Investigation of Nanoporous Superalloy Membranes for the Production of Nanoemulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manufacturing of the Superalloy Membranes
2.1.1. Homogenization and Precipitation Heat Treatment
2.1.2. Coarsening of the γ′-Phase and Electro-Chemical Phase Extraction
2.1.3. Superalloy Membrane Characterization
2.2. Premix Membrane Emulsification
2.2.1. Pre-Emulsion Composition and Preparation
2.2.2. Instrumented Small-Scale Extruder
2.2.3. Experimental Set up
2.2.4. Droplet Size Determination
3. Results and Discussion
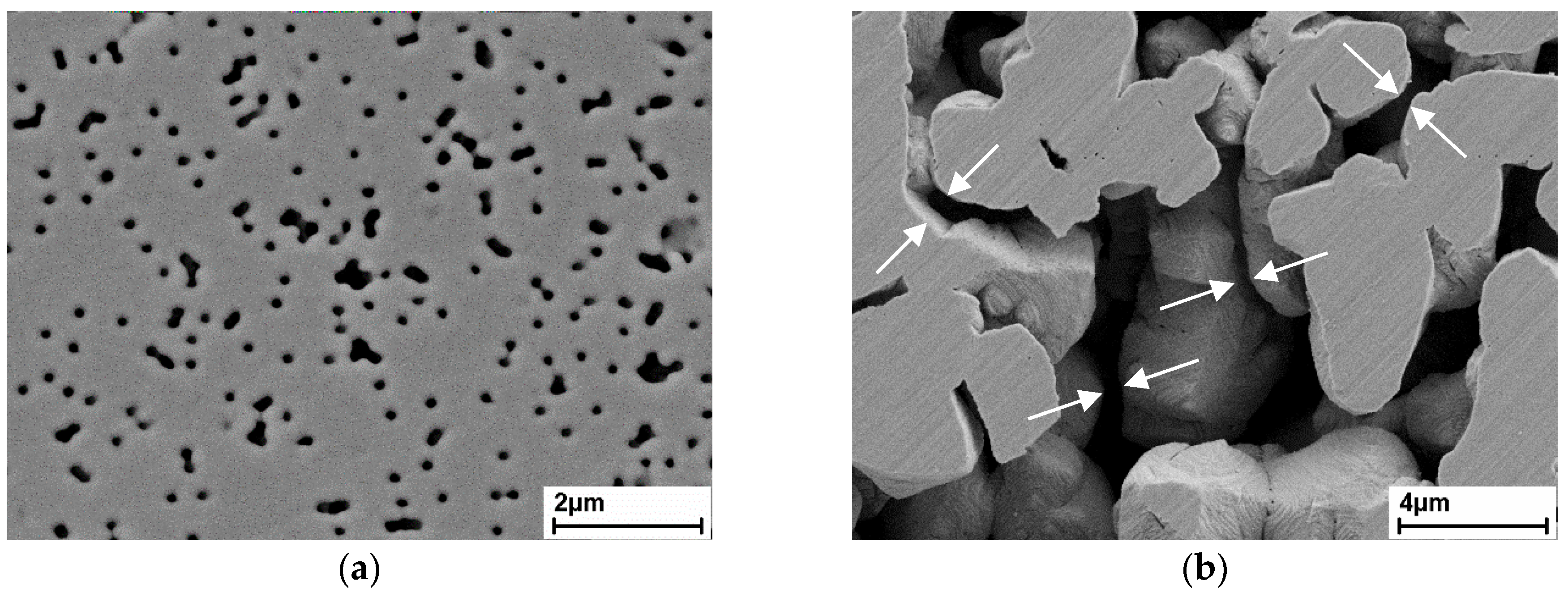
3.1. Microstructure of the Membranes
3.2. Flow Resistance and Energy Dissipation in Premix Membrane Emulsification
3.3. Emulsification Performance at Low Flow Rate (0.5 mL/s)
3.4. Emulsification Performance at Intermediate Flow Rate (1.0 mL/s)
3.5. Emulsification Performance at High Flow Rate (1.4 mL/s)
3.6. Comparison of Flow Rates and Membrane Structures
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Schubert, H.; Engel, R. Product and Formulation Engineering of Emulsions. Chem. Eng. Res. Des. 2004, 82, 1137–1143. [Google Scholar] [CrossRef]
- Cornier, J.; Owen, A.; Kwade, A.; van de Voorde, M. Pharmaceutical Nanotechnology. Innovation and Production; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017. [Google Scholar]
- Jahnke, S. The theory of high-pressure homogenization. In Emulsions and Nanosuspensions for the Formulation of Poorly Soluble Drugs: Based on the Invited Lectures and Communications Presented at the Colloidal Drug Carriers—CDC—3rd Expert Meeting, Berlin (Germany), 29–31 May 1997; 34 Tables; Müller, R.H., Ed.; Medpharm Scientific Publ.: Stuttgart, Germany, 1998; pp. 177–200. [Google Scholar]
- Vladisavljević, G.T.; Williams, R.A. Recent developments in manufacturing emulsions and particulate products using membranes. Adv. Colloid Interface Sci. 2005, 113, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Shuto, I.; Hagura, Y. Characteristics of the Membrane Emulsification Method Combined with Preliminary Emulsification for Preparing Corn Oil-in-Water Emulsions. FSTI 1996, 2, 43–47. [Google Scholar] [CrossRef]
- Trentin, A.; Ferrando, M.; López, F.; Güell, C. Premix membrane O/W emulsification: Effect of fouling when using BSA as emulsifier. Desalination 2009, 245, 388–395. [Google Scholar] [CrossRef]
- Rösler, J.; Mukherji, D. Design of Nanoporous Superalloy Membranes for Functional Applications. Adv. Eng. Mater. 2003, 5, 916–918. [Google Scholar] [CrossRef]
- Rösler, J.; Näth, O.; Jäger, S.; Schmitz, F.; Mukherji, D. Fabrication of nanoporous Ni-based superalloy membranes. Acta Mater. 2005, 53, 1397–1406. [Google Scholar] [CrossRef]
- Rösler, J.; Näth, O. Mechanical behaviour of nanoporous superalloy membranes. Acta Mater. 2010, 58, 1815–1828. [Google Scholar] [CrossRef]
- Rösler, J.; Krause, W.; Hinze, B. A Concept for the Control of Pore Size in Superalloy Membranes. Metals 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Hinze, B. Verfahren zur Materialauswahl für akustische Liner im Heißgaspfad von Flugtriebwerken. Ph.D. Thesis, Technische Universität Braunschweig, Niedersächsisches Forschungszentrum für Luftfahrt, Braunschweig, Germany, January 2015. [Google Scholar]
- Näth, O. Nanoporöse Strukturen auf Nickelbasis: Herstellung, Eigenschaften und Anwendungspotenzial. Ph.D. Thesis, Technische Universität Braunschweig, Braunschweig, Germany, October 2008. [Google Scholar]
- Durand-Charre, M. The Microstructure of Superalloys; Gordon & Breach: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Tien, J.K.; Copley, S.M. The effect of uniaxial stress on the periodic morphology of coherent gamma prime precipitates in nickel-base superalloy crystals. Metall. Trans. 1971, 2, 215–219. [Google Scholar] [CrossRef]
- Pollock, T.M.; Argon, A.S. Directional coarsening in nickel-base single crystals with high volume fractions of coherent precipitates. Acta Metall. Mater. 1994, 42, 1859–1874. [Google Scholar] [CrossRef]
- Harris, K.; Erickson, G.L.; Sikkenga, S.L.; Brentnall, W.D.; Aurrecoechea, J.M.; Kubarych, K.G. Development of the Rhenium Containing Superalloys CMSX-4® & CM 186 LC® for Single Crystal Blade and Directionally Solidified Vane Applications in Advanced Turbines. In Superalloys 1992; Antolovich, S.D., Stusrud, R.W., Mackay, R.A., Anton, D.L., Khan, T., Kissinger, R.D., Klarstrom, D.L., Eds.; TMS: Warrendale, PA, USA, 1992; pp. 297–306. [Google Scholar]
- Sutera, S.P.; Skalak, R. The History of Poiseuille’s Law. Annu. Rev. Fluid Mech. 1993, 25, 1–20. [Google Scholar] [CrossRef]
- Schatt, W.; Wieters, K.-P.; Kieback, B. Pulvermetallurgie. Technologien und Werkstoffe, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Gehrmann, S.; Bunjes, H. Instrumented small scale extruder to investigate the influence of process parameters during premix membrane emulsification. Chem. Eng. J. 2016, 284, 716–723. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Kwade, A.; Schwedes, J. Chapter 6 Wet Grinding in Stirred Media Mills. In Particle Breakage; Salman, A.D., Ghadiri, M., Hounslow, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 251–382. [Google Scholar]
- DeRoussel, P.; Khakhar, D.V.; Ottino, J.M. Mixing of viscous immiscible liquids. Part 2: Overemulsification—Interpretation and use. Chem. Eng. Sci. 2001, 56, 5531–5537. [Google Scholar] [CrossRef]
- Gehrmann, S.; Bunjes, H. Preparation of Nanoemulsions by Premix Membrane Emulsification: Which Parameters Have a Significant Influence on the Resulting Particle Size? J. Pharm. Sci. 2017, 106, 2068–2076. [Google Scholar] [CrossRef] [PubMed]





| Manufacturing Process Steps | TM-γ′-Membrane | LF-γ′-Membrane | LF-γ-Membrane |
|---|---|---|---|
| Homogenization heat treatment | 1277 °C for 2 h 1288 °C for 3 h 1296 °C for 3 h 1304 °C for 2 h 1313 °C for 2 h 1316 °C for 2 h 1318 °C for 2 h 1321 °C for 2 h | 1277 °C for 2 h 1288 °C for 3 h 1296 °C for 3 h 1306 °C for 3 h 1315 °C for 3 h 1318 °C for 2 h 1321 °C for 2 h | |
| Cooling from homogenization | AC to room temperature | FC 0.2 °C/min to 1080 °C | |
| Precipitation heat treatment | 1080 °C for 4 h, AC | ||
| 870 °C for 16 h, AC | |||
| Coarsening of γ′ | 1000 °C, 170 MPa, FC | 1080 °C for 1000 h, AC | |
| Extracted phase | γ-phase | γ′-phase | |
| Mem-Brane | ε [%] | s [µm] | [mL/s] | [bar] | [%] | () [nm] | () [] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LF-γ | 51 | 300 | 0.5 | 2.3 | 2.3 | 0 | 866 | 866 | 887 | 138.3 | 138.3 | 145.2 |
| 1 | 5.3 | 4.9 | −7.5 | 673 | 707 | 699 | 83.6 | 92.2 | 90.2 | |||
| 1.4 | 6.9 | 6.6 | −4.3 | 680 | 698 | 691 | 85.3 | 89.9 | 87.9 | |||
| PE | 9 | 1–2 | 0.5 | 9.4 | 13.9 | 47.9 | 195 | 157 | 173 | 1.1 | 0.7 | 0.8 |
| 1 | 18 | 31 | 72.2 | 194 | 146 | 165 | 1.1 | 0.6 | 0.8 | |||
| 1.4 | 19.4 | 39 | 101 | 221 | 153 | 169 | 1.4 | 0.7 | 0.8 | |||
| TM-γ′ | 26 | 300 | 0.5 | 27.2 | 23.5 | −13.6 | 291 | 314 | 308 | 6.9 | 8 | 7.7 |
| 1 | 45.4 | 46.3 | 2 | 316 | 313 | 319 | 8.1 | 7.9 | 8.3 | |||
| 1.4 | 76 | 82 | 7.9 | 287 | 277 | 285 | 6.7 | 6.2 | 6.6 | |||
| LF-γ′ | 41 | 300 | 0.5 | 35.2 | 38.6 | 9.7 | 203 | 193 | 196 | 5.3 | 4.8 | 4.9 |
| 1 | 75.4 | 77.8 | 3.2 | 194 | 191 | 194 | 4.8 | 4.7 | 4.8 | |||
| 1.4 | 108.1 | 104.5 | −3.3 | 192 | 195 | 194 | 4.7 | 4.9 | 4.8 | |||
| Membrane | ||||||||
|---|---|---|---|---|---|---|---|---|
| [nm] | Span [-] | [nm] | Span [-] | [nm] | Span [-] | [nm] | Span [-] | |
| PE | 413 | 1.06 | 223 | 0.96 | 176 | 0.64 | 157 | 0.60 |
| TM-γ′ | 434 | 0.56 | 372 | 0.45 | 352 | 0.44 | 316 | 0.48 |
| LF-γ′ | 459 | 0.99 | 343 | 0.85 | 287 | 1.13 | 253 | 1.20 |
| Membrane | ||||||||
|---|---|---|---|---|---|---|---|---|
| [nm] | Span [-] | [nm] | Span [-] | [nm] | Span [-] | [nm] | Span [-] | |
| PE | 364 | 2.42 | 200 | 0.85 | 163 | 0.63 | 154 | 0.47 |
| TM-γ′ | 344 | 0.99 | 120 | 2.41 | 183 | 0.75 | 160 | 0.67 |
| LF-γ′ | 461 | 6.83 | 275 | 1.16 | 127 | 1.95 | 184 | 0.80 |
| Membrane | ||||||||
|---|---|---|---|---|---|---|---|---|
| [nm] | Span [-] | [nm] | Span [-] | [nm] | Span [-] | [nm] | Span [-] | |
| PE | 379 | 2.75 | 183 | 0.70 | 164 | 0.68 | 153 | 0.56 |
| TM-γ′ | 324 | 1.08 | 157 | 1.36 | 156 | 0.78 | 143 | 0.52 |
| LF-γ′ | 440 | 7.10 | 268 | 1.17 | 135 | 1.79 | 185 | 0.76 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohnke, M.; Finke, J.H.; Kwade, A.; Rösler, J. Investigation of Nanoporous Superalloy Membranes for the Production of Nanoemulsions. Metals 2018, 8, 361. https://doi.org/10.3390/met8050361
Kohnke M, Finke JH, Kwade A, Rösler J. Investigation of Nanoporous Superalloy Membranes for the Production of Nanoemulsions. Metals. 2018; 8(5):361. https://doi.org/10.3390/met8050361
Chicago/Turabian StyleKohnke, Maren, Jan Henrik Finke, Arno Kwade, and Joachim Rösler. 2018. "Investigation of Nanoporous Superalloy Membranes for the Production of Nanoemulsions" Metals 8, no. 5: 361. https://doi.org/10.3390/met8050361





