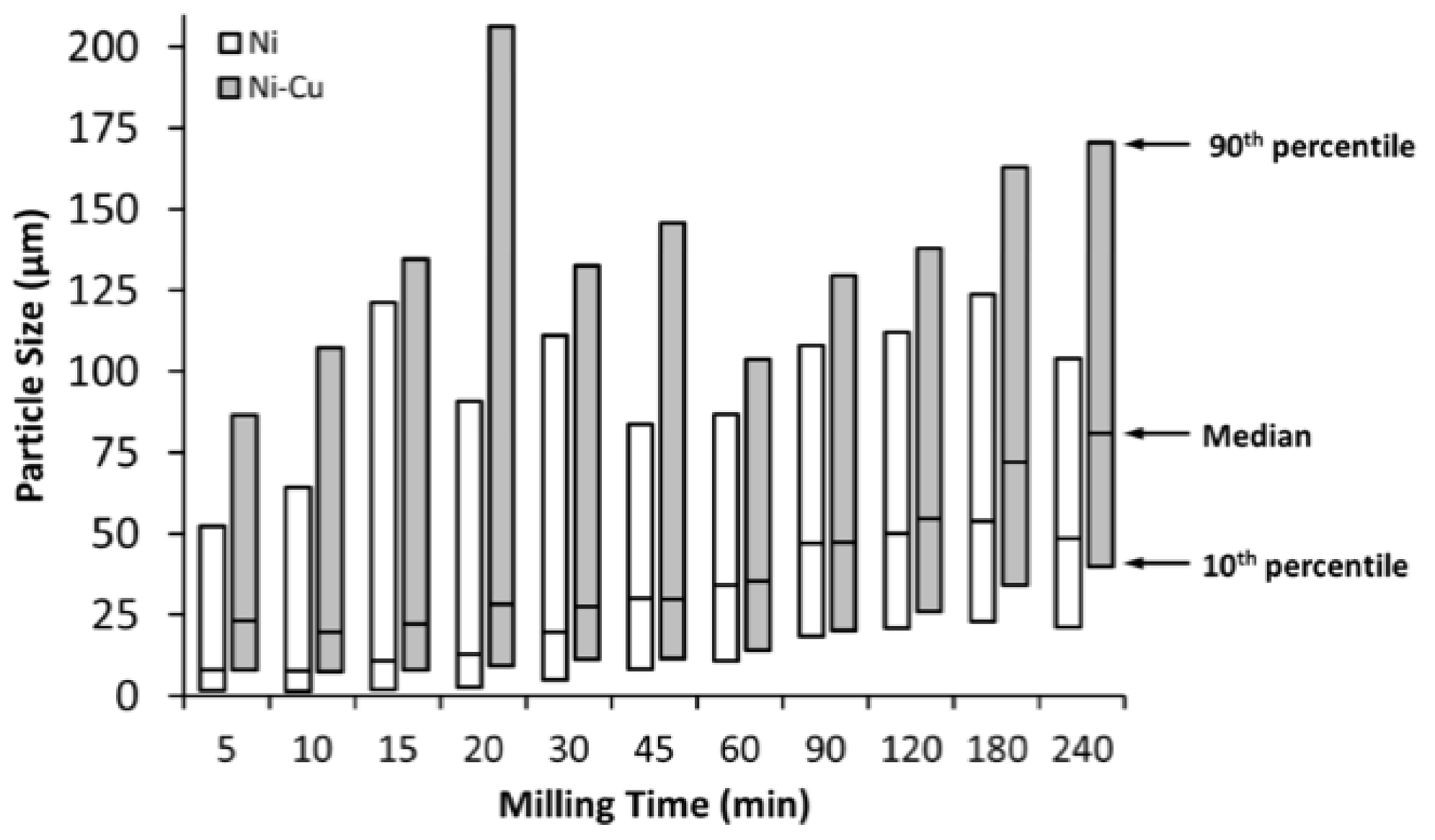
3.1. Milling Duration and Catalyst Microstructure
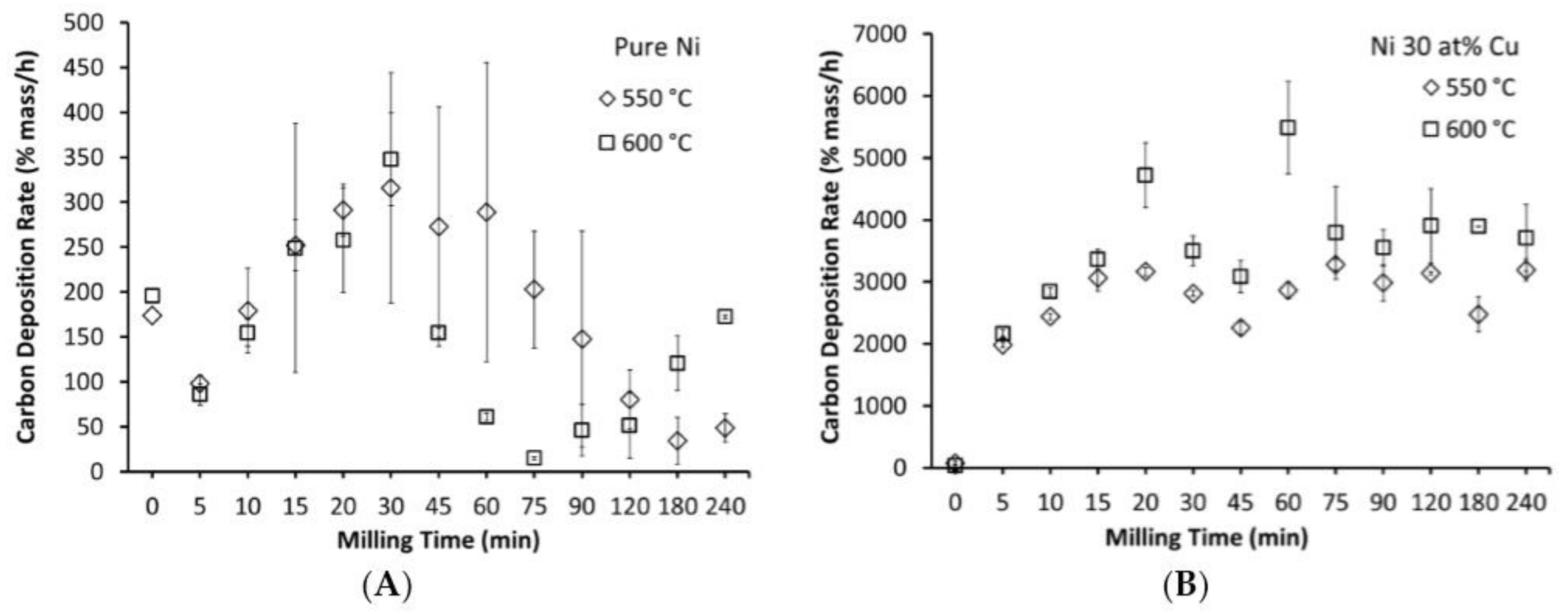
3.2. Milling Duration and Carbon Deposition
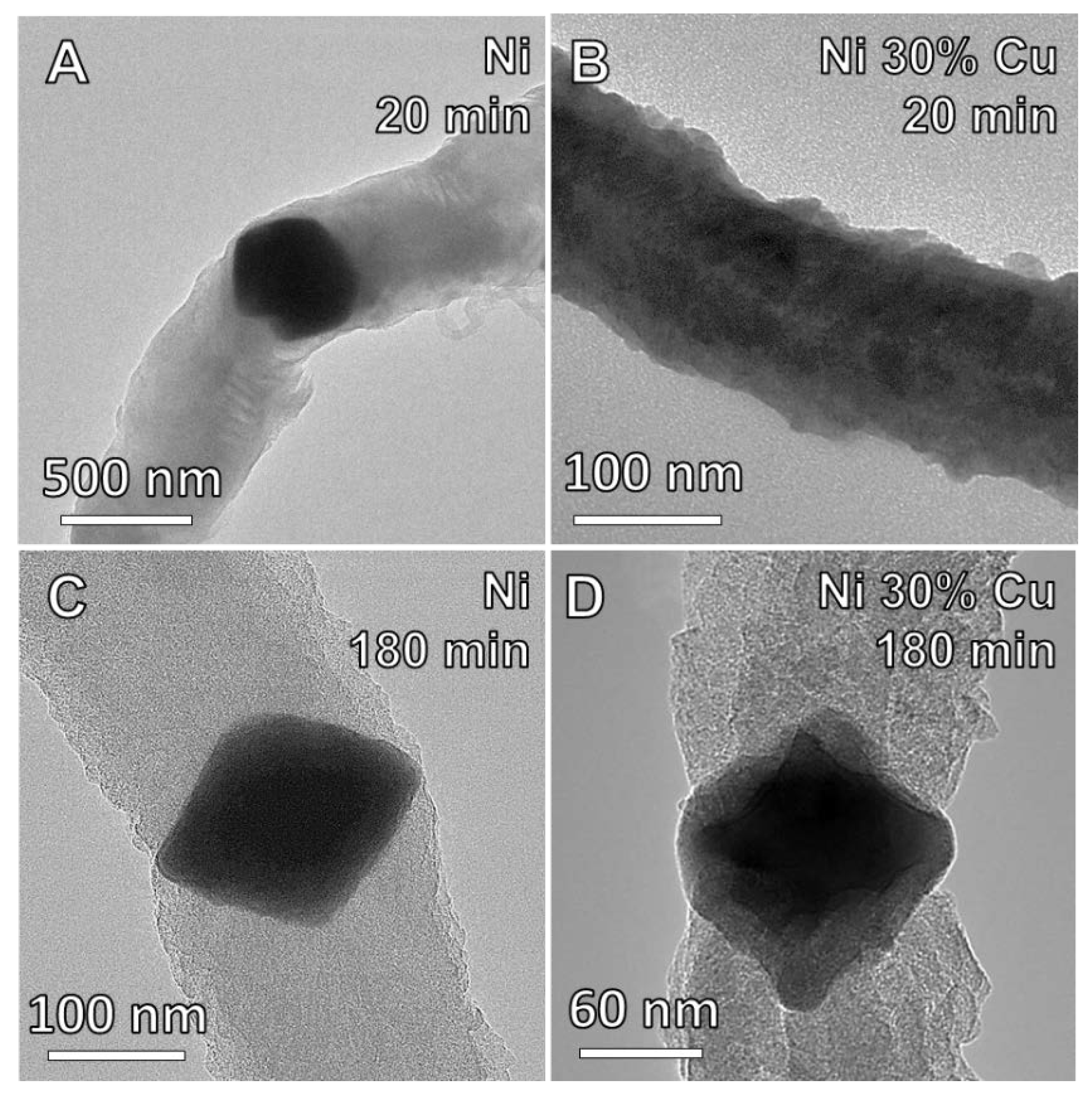
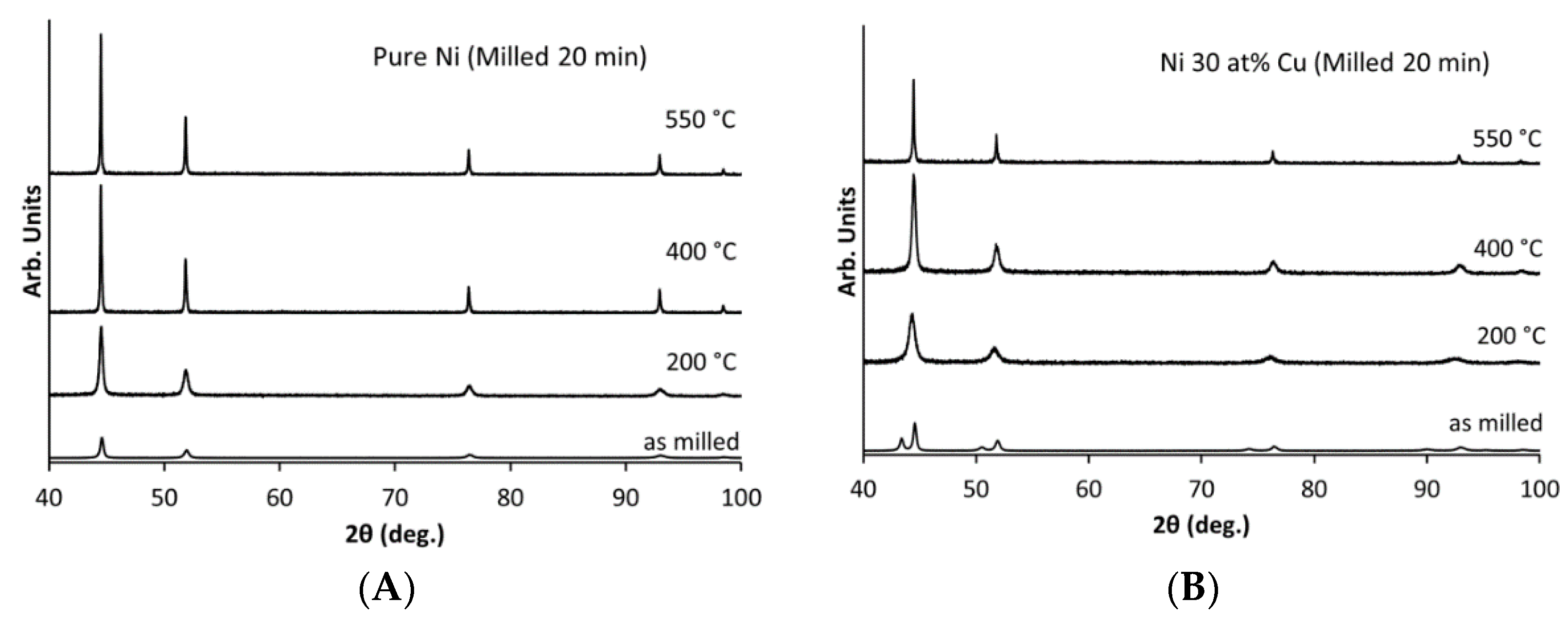
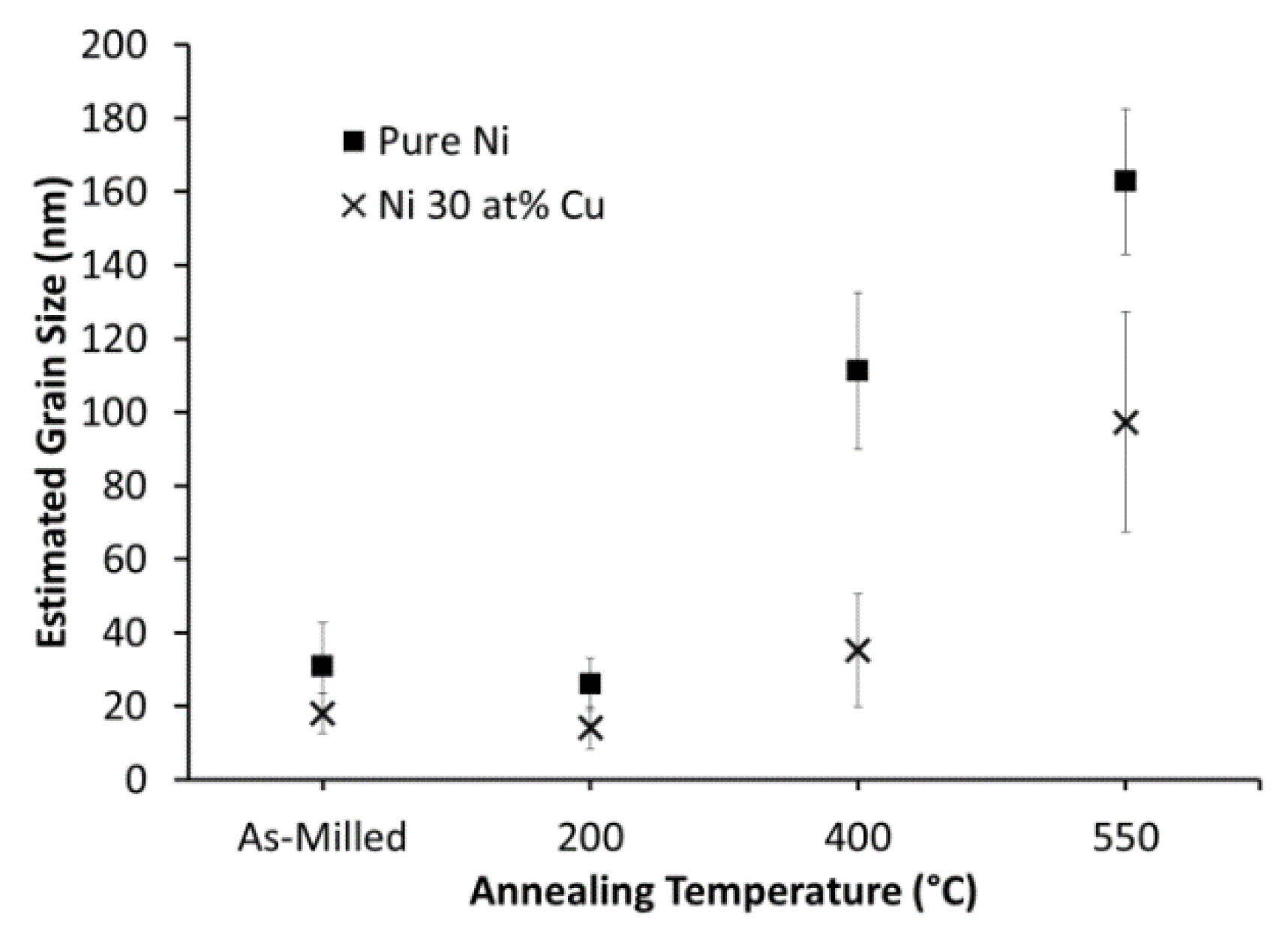
3.3. The Effects of Thermal Processing on Catalyst Microstructure
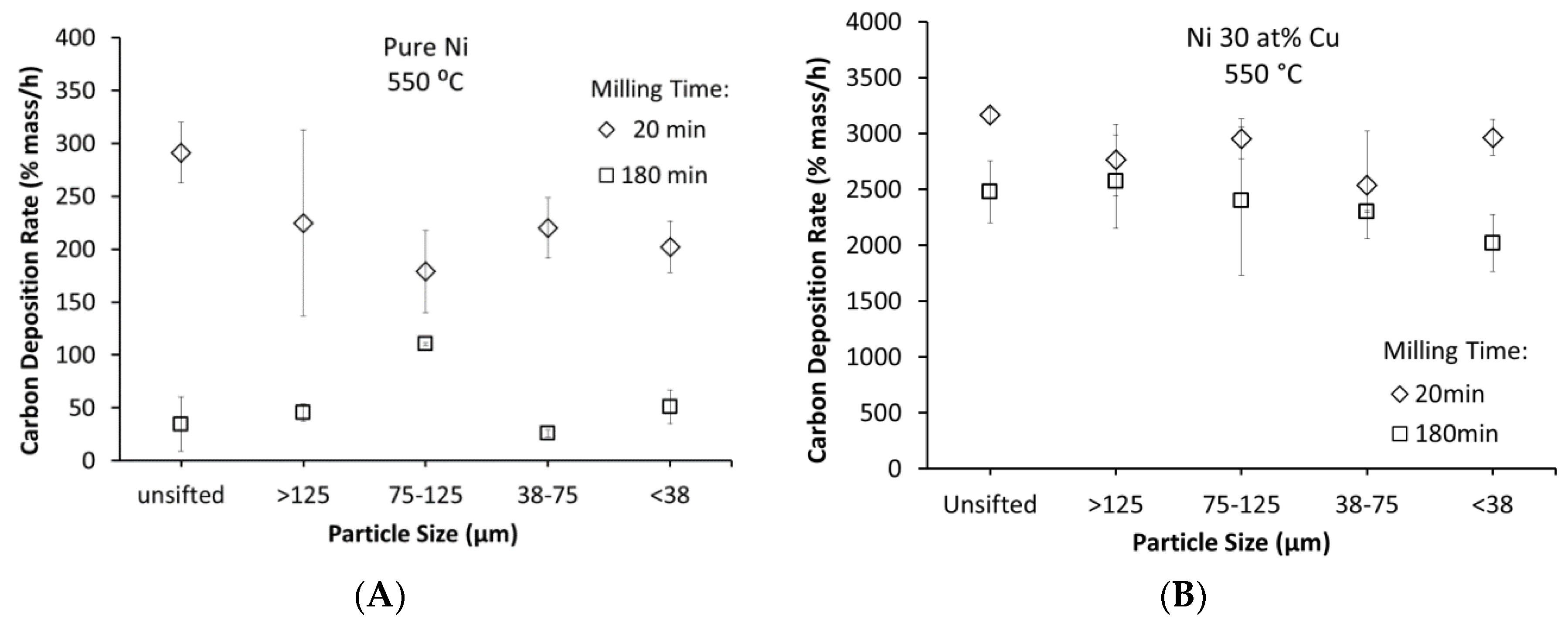
3.4. Particle Size and Deposition Kinetics
4. Discussion
4.1. Catalyst Development
4.2. Carbon Deposition
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klein, K.L.; Melechko, A.V.; Rack, P.D.; Fowlkes, J.D.; Meyer, H.M.; Simpson, M.L. Cu–Ni composition gradient for the catalytic synthesis of vertically aligned carbon nanofibers. Carbon 2005, 43, 1857–1863. [Google Scholar] [CrossRef]
- Rodriguez, N.M. A review of catlytically grown carbon nanofibers. J. Mater. Res. 1993, 8, 3233–3250. [Google Scholar] [CrossRef]
- DeJong, K.P.; Geus, J.W. Carbon nanofiber: Synthesis and applications. Catal. Rev. 2000, 42, 481–510. [Google Scholar] [CrossRef]
- Chambers, A.; Park, C.; Baker, R.T.K.; Rodriguez, N.M. Hydrogen storage in graphite nanofibers. J. Phys. Chem. B 1998, 102, 4253–4256. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.; Lee, K.M.; Jeong, S.Y.; Lee, E.; Baeck, S.H.; Shim, S.E. Electrochemical improvement due to alignment of carbon nanofibers fabricated by electrospinning as an electrode for supercapacitor. Carbon 2016, 99, 607–618. [Google Scholar] [CrossRef]
- Hammel, E.; Tang, X.; Trampert, M.; Schmitt, T.; Mauthner, K.; Eder, A.; Potschke, P. Carbon nanofibers for composite applications. Carbon 2004, 42, 1153–1158. [Google Scholar] [CrossRef]
- Wang, G.; Dong, Q.; Ling, Z.; Pan, C.; Yu, C.; Qiu, J. Hierarchical activated carbon nanofiber webs with tuned structure fabricated by electrospinning for capacitive deionization. J. Mat. Chem. 2012, 22, 21819–21823. [Google Scholar] [CrossRef]
- Faccini, M.; Borja, G.; Boerrigter, M.; Mart, D.M.; Crespiera, S.M.; Vázquez-Campos, S.; Aubouy, L.; Amantia, D. Electrospun carbon nanofiber membranes for filtration of nanoparticles from water. J. Nanomater. 2015, 2015, 247471. [Google Scholar] [CrossRef]
- Rodriguez, N.M.; Kim, M.-S.; Baker, R.T.K. Carbon nanofibers: A unique catalyst support medium. J. Phys. Chem. 1994, 98, 13108–13111. [Google Scholar] [CrossRef]
- See, C.H.; Harris, A.T. A review of carbon nanotube synthesis via fluidized-bed chemical vapor deposition. Ind. Eng. Chem. Res. 2007, 46, 997–1012. [Google Scholar] [CrossRef]
- Meyyappan, M.; Delzeit, L.; Cassell, A.; Hash, D. Carbon nanotube growth by PECVD: A review. Plasma Sources Sci. Technol. 2003, 12, 205–216. [Google Scholar] [CrossRef]
- Rodriguez, N.M.; Chambers, A.; Baker, R.T.K. Catalytic engineering of carbon nanostructures. Langmuir 1995, 11, 3862–3866. [Google Scholar] [CrossRef]
- Baird, T.; Fryer, J.R.; Grant, B. Structure of filamentous carbon. Nature 1971, 233, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Atwater, M.A.; Phillips, J.; Doorn, S.K.; Luhrs, C.C.; Fernandez, Y.; Menendez, J.A.; Leseman, Z.C. The production of carbon nanofibers and thin films on palladium catalysts from ethylene–oxygen mixtures. Carbon 2009, 47, 2269–2280. [Google Scholar] [CrossRef]
- Atwater, M.A.; Phillips, J.; Leseman, Z.C. Formation of carbon nanofibers and thin films catalyzed by palladium in ethylene-hydrogen mixtures. J. Phys. Chem. C 2010, 114, 5804–5810. [Google Scholar] [CrossRef]
- Atwater, M.A.; Phillips, J.; Leseman, Z.C. The effect of powder sintering on the palladium-catalyzed formation of carbon nanofibers from ethylene–oxygen mixtures. Carbon 2010, 48, 1932–1938. [Google Scholar] [CrossRef]
- Chambers, A.; Rodriguez, N.M.; Baker, R.T.K. Modification of the catalytic behavior of cobalt by the addition of copper. J. Phys. Chem. 1995, 99, 10581–10589. [Google Scholar] [CrossRef]
- Chambers, A.; Rodriguez, N.M.; Baker, R.T.K. Influence of copper on the structural characteristics of carbon nanofibers produced from the cobalt-catalyzed decomposition of ethylene. J. Mater. Res. 1996, 11, 430–438. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Ivanob, E.; Boldyrev, V.V. The science and technology of mechanical alloying. Mater. Sci. Eng. A 2001, 304–306, 151–158. [Google Scholar] [CrossRef]
- Guevara, L.; Wanner, C.; Welsh, R.; Atwater, M. Using mechanical alloying to create bimetallic catalysts for vapor-phase carbon nanofiber synthesis. Fibers 2015, 3, 394–410. [Google Scholar] [CrossRef]
- Rodriguez, N.M.; Kim, M.S.; Baker, R.T.K. Deactivation of copper-nickel catalysts due to changes in surface composition. J. Catal. 1993, 140, 16–29. [Google Scholar] [CrossRef]
- Kim, M.S.; Rodriguez, N.M.; Baker, R.T.K. The interaction of hydrocarbons with copper-nickel and nickel in the formation of carbon filaments. J. Catal. 1991, 131, 60–73. [Google Scholar] [CrossRef]
- Atwater, M.A.; Phillips, J.; Leseman, Z.C. Accelerated growth of carbon nanofibers using physical mixtures and alloys of Pd and Co in an ethylene–hydrogen environment. Carbon 2011, 49, 1058–1066. [Google Scholar] [CrossRef]
- Atwater, M.A.; Welsh, R.J.; Edwards, D.S. Direct synthesis of nanofibrous nonwoven carbon components: Initial observations, capabilities, and challenges. J. Micro Nano Manuf. 2016, 4, 041004. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzak, L. (Eds.) Cu-Ni phase diagram. In Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1990; Volume 2, pp. 1442–1445. [Google Scholar]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001; p. 170. [Google Scholar]
- Miettinen, J. Thermodynamic description of Cu–Mg–Ni and Cu–Mg–Zn systems. Calphad 2008, 32, 389–398. [Google Scholar] [CrossRef]
- Zeifert, B.; Salmones, J.; Hernández, J.; Reynoso, R.; Nava, N.; Cabañas-Moreno, J.; Aguilar-Rǭos, G. Preparation of iron–nickel catalysts by mechanical alloying. Mater. Lett. 2000, 43, 244–248. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström–Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloys Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Esawi, A.; Morsi, K. Dispersion of carbon nanotubes (CNTs) in aluminum powder. Compos. Part A 2007, 38, 646–650. [Google Scholar] [CrossRef]
- Ahn, C.; Ye, Y.; Ratnakumar, B.; Witham, C.; Bowman, R., Jr.; Fultz, B. Hydrogen desorption and adsorption measurements on graphite nanofibers. Appl. Phys. Lett. 1998, 73, 3378–3380. [Google Scholar] [CrossRef]
- Callister, W.D. Materials Science and Engineering: An introduction, 7th ed.; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Ames, M.; Markmann, J.; Karos, R.; Michels, A.; Tschope, A.; Birringer, R. Unraveling the nature of room temperature grain growth in nanocrystalline materials. Acta Mater. 2008, 56, 4255–4266. [Google Scholar] [CrossRef]
- Koch, C.C.; Scattergood, R.O.; Darling, K.A.; Semones, J.E. Stabilization of nanocrystalline grain sizes by solute additions. J. Mater. Sci. 2008, 43, 7264–7272. [Google Scholar] [CrossRef]
- Atwater, M.A.; Bahmanpour, H.; Scattergood, R.O.; Koch, C.C. The thermal stability of nanocrystalline cartridge brass and the effect of zirconium additions. J. Mater. Sci. 2013, 48, 220–226. [Google Scholar] [CrossRef]
- Detor, A.J.; Schuh, C.A. Microstructural evolution during the heat treatment of nanocrystalline alloys. J. Mater. Res. 2007, 22, 3233–3248. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Lim, S.; Song, Y.; Ota, Y.; Qiao, W.; Tanaka, A.; Mochida, I. Koh activation of carbon nanofibers. Carbon 2004, 42, 1723–1729. [Google Scholar] [CrossRef]
- Ramos, A.; Camean, I.; Garcıa, A.B. Graphitization thermal treatment of carbon nanofibers. Carbon 2013, 59, 2–32. [Google Scholar] [CrossRef]
- Qi, X.; Ruan, X.; Pan, C. Graphitization of solid carbon nanofibers at an unexpectedly low temperature. Mater. Lett. 2007, 61, 4272–4275. [Google Scholar] [CrossRef]
- Atwater, M.A.; Mousavi, A.K.; Phillips, J.; Leseman, Z.C. Direct synthesis of nanoscale carbon nonwovens by catalytic deposition. Carbon 2013, 57, 363–370. [Google Scholar] [CrossRef]
- Atwater, M.A.; Welsh, R.J.; Edwards, D.S.; Guevara, L.N.; Nelson, C.B.; Stone, B.T. Multiscale design of nanofibrous carbon aerogels: Synthesis, properties and comparisons with other low-density carbon materials. Carbon 2017, 124, 588–598. [Google Scholar] [CrossRef]
- Jablonski, G.A.; Geurts, F.W.; Sacco, A., Jr.; Biederman, R.R. Carbon deposition over Fe, Ni, and Co foils from CO-H2-CH4-CO2-H2O, CO-CO2, CH4-H2, and CO-H2-H2O gas mixtures: I. Morphology. Carbon 1992, 30, 87–98. [Google Scholar] [CrossRef]
- Jablonski, G.A.; Geurts, F.W.; Sacco, A., Jr. Carbon deposition over Fe, Ni, and Co foils from CO-H2-CH4-CO2-H2O, CO-CO2, CH4-H2, and CO-H2-H2O gas mixtures: II: Kinetics. Carbon 1992, 30, 99–106. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guevara, L.; Welsh, R.; Atwater, M.A. Parametric Effects of Mechanical Alloying on Carbon Nanofiber Catalyst Production in the Ni-Cu System. Metals 2018, 8, 286. https://doi.org/10.3390/met8040286
Guevara L, Welsh R, Atwater MA. Parametric Effects of Mechanical Alloying on Carbon Nanofiber Catalyst Production in the Ni-Cu System. Metals. 2018; 8(4):286. https://doi.org/10.3390/met8040286
Chicago/Turabian StyleGuevara, Laura, Roger Welsh, and Mark A. Atwater. 2018. "Parametric Effects of Mechanical Alloying on Carbon Nanofiber Catalyst Production in the Ni-Cu System" Metals 8, no. 4: 286. https://doi.org/10.3390/met8040286