Template-Assisted Fabrication of Nanostructured Tin (β-Sn) Arrays for Bulk Microelectronic Packaging Devices
Abstract
:1. Introduction
2. Experimental Details
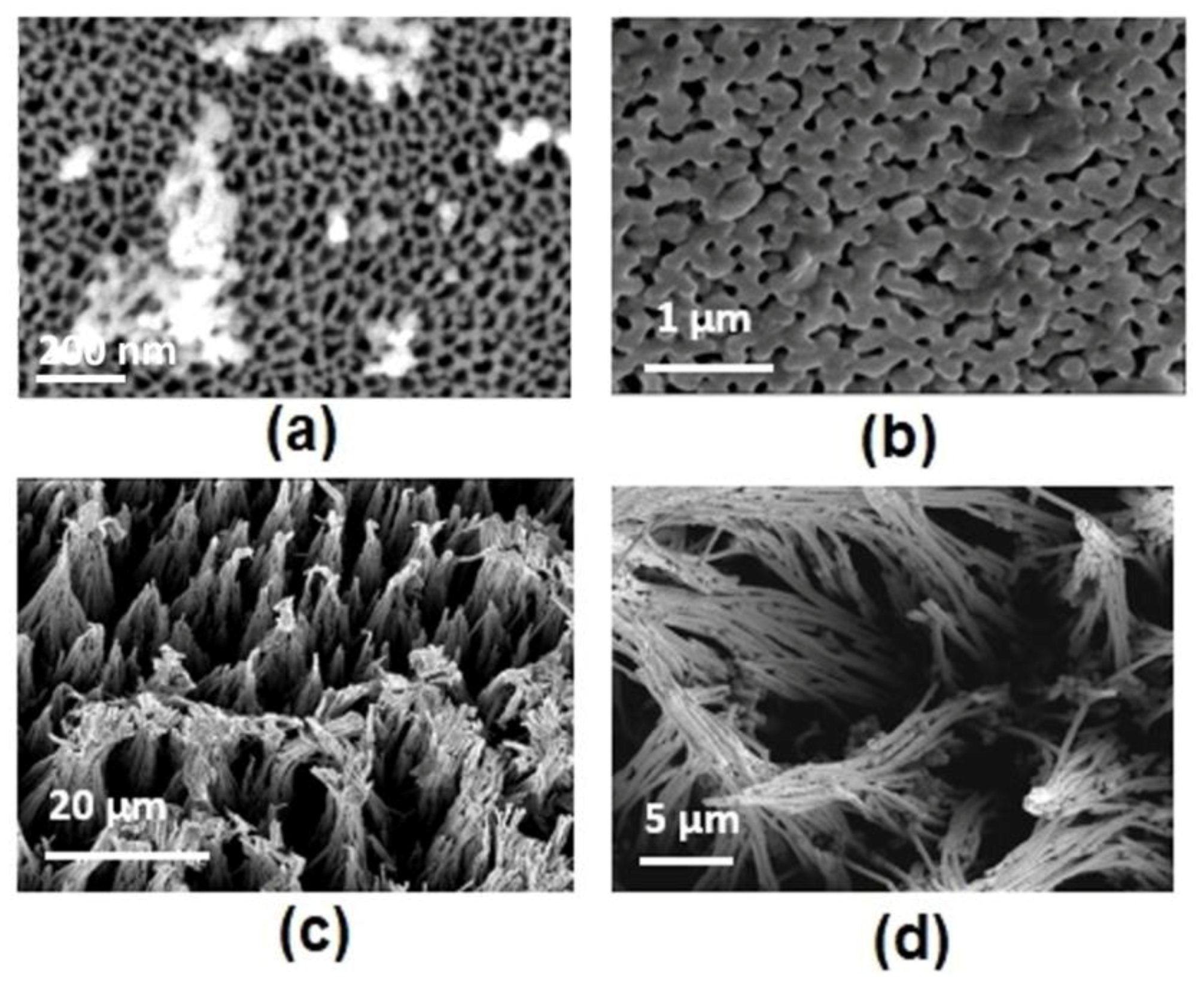
2.1. AAO Template Preparation
2.2. Bath Preparation
- Stannous Sulfate, SnSO4 (50 g/L, 99.9% pure);
- Sulfuric Acid, H2SO4 (170 g/L, 99.999% pure);
- Polyethylene glycol (PEG), H–(O–CH2–CH2)n–OH, n = 10,000 (g/L, 99.99% pure);
- Glutaraldehyde, OCH(CH2)3CHO (0.025 g/L, 99.99% pure).
2.3. The Plating Cell
2.4. The Pulse Plating Experiment
2.5. Recovery of Nanostructures
3. Microstructural Characterization
3.1. Phase Analysis
3.2. Nanostructure Morphology
4. Results and Discussion
4.1. Phase Evolution
4.2. Compositional Analysis
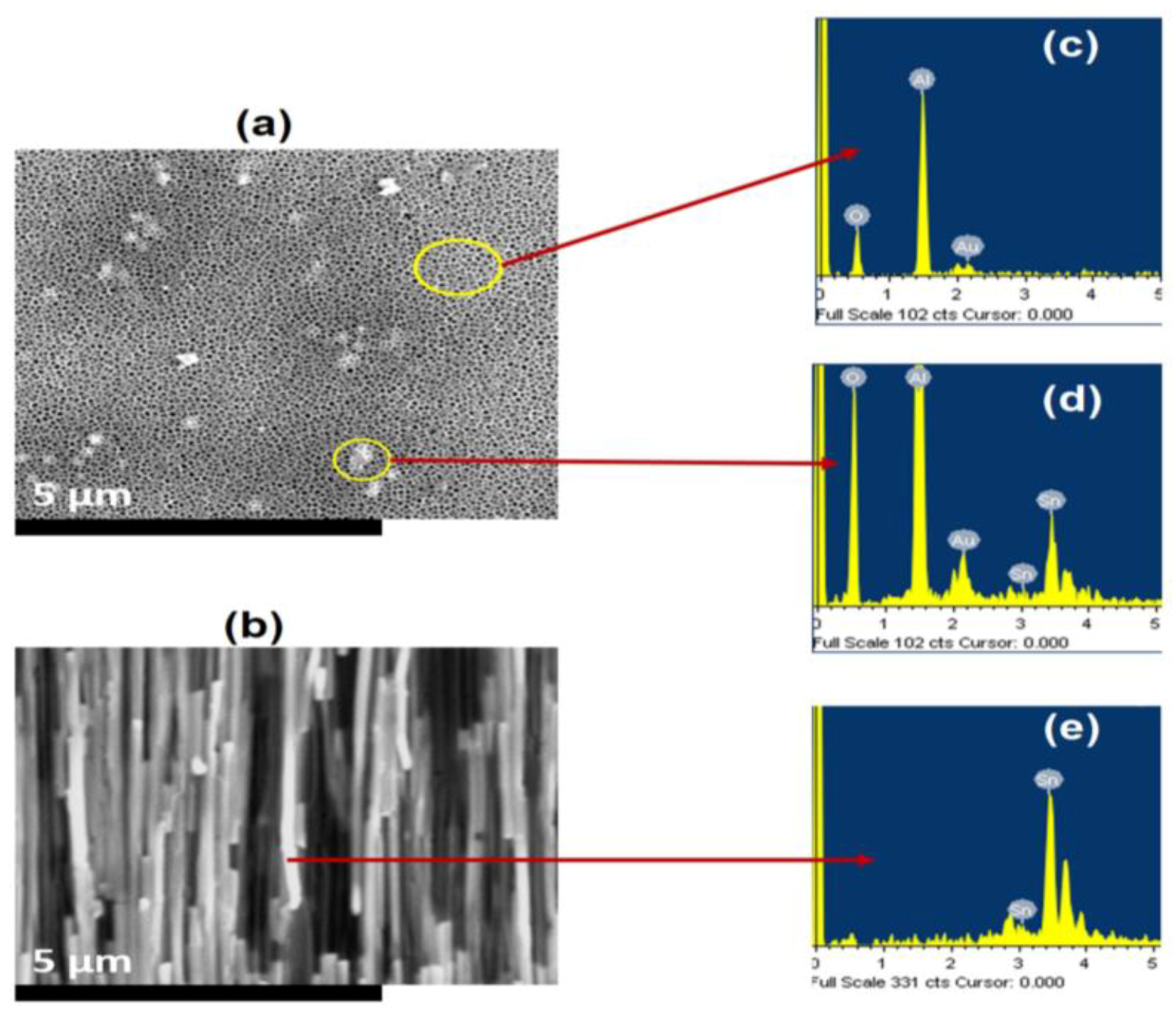
4.3. Morphology of Nanostructures
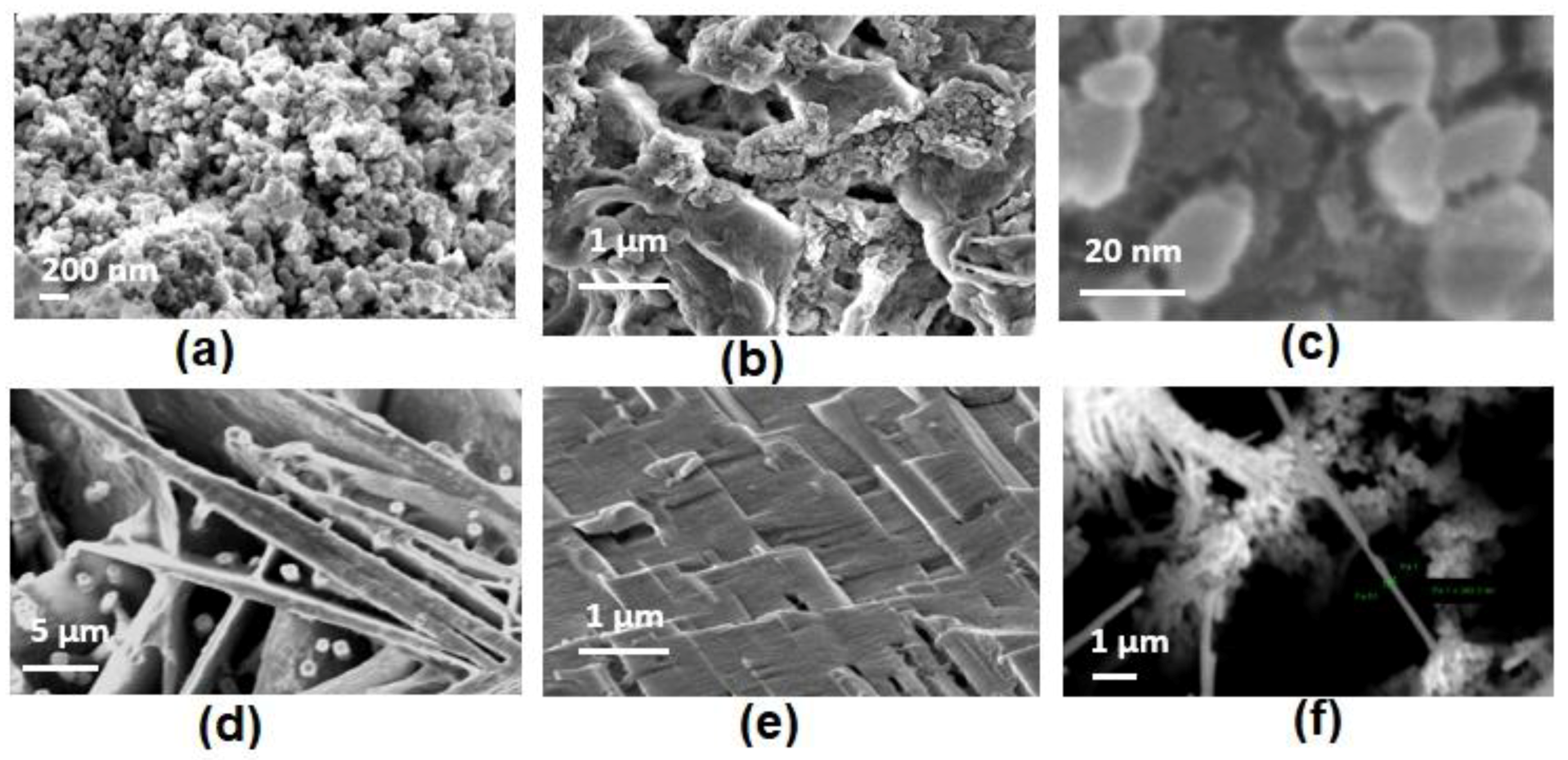
4.3.1. Formation of Short Nanorods (−0.5 V)
4.3.2. Formation of Large Nanorods/Nanowires (−1.1 V)
4.3.3. Formation of Nano-Rods, Nanoparticles, and Nanoplates (−3.2 V)
5. Conclusions
- 1D Sn nanostructures arrays were produced successfully via the template assisted plating approach from aqueous sulfate bath.
- It was found that the nanostructure shape, size, and morphology were severely affected in response to various deposition potential.
- Low deposition potential of −0.5 V causes formation of very short nanowires (1–2 µm, dia 140–160 nm) and incomplete filling of the AAO template pores is found.
- At a medium deposition potential, −1.1 V, the growth rate of nanostructures was further increased to (4–7 µm length, dia 200 nm). Most of the template pores were filled completely
- As the deposition potential was raised to a sufficiently high value, −3.2 V, multiple nanostructures were formed. The nanoparticles were formed due to the dendritic deposition of Sn inside the AAO template pores (20 nm).
- The multiple nanostructures (nanoplates, nanorods, and nanoparticles) were formed on account of the tip effect of Sn nuclei inside the AAO template. It can be suggested that an optimum deposition potential is needed to exploit the use of each kind of nanostructure and can be potentially controlled for mass production.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hernandez-Velez, M. Nanowires and 1D arrays fabrication: An overview. Thin Solid Films 2006, 495, 51–63. [Google Scholar] [CrossRef]
- Vazquez, M.; Pirota, K.; Hernandez-Velez, M.; Prida, V.M.; Navas, D.; Sanz, R.; Batallan, F.; Velazquez, J. Magnetic properties of densely packed arrays of Ni nanowires as a function of their diameter and lattice parameter. J. Appl. Phys. 2004, 95, 6642–6644. [Google Scholar] [CrossRef] [Green Version]
- Djenizian, T.; Hanzu, I.; Eyraud, M.; Santinacci, L. Electrochemical fabrication of tin nanowires: A short review. C. R. Chim. 2008, 11, 995–1003. [Google Scholar] [CrossRef]
- Pang, S.K.; Yung, K.C. A green approach to synthesis of nanoparticles of Sn-3.0Ag-0.5Cu lead-free solder alloy. Mater. Trans. 2012, 53, 1770–1774. [Google Scholar] [CrossRef]
- McAlpine, M.C.; Friedman, R.S.; Jin, S.; Lin, K.H.; Wang, W.U.; Lieber, C.M. High-Performance Nanowire Electronics and Photonics on Glass and Plastic Substrates. Nano Lett. 2003, 3, 1531–1535. [Google Scholar] [CrossRef]
- Bouzigues, C.; Gacoin, T.; Alexandrou, A. Biological applications of rare-earth based nanoparticles. ACS Nano 2011, 5, 8488–8505. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Sharma, A.; Kim, J. Reclamation of diesel oil from sea water using magnetic Au/Fe2O3 nanocomposites prepared by chemical precipitation. Mater. Res. Express 2018, 5, 015047. [Google Scholar] [CrossRef]
- Chena, Z.G.; Hana, G.; Yanga, L.; Chenga, L.; Zoua, J. Nanostructured thermoelectric materials: Current research and future challenge. Prog. Nat. Sci. Mater. Int. 2012, 22, 535–549. [Google Scholar] [CrossRef]
- Aghiu, M.A.; Gorea, M.; Kristaly, F.; Tomoaia-Cotisel, M. A new method for synthesis of forsterite nanomaterials for bioimplants. Ceram. Silik. 2014, 58, 303–307. [Google Scholar]
- Sharma, B.; Sharma, A.; Kim, J.S. Recent Advances on H 2 Sensor Technologies Based on MOX and FET Devices: A Review. Sens. Actuators B Chem. 2018, 262, 758–770. [Google Scholar] [CrossRef]
- Nguyen, V.H. Nanomaterials: A guide to fabrication and applications. Adv. Nat. Sci Nanosci. Nanotechnol. 2017, 8, 020401. [Google Scholar]
- Schlesinger, M.; Paunovic, M. Modern Electroplating, 5th ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Sharma, A.; Cheon, C.S.; Jung, J.P. Recent progress in electroless plating of copper. J. Microelectron. Packag. Soc. 2016, 23, 1–6. [Google Scholar] [CrossRef]
- Li, R.; Sun, X.; Zhou, X.; Cai, M.; Sun, X. Aligned Heterostructures of Single-Crystalline Tin Nanowires Encapsulated in Amorphous Carbon Nanotubes. J. Phys. Chem. C 2007, 111, 9130–9135. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, X.; Zhao, Y.; Jia, N.; Liu, L.; Yan, M.; Jiang, Z. Single crystal tin nano-rod arrays electrodeposited by a soft template. Chem. Commun. 2005, 4941–4942. [Google Scholar] [CrossRef] [PubMed]
- Qui, L.; Vilas, G.P.; Jose, C.M.; Aharon, G. Synthesis of tin nanorods via a sonochemical method combined with a polyol process. Ultrason. Sonochem. 2005, 12, 243–247. [Google Scholar]
- Johansson, A. Template-Based Fabrication of Nanostructured Materials. Ph.D. Thesis, Upssala University, Upssala, Sweden, 2006. [Google Scholar]
- Sharma, A.; Jang, Y.J.; Jung, J.P. Effect of KOH to Na2SiO3 ratio on microstructure and hardness of plasma electrolytic oxidation coatings on AA 6061 alloy. J. Mater. Eng. Perform. 2017, 26, 5032–5042. [Google Scholar] [CrossRef]
- Thierry, D.; Ilie, H.; Yesudas, D.P.; Florence, V.; Philippe, K. Electrochemical fabrication of Sn nanowires on titania nanotube guide layers. Nanotechnology 2008, 19, 205601. [Google Scholar]
- Lin, J.Y.; Yu, L.H.; Huang, J.J.; Wu, J.R. Effect of silver nitrate concentration of silver nanowires synthesized using a polyol method and their application as transparent conductive films. Thin Solid Films 2015, 584, 243–247. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharya, S.; Das, S.; Das, K. A study on the effect of pulse electrodeposition parameters on the morphology of pure tin coatings. Metall. Mater. Trans. A 2014, 45, 4610–4622. [Google Scholar] [CrossRef]
- Rashidi, A.M.; Amadeh, A. The effect of current density on the grain size of electrodeposited nanocrystalline nickel coatings. Surf. Coat. Technol. 2008, 202, 3772–3776. [Google Scholar] [CrossRef]
- Schlesinger, M.; Paunovic, M. Fundamental of Electrochemical Deposition, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Zhang, H.; Jiang, F.; Zhou, R.; Du, Y.; Yang, P.; Wang, C.; Xu, J. Effect of deposition potential on the structure and electrocatalytic behavior of Pt micro/nanoparticles. Int. J. Hydrogen Energy 2011, 36, 15052–15059. [Google Scholar] [CrossRef]
- Schmitz, E.P.S.; Quinaia, S.P.; Garcia, J.R.; de Andrade, C.K.; Lopes, M.C. Influence of commercial organic additives on the nickel electroplating. Int. J. Electrochem. Sci. 2016, 11, 983–997. [Google Scholar]
- Shin, H.S.; Song, J.Y.; Yu, J. Lattice Deformation of Sn Nanowires for the Application to Nano-Interconnection Technology. In Proceedings of the 60th International Conference on ‘IEEE Electronic Components and Technology’, Las Vegas, NV, USA, 1–4 June 2010; pp. 1861–1865. [Google Scholar]
- Cui, Q.; Rajathurai, K.; Jia, W.; Li, X.; Gao, F.; Lei, Y.; Gu, Z. Synthesis of Single Crystalline Tin Nanorods and Their Application as Nanosoldering Materials. J. Phys. Chem. C 2010, 114, 21938–21942. [Google Scholar] [CrossRef]
- Cai, H.; Wang, W.; Liu, P.; Wang, G.; Liu, A.; He, Z.; Cheng, Z.; Zhang, S.; Xia, M. Enhanced synthesis of Sn nanowires with aid of Se atom via physical vapor transport. J. Cryst. Growth 2015, 420, 42–46. [Google Scholar] [CrossRef]
- Feng, B.; Faruque, F.; Bao, P.; Chien, A.T.; Kumar, S.; Peterson, G.P. Double-sided tin nanowire arrays for advanced thermal interface materials. Appl. Phys. Lett. 2013, 102, 093105. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Lan, Q.; Ma, H.; Qu, K.; Inkson, B.J.; Mellors, N.J.; Xue, D.; Peng, Y. Nanoscale characterization of 1D Sn-3.5Ag nanosolders and their application into nanowelding at the nanoscale. Nanotechnology 2014, 25, 425301. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, G.; Yang, H.; Wang, W.; Luo, S.; Dai, S.; Qiu, M. Sn nanowires can be used in low temperature soldering. Appl. Phys. Lett. 2016, 108, 193101. [Google Scholar] [CrossRef]
- Tian, Y.T.; Meng, G.W.; Gao, T.; Sun, S.H.; Xie, T.; Peng, X.S.; Ye, C.H.; Zhang, L.D. Alumina nanowire arrays standing on a porous anodic alumina membrane. Nanotechnology 2004, 15, 189–191. [Google Scholar] [CrossRef]
- Xiao, Z.L.; Han, C.Y.; Welp, U.; Wang, H.H.; Kwok, W.K.; Willing, G.A.; Hiller, J.M.; Cook, R.E.; Miller, D.J.; Crabtree, G.W. Fabrication of alumina nanotubes and nanowires by etching porous alumina Membranes. Nano Lett. 2002, 2, 1293–1297. [Google Scholar] [CrossRef]
- Ertan, A.; Tiwari, S.N.; Talu, O. Electrodeposition of Nickel Nanowires and Nanotubes Using Various Templates. J. Exp. Nanosci. 2008, 3, 287–295. [Google Scholar] [CrossRef]
- Shin, S.; Al-Housseiny, T.T.; Kim, B.S.; Cho, H.H.; Stone, H.A. The Race of Nanowires: Morphological Instabilities and a Control Strategy. Nano Lett. 2014, 14, 4395–4399. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, M.S.; Pushpavanam, M. Pulse and pulse reverse plating—Conceptual, advantages and applications. Electrochim. Acta 2008, 53, 3313–3322. [Google Scholar] [CrossRef]
- Inguanta, R.; Ferrara, G.; Piazza, S.; Sunseri, C. Nanostructures fabrication by template deposition into anodic alumina membranes. Chem. Eng. Trans. 2009, 17, 957–962. [Google Scholar]
- Shin, S.; Kim, B.S.; Kim, K.M.; Kong, B.H.; Cho, H.K.; Cho, H.H. Tuning the morphology of copper nanowires by controlling the growth processes in electrodeposition. J. Mater. Chem. 2011, 21, 17967–17971. [Google Scholar] [CrossRef]




| Parameters | Values |
|---|---|
| pH | 0.6 |
| Applied potential | −0.5 V, −1.1 V, −3.2 V |
| Off time potential | 0 |
| On-time, Off-time | 0.001 s, 0.01 s |
| Temperature | 26 °C |
| Duty Cycle | 10% |
| Frequency | 100 Hz |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Srivastava, A.K.; Jeon, Y.; Ahn, B. Template-Assisted Fabrication of Nanostructured Tin (β-Sn) Arrays for Bulk Microelectronic Packaging Devices. Metals 2018, 8, 347. https://doi.org/10.3390/met8050347
Sharma A, Srivastava AK, Jeon Y, Ahn B. Template-Assisted Fabrication of Nanostructured Tin (β-Sn) Arrays for Bulk Microelectronic Packaging Devices. Metals. 2018; 8(5):347. https://doi.org/10.3390/met8050347
Chicago/Turabian StyleSharma, Ashutosh, Ashok K. Srivastava, Yongho Jeon, and Byungmin Ahn. 2018. "Template-Assisted Fabrication of Nanostructured Tin (β-Sn) Arrays for Bulk Microelectronic Packaging Devices" Metals 8, no. 5: 347. https://doi.org/10.3390/met8050347





