Magnesium-β-Tricalcium Phosphate Composites as a Potential Orthopedic Implant: A Mechanical/Damping/Immersion Perspective
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Processing
2.2. Material Characterization
2.2.1. Density Measurements
2.2.2. Microstructural Characterization
2.2.3. Damping and Elastic Modulus
2.2.4. Mechanical Properties
2.2.5. Immersion Studies
3. Results and Discussion
3.1. Density and Porosity
3.2. Microstructural Characterisation
3.3. Microhardness
3.4. Damping Characteristics and Elastic Modulus
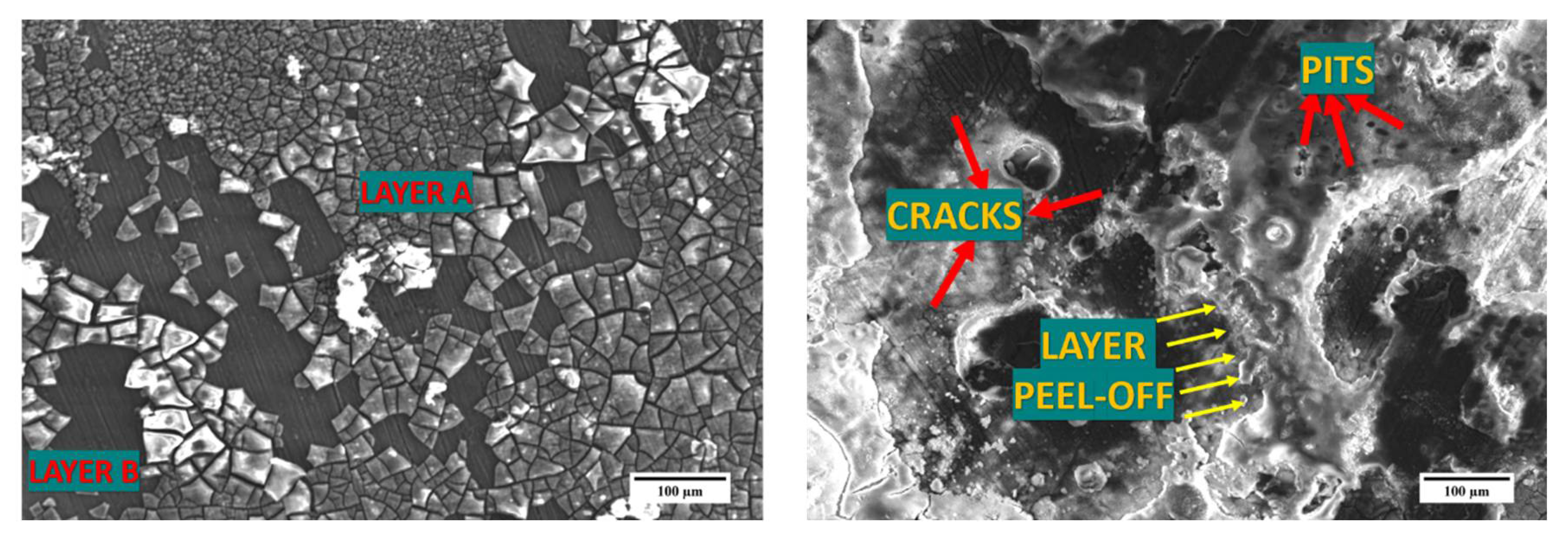
3.5. Immersion Studies
3.6. Compression Properties
4. Conclusions
- Near dense Mg-β-TCP composites were successfully synthesized with blend-press-sinter powder metallurgy technique with a porosity of less than 1%.
- The microhardness of pure Mg increased due to the presence of β-TCP particles with ~17.39% enhancement realized in the case of Mg-1.5 TCP composite.
- Mg-1.5 TCP composite exhibited a compressive yield strength, ultimate compressive strength, compressive fracture strain, and total energy absorbed under compression loading of ~103 MPa (↑~34%), ~240 MPa (↑~53%), ~19.3% (↑~22%), and ~29.2 MJ/m3 (↑~64%), respectively. The enhancements with respect to the base pure Mg are significant in all of the cases.
- The damping response of pure Mg enhanced with the addition of β-TCP particles, with Mg-1.5 TCP composite exhibiting the best damping capacity (~15.7% increase as compared to pure Mg) and damping loss rate (~113% increase compared to pure Mg) values.
- The presence of β-TCP particles assisted in the corrosion protection of pure Mg. The pH values stabilized earlier for the composites as compared to pure Mg and displayed lower corrosion rate values, which a superior ~9 times protection displayed by the Mg-1.5 TCP composite as compared to pure Mg.
Author Contributions
Conflicts of Interest
References
- Kuśnierczyk, K.; Basista, M. Recent advances in research on magnesium alloys and magnesium–calcium phosphate composites as biodegradable implant materials. J. Biomater. Appl. 2017, 31, 878–900. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Ma, P.X. Tissue Engineering Using Ceramics and Polymers; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Parande, G.; Manakari, V.; Meenashisundaram, G.K.; Gupta, M. Enhancing the hardness/compression/damping response of magnesium by reinforcing with biocompatible silica nanoparticulates. Int. J. Mater. Res. 2016, 107, 1091–1099. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.-L.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of recent developments in the field of magnesium corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.-L.; Cao, F.; Shi, Z.; Bowen, P.K. Advances in mg corrosion and research suggestions. J. Mages. Alloys 2013, 1, 177–200. [Google Scholar] [CrossRef]
- Tan, L.; Yu, X.; Wan, P.; Yang, K. Biodegradable materials for bone repairs: A review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Atrens, A.; Liu, M.; Abidin, N.I.Z. Corrosion mechanism applicable to biodegradable magnesium implants. Mater. Sci. Eng. B 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- Lu, Y.; Tan, L.; Xiang, H.; Zhang, B.; Yang, K.; Li, Y. Fabrication and characterization of Ca–Mg–P containing coating on pure magnesium. J. Mater. Sci. Technol. 2012, 28, 636–641. [Google Scholar] [CrossRef]
- Witte, F.; Feyerabend, F.; Maier, P.; Fischer, J.; Störmer, M.; Blawert, C.; Dietzel, W.; Hort, N. Biodegradable magnesium–hydroxyapatite metal matrix composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.; Han, Y. The microstructure, mechanical and corrosion properties of calcium polyphosphate reinforced ZK60A magnesium alloy composites. J. Alloys Compd. 2010, 504, 585–593. [Google Scholar] [CrossRef]
- Gu, X.; Zhou, W.; Zheng, Y.; Dong, L.; Xi, Y.; Chai, D. Microstructure, mechanical property, bio-corrosion and cytotoxicity evaluations of Mg/Ha composites. Mater. Sci. Eng. C 2010, 30, 827–832. [Google Scholar] [CrossRef]
- Koepp, H.E.; Schorlemmer, S.; Kessler, S.; Brenner, R.E.; Claes, L.; Günther, K.P.; Ignatius, A.A. Biocompatibility and osseointegration of β-TCP: Histomorphological and biomechanical studies in a weight-bearing sheep model. J. Biomed. Mater. Res. Part B 2004, 70, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Dieringa, H.; Fuskova, L.; Fechner, D.; Blawert, C. Mechanical and corrosion behaviour of a hydroxyapatite reinforced magnesium alloy WE43. In Proceedings of the 17th International Conference on Composite Materials, ICCM, Edinburgh, UK, 27–31 July 2009. [Google Scholar]
- Yan, Y.; Kang, Y.; Li, D.; Yu, K.; Xiao, T.; Deng, Y.; Dai, H.; Dai, Y.; Xiong, H.; Fang, H. Improvement of the mechanical properties and corrosion resistance of biodegradable β-Ca3(PO4)2/Mg-Zn composites prepared by powder metallurgy: The adding β-Ca3(PO4)2, hot extrusion and aging treatment. Mater. Sci. Eng. C 2017, 74, 582–596. [Google Scholar] [CrossRef] [PubMed]
- He, S.-Y.; Sun, Y.; Chen, M.-F.; Liu, D.-B.; Ye, X.-Y. Microstructure and properties of biodegradable β-TCP reinforced Mg-Zn-Zr composites. Trans. Nonferr. Met. Soc. China 2011, 21, 814–819. [Google Scholar] [CrossRef]
- Liu, D.B.; Huang, Y.; Prangnell, P.B. Microstructure and performance of a biodegradable Mg–1Ca–2Zn–1TCP composite fabricated by combined solidification and deformation processing. MatL 2012, 82, 7–9. [Google Scholar] [CrossRef]
- Famery, R.; Richard, N.; Boch, P. Preparation of α- and β-tricalcium phosphate ceramics, with and without magnesium addition. Ceram. Int. 1994, 20, 327–336. [Google Scholar] [CrossRef]
- Matli, P.R.; Ubaid, F.; Shakoor, R.A.; Parande, G.; Manakari, V.; Yusuf, M.; Mohamed, A.M.A.; Gupta, M. Improved properties of Al–Si3N4 nanocomposites fabricated through a microwave sintering and hot extrusion process. RSC Adv. 2017, 7, 34401–34410. [Google Scholar] [CrossRef]
- ASTM Standard. E384, Standard Test Method for Microindentation Hardness of Materials; ASTM International: West Conshohocken, PA, USA, 2000. [Google Scholar]
- ASTM Standard. E9-09. Standard Test Methods of Compression Testing of Metallic Materials at Room Temperature; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Wong, W.; Gupta, M. Using microwave energy to synthesize light weight/energy saving magnesium based materials: A review. Technologies 2015, 3, 1–18. [Google Scholar] [CrossRef]
- Parande, G.; Manakari, V.; Meenashisundaram, G.K.; Gupta, M. Enhancing the tensile and ignition response of monolithic magnesium by reinforcing with silica nanoparticulates. J. Mater. Res. 2017, 32, 2169–2178. [Google Scholar] [CrossRef]
- Wang, X.; Wu, K.; Zhang, H.; Huang, W.; Chang, H.; Gan, W.; Zheng, M.; Peng, D. Effect of hot extrusion on the microstructure of a particulate reinforced magnesium matrix composite. Mater. Sci. Eng. A 2007, 465, 78–84. [Google Scholar] [CrossRef]
- Kujur, M.S.; Mallick, A.; Manakari, V.; Parande, G.; Tun, K.S.; Gupta, M. Significantly enhancing the ignition/compression/damping response of monolithic magnesium by addition of SM2O3 nanoparticles. Metals 2017, 7, 357. [Google Scholar] [CrossRef]
- Anilchandra, A.R.; Surappa, M.K. Microstructure and damping behaviour of consolidated magnesium chips. Mater. Sci. Eng. A 2012, 542, 94–103. [Google Scholar] [CrossRef]
- Carreño-Morelli, E.; Urreta, S.E.; Schaller, R. Mechanical spectroscopy of thermal stress relaxation at metal–ceramic interfaces in aluminium-based composites. Acta Mater. 2000, 48, 4725–4733. [Google Scholar] [CrossRef]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.; Staiger, M.P.; Dias, G.J. Magnesium biomaterials for orthopedic application: A review from a biological perspective. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1316–1331. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-H.; Chen, M.-S.; Lin, L.-H.; Lin, M.-H.; Wu, C.-Z.; Ou, K.-L.; Yu, C.-H. Effect of heat treatment on the microstructures and damping properties of biomedical Mg–Zr alloy. J. Alloys Compd. 2011, 509, 813–819. [Google Scholar] [CrossRef]
- Wang, B.; Xu, D.; Dong, J.; Ke, W. Effect of corrosion product films on the in vitro degradation behavior of Mg-3% Al-1% Zn (in wt %) alloy in hank’s solution. J. Mater. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Gupta, M.; Meenashisundaram, G.K. Insight into Designing Biocompatible Magnesium Alloys and Composites: Processing, Mechanical and Corrosion Characteristics; Springer: Berlin, Germany, 2015. [Google Scholar]
- Cheng, M.; Chen, J.; Yan, H.; Su, B.; Yu, Z.; Xia, W.; Gong, X. Effects of minor sr addition on microstructure, mechanical and bio-corrosion properties of the Mg-5Zn based alloy system. J. Alloys Compd. 2017, 691, 95–102. [Google Scholar] [CrossRef]
- Taltavull, C.; Shi, Z.; Torres, B.; Rams, J.; Atrens, A. Influence of the chloride ion concentration on the corrosion of high-purity Mg, Ze41 and Az91 in buffered hank’s solution. J. Mater. Sci. Mater. Med. 2014, 25, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Meenashisundaram, G.K.; Nai, M.H.; Gupta, M. Effects of ti and TIB2 nanoparticulates on room temperature mechanical properties and in vitro degradation of pure mg. In Magnesium Technology 2015; Manuel, M.V., Singh, A., Alderman, M., Neelameggham, N.R., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 413–418. [Google Scholar]
- Ma, X.; Dong, L.; Wang, X. Microstructure, mechanical property and corrosion behavior of co-continuous β-tcp/mgca composite manufactured by suction casting. Mater. Des. 2014, 56, 305–312. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Xie, M.; Qu, L.; Zhang, P.; Li, X. Structure, mechanical property and corrosion behaviors of (ha+ β-TCP)/Mg–5Sn composite with interpenetrating networks. Mater. Sci. Eng. C 2015, 56, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Mostaed, E.; Hashempour, M.; Fabrizi, A.; Dellasega, D.; Bestetti, M.; Bonollo, F.; Vedani, M. Microstructure, texture evolution, mechanical properties and corrosion behavior of ecap processed ZK60 magnesium alloy for biodegradable applications. J. Mech. Behav. Biomed. Mater. 2014, 37, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Ahmadkhaniha, D.; Järvenpää, A.; Jaskari, M.; Sohi, M.H.; Zarei-Hanzaki, A.; Fedel, M.; Deflorian, F.; Karjalainen, L. Microstructural modification of pure Mg for improving mechanical and biocorrosion properties. J. Mech. Behav. Biomed. Mater. 2016, 61, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Song, G.-L.; Yang, S.; Outeiro, J.; Dillon, O., Jr.; Puleo, D.; Jawahir, I. Grain refined and basal textured surface produced by burnishing for improved corrosion performance of AZ31B Mg alloy. Corros. Sci. 2012, 57, 192–201. [Google Scholar] [CrossRef]
- Zainal Abidin, N.I.; Atrens, A.D.; Martin, D.; Atrens, A. Corrosion of high purity Mg, Mg2Zn0.2Mn, ZE41 and AZ91 in hank’s solution at 37 °C. Corros. Sci. 2011, 53, 3542–3556. [Google Scholar] [CrossRef]
- Geng, F.; Tan, L.; Jin, X.; Yang, J.; Yang, K. The preparation, cytocompatibility, and in vitro biodegradation study of pure β-TCP on magnesium. J. Mater. Sci. Mater. Med. 2009, 20, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Parande, G.; Manakari, V.; Kopparthy, S.D.S.; Gupta, M. Utilizing low-cost eggshell particles to enhance the mechanical response of Mg-2.5Zn magnesium alloy matrix. Adv. Eng. Mater. 2017, 1700919. [Google Scholar] [CrossRef]
- Tun, K.; Zhang, Y.; Parande, G.; Manakari, V.; Gupta, M. Enhancing the hardness and compressive response of magnesium using complex composition alloy reinforcement. Metals 2018, 8, 276. [Google Scholar] [CrossRef]
- Hermawan, H. Biodegradable metals: State of the art. In Biodegradable Metals; Springer: Berlin, Germany, 2012; pp. 13–22. [Google Scholar]








| Material | Theoretical Density (g cm−3) | Experimental Density (g cm−3) | Porosity (%) | Grain Size (µm) | Hardness (Hv) |
|---|---|---|---|---|---|
| Pure Mg | 1.74 | 1.7363 ± 0.002 | 0.21 | 34 ± 2 | 46 ± 3 |
| Mg-0.5 TCP | 1.7412 | 1.7371 ± 0.0147 | 0.23 | 18 ± 2 (↓47%) | 52 ± 2 (↑13.04%) |
| Mg-1.0 TCP | 1.7424 | 1.7381 ± 0.0067 | 0.24 | 13 ± 1 (↓61%) | 54 ± 3 (↑17.39%) |
| Mg-1.5 TCP | 1.7449 | 1.7387 ± 0.0048 | 0.28 | 10 ± 1 (↓70%) | 54 ± 1 (↑17.39%) |
| Material | Plane | I/Imax |
|---|---|---|
| Pure Mg | Prismatic | 1.00 |
| Basal | 0..29 | |
| Pyramidal | 0.36 | |
| Mg-0.5 TCP | Prismatic | 0.34 |
| Basal | 0.76 | |
| Pyramidal | 1.00 | |
| Mg-1.0 TCP | Prismatic | 0.22 |
| Basal | 0.98 | |
| Pyramidal | 1.00 | |
| Mg-1.5 TCP | Prismatic | 0.23 |
| Basal | 0.58 | |
| Pyramidal | 1.00 |
| Material | Damping Loss Rate (L) | Damping Capacity (Q−1) (× 10−4) | Elastic Modulus (GPa) |
|---|---|---|---|
| Pure Mg | 8.3 ± 0.2 | 6.56 ± 0.2 | 44.7 ± 0.2 |
| Mg-0.5TCP | 15.7 ± 0.9 (↑89%) | 6.94 ± 0.2 (↑5.7%) | 43.7 ± 0.4 |
| Mg-1.0 TCP | 17.4 ± 0.7 (↑109%) | 6.96 ± 0.3 (↑6.0%) | 43.5 ± 0.08 |
| Mg-1.5 TCP | 17.7 ± 0.5 (↑113%) | 7.59 ± 0.2 (↑15.7%) | 43.7 ± 0.6 |
| Material | Corrosion Rate (mm/Year) | pH |
|---|---|---|
| Pure Mg | 1.95 | 10.41 |
| Mg-0.5 TCP | 0.23 | 10.23 |
| Mg-1.0 TCP | 0.92 | 10.11 |
| Mg-1.5 TCP | 0.21 | 9.92 |
| Pure Mg [32] | 2.08 | - |
| Mg1Ca [32] | 3.16 | |
| Mg1Ca1Zn [32] | 2.13 | |
| Mg1Ca3Zn [32] | 2.92 | |
| Mg5Zn [33] | 2.25 | |
| Mg5Zn0.2Sr [33] | 1.75 | |
| Mg3Sr [32] | 0.75 | |
| ZE41 [34] | 2.04 | |
| AZ91 [34] | 3.56 |
| Material | 0.2 CYS (MPa) | UCS (MPa) | Fracture Strain (%) | Energy Absorbed (MJ/m3) |
|---|---|---|---|---|
| Pure Mg | 77 ± 5 | 156 ± 7 | 15.8 ± 0.3 | 17.7 ± 0.7 |
| Mg-0.5 TCP | 92 ± 1 (↑19%) | 258 ± 4 (↑65%) | 18.5 ± 0.6 (↑17%) | 28.3 ± 1.3 (↑59%) |
| Mg-1.0 TCP | 96 ± 2 (↑24%) | 223 ± 7 (↑42%) | 17.2 ± 0.7 (↑9%) | 23.2 ± 2.9 (↑31%) |
| Mg-1.5 TCP | 103 ± 7 (↑34%) | 240 ± 7 (↑53%) | 19.3 ± 0.5 (↑22%) | 29.2 ± 2.4 (↑64%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parande, G.; Manakari, V.; Gupta, H.; Gupta, M. Magnesium-β-Tricalcium Phosphate Composites as a Potential Orthopedic Implant: A Mechanical/Damping/Immersion Perspective. Metals 2018, 8, 343. https://doi.org/10.3390/met8050343
Parande G, Manakari V, Gupta H, Gupta M. Magnesium-β-Tricalcium Phosphate Composites as a Potential Orthopedic Implant: A Mechanical/Damping/Immersion Perspective. Metals. 2018; 8(5):343. https://doi.org/10.3390/met8050343
Chicago/Turabian StyleParande, Gururaj, Vyasaraj Manakari, Harshit Gupta, and Manoj Gupta. 2018. "Magnesium-β-Tricalcium Phosphate Composites as a Potential Orthopedic Implant: A Mechanical/Damping/Immersion Perspective" Metals 8, no. 5: 343. https://doi.org/10.3390/met8050343






