Phosphorus-Containing Mineral Evolution and Thermodynamics of Phosphorus Vaporization during Carbothermal Reduction of High-Phosphorus Iron Ore
Abstract
:1. Introduction
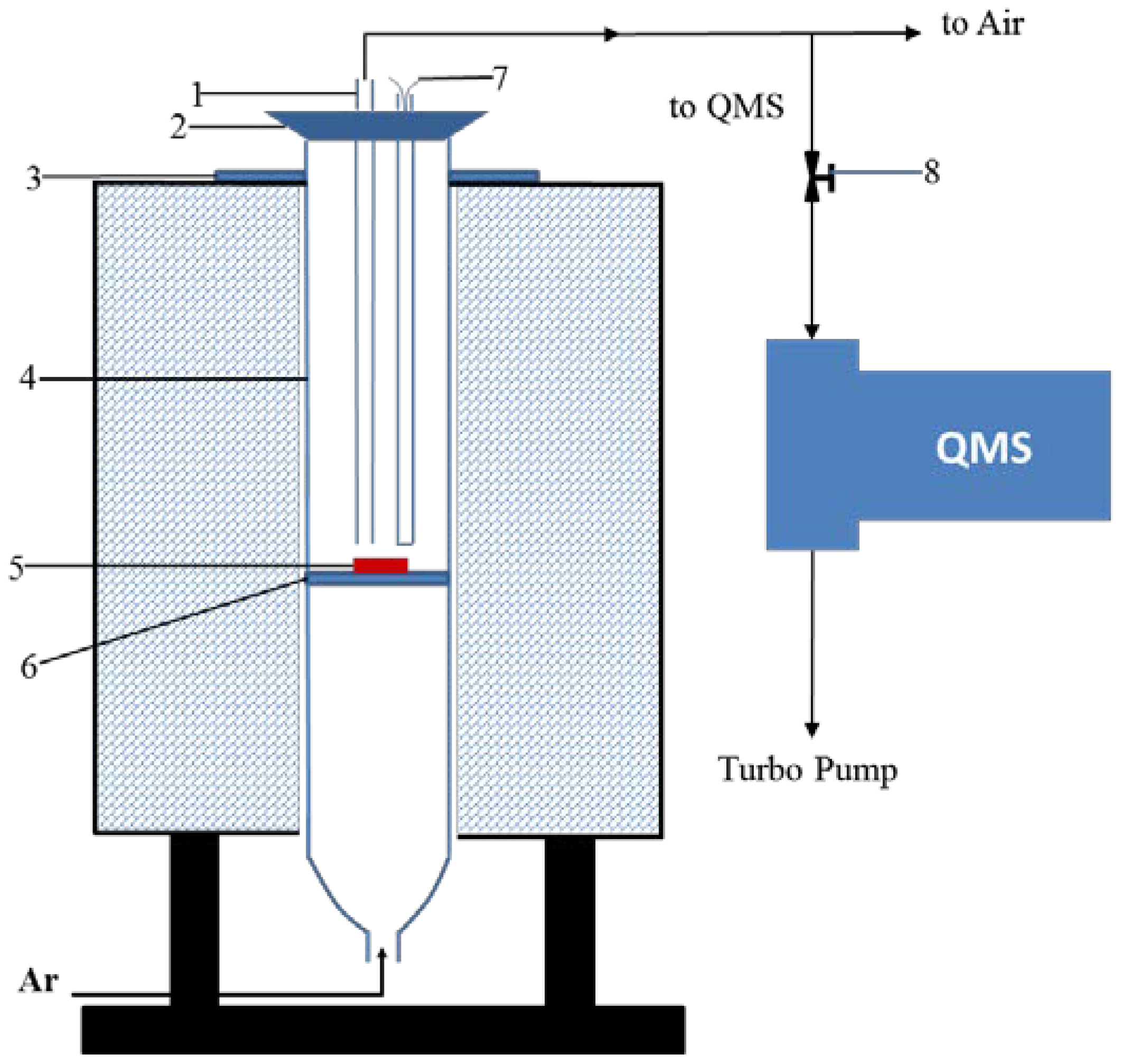
2. Experiments
2.1. Experimental Materials
2.2. Experimental Procedure
3. Results and Discussion
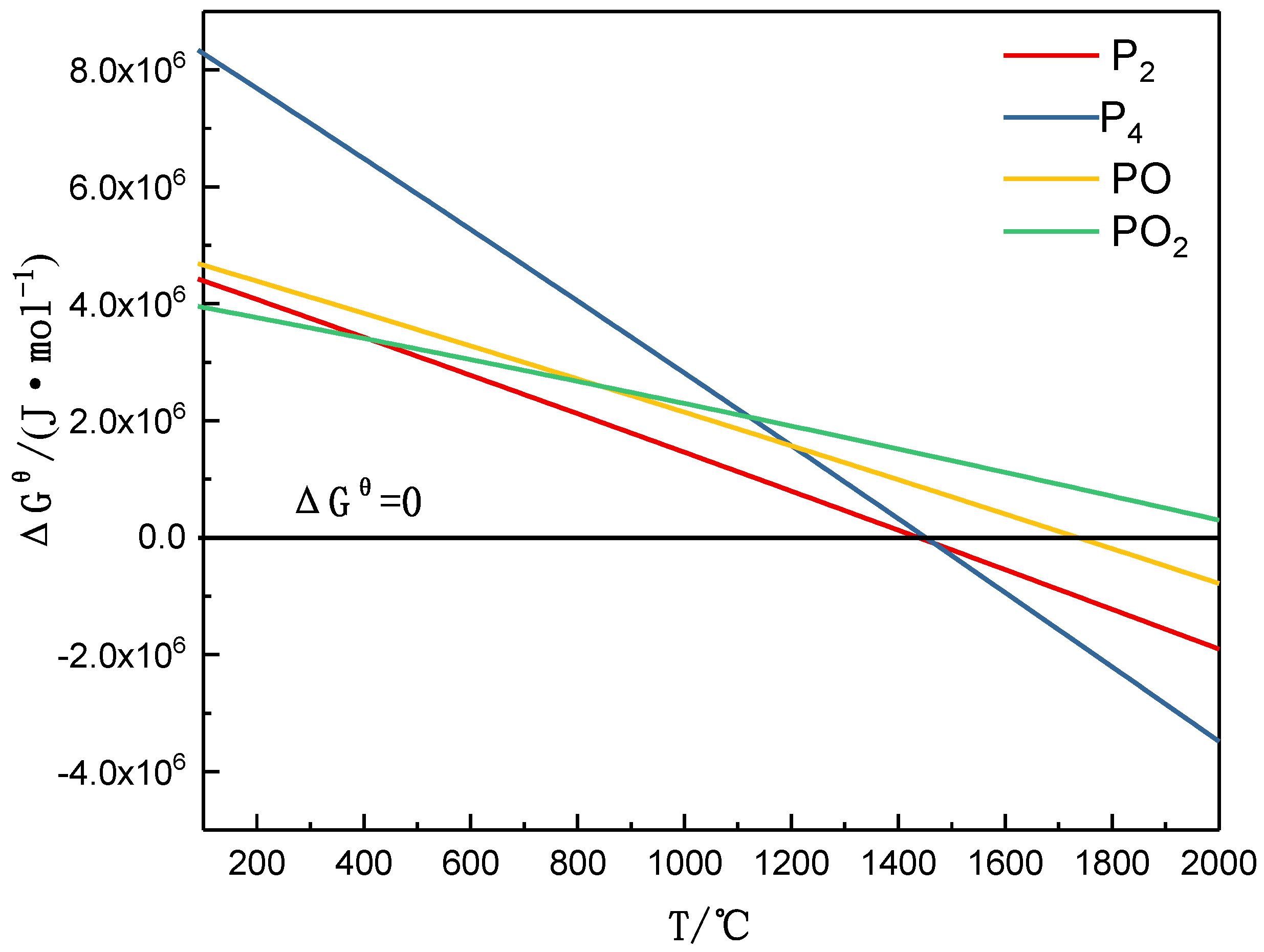
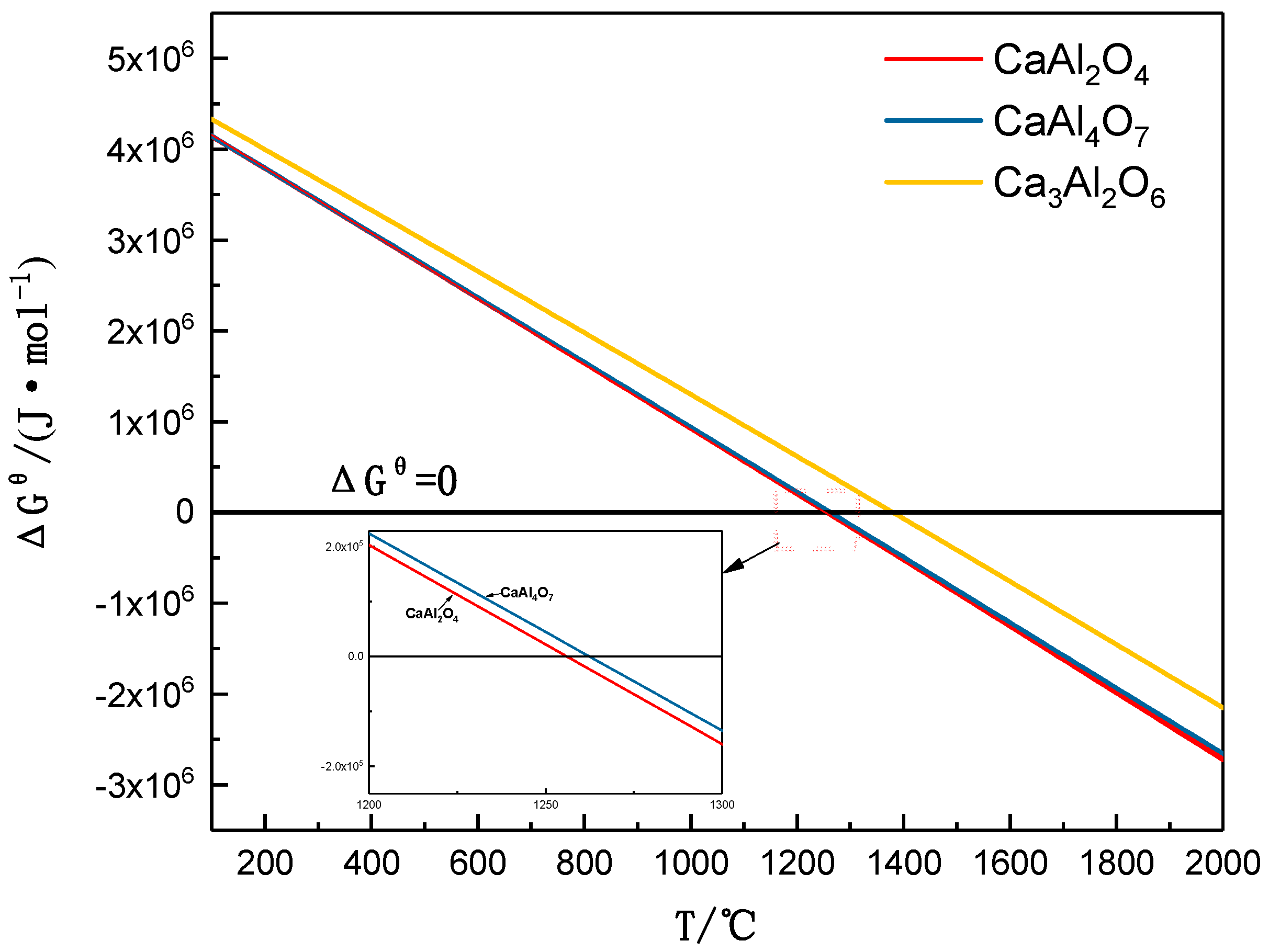
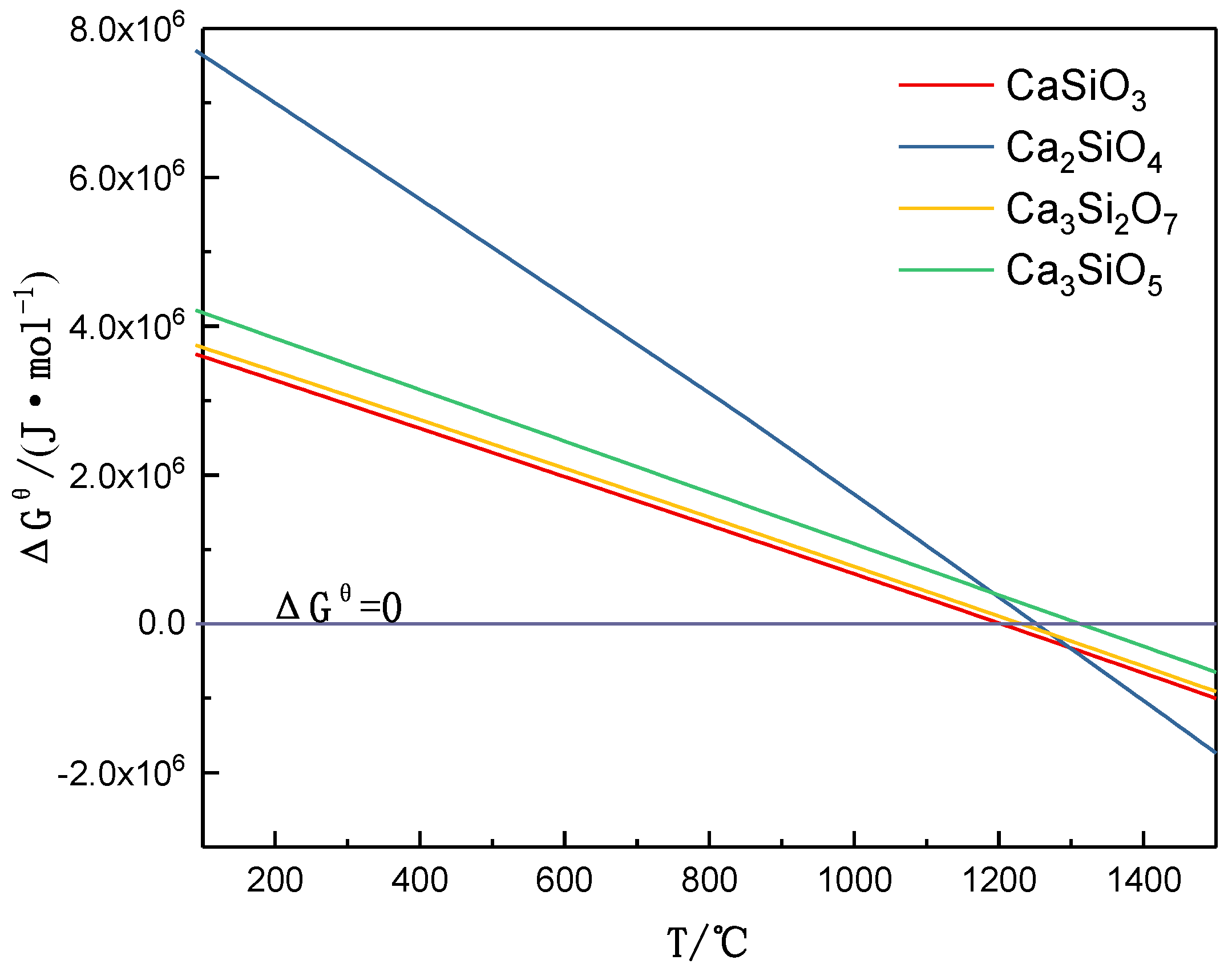
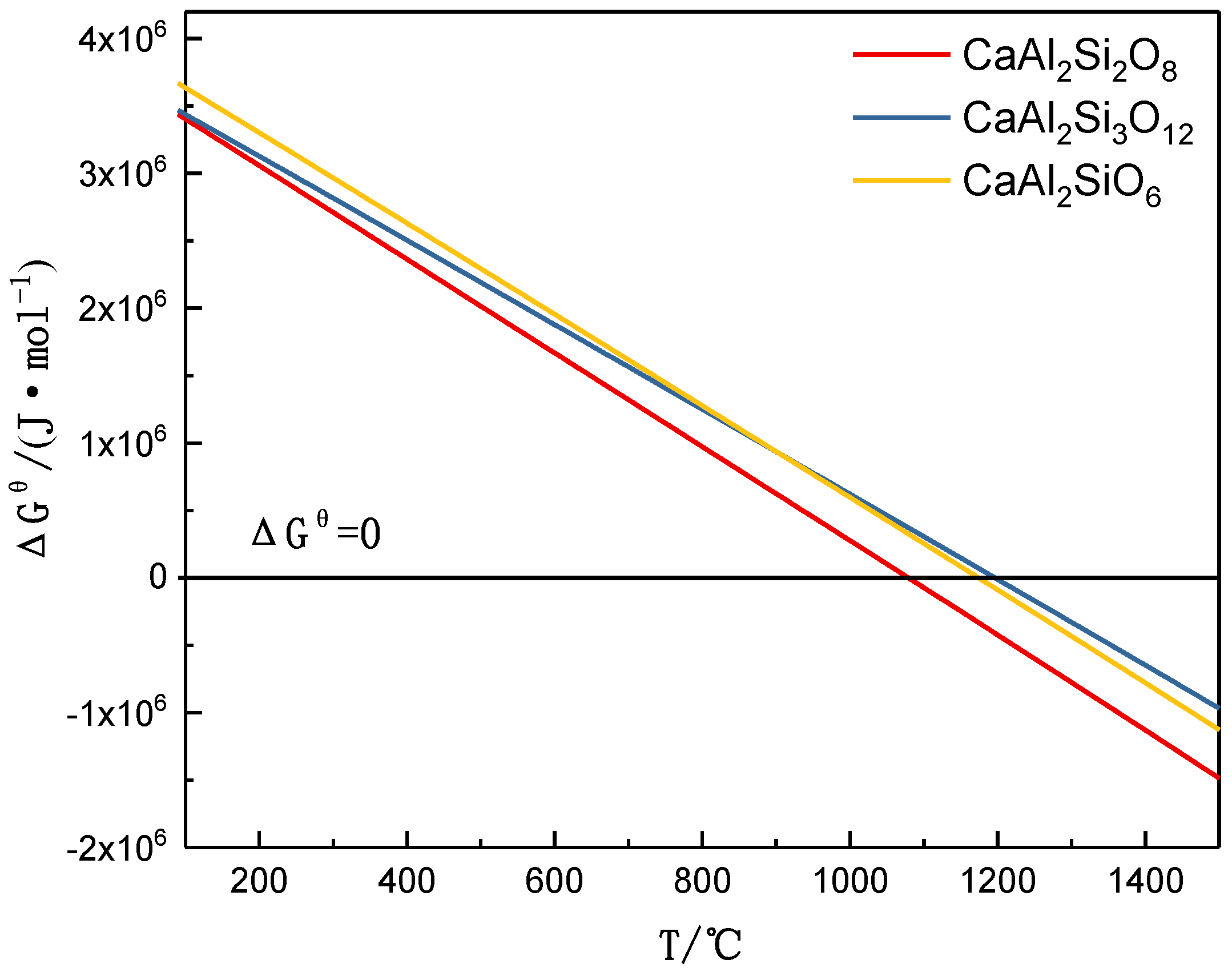
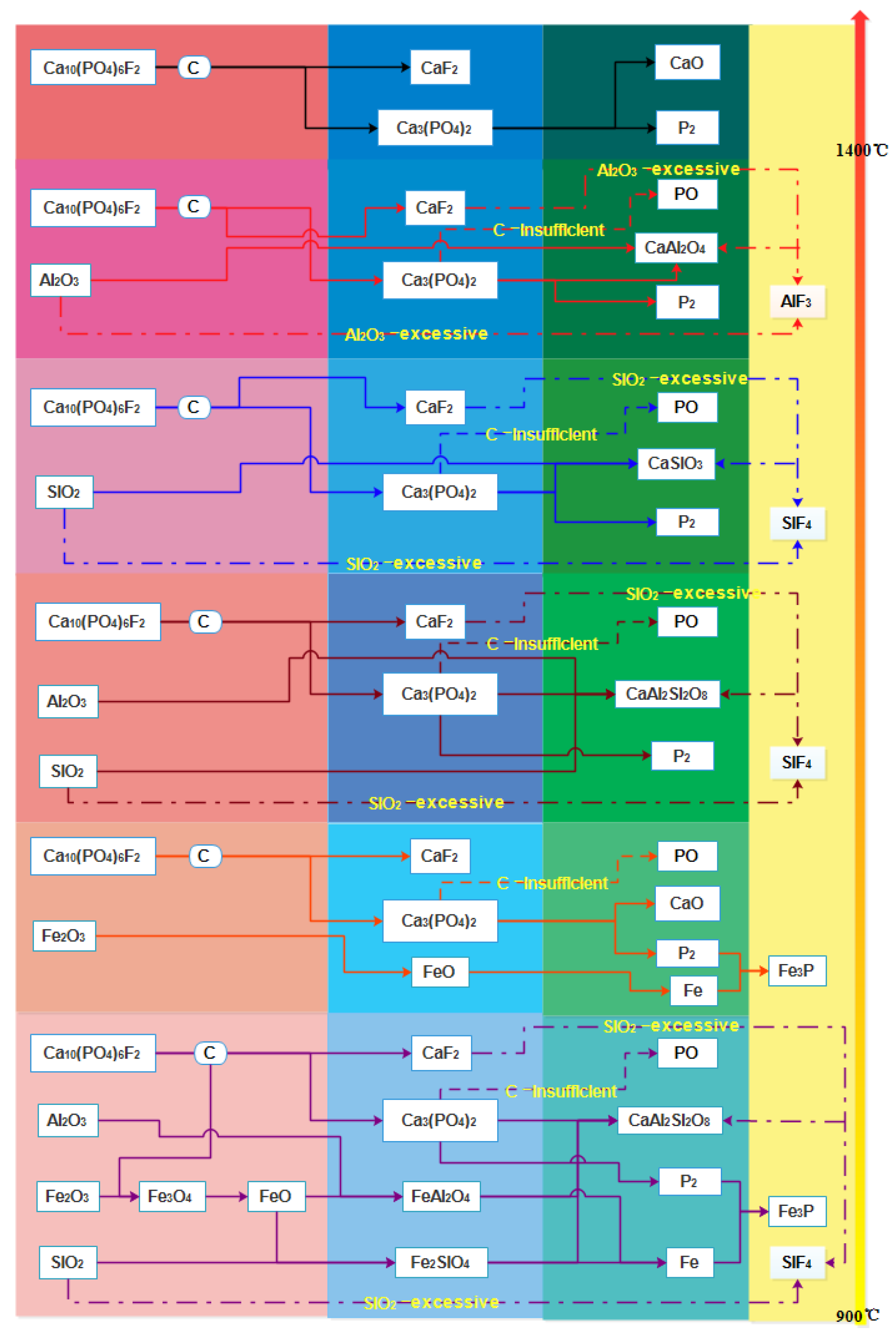
3.1. Mineral Reaction Summary and Thermodynamics Discussion of High-Phosphorus Iron Ore Reduction
ΔGθ = 1.67425E7 − 15945.22T.
ΔGθ = 7.14216E6 − 7002.12T.
3Ca3Al2Si3O12(s),
9CaAl2Si2O8 + 2Fe(s) + Fe3P,
ΔGθ = 4.53867E6 − 4890.81T.
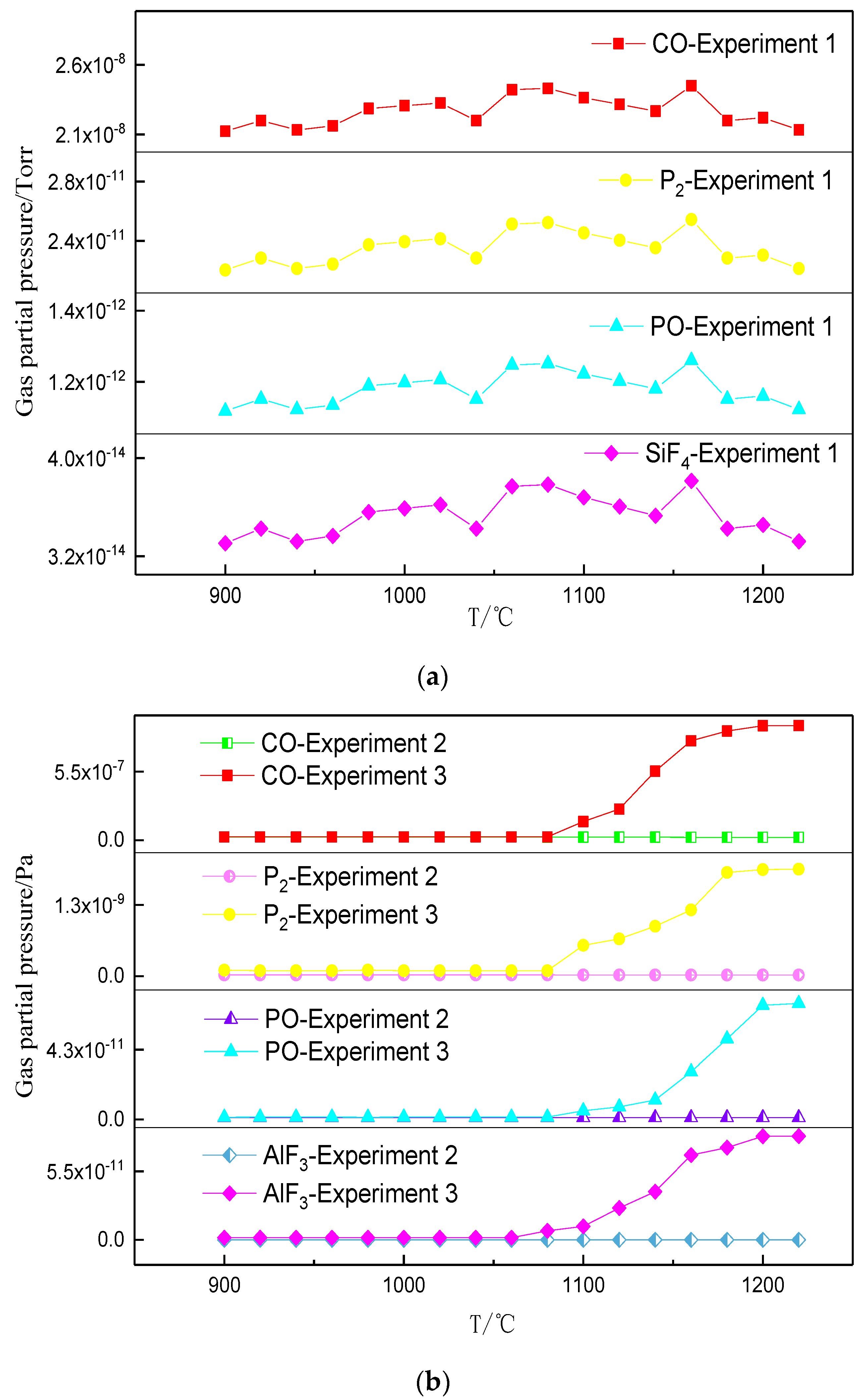
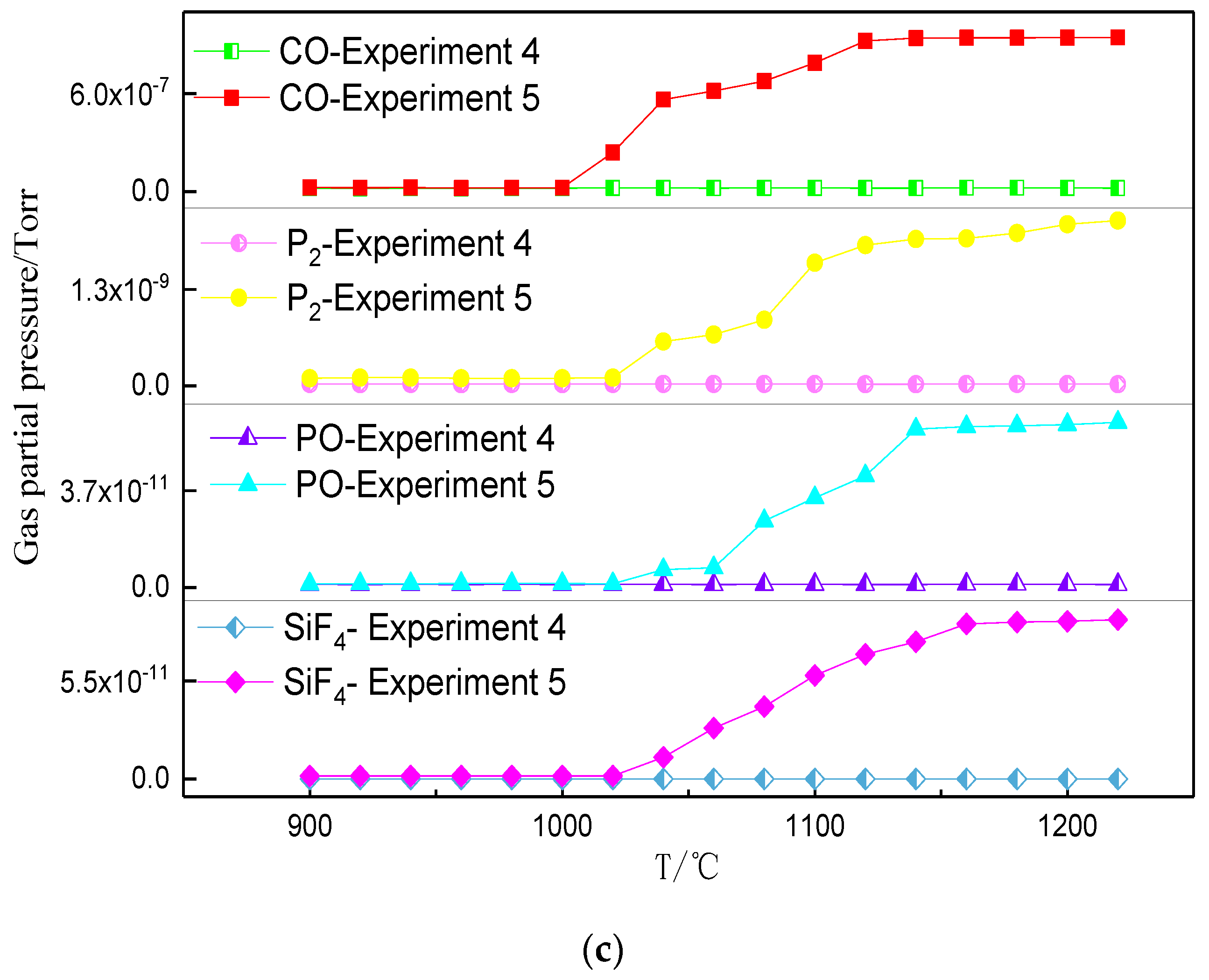
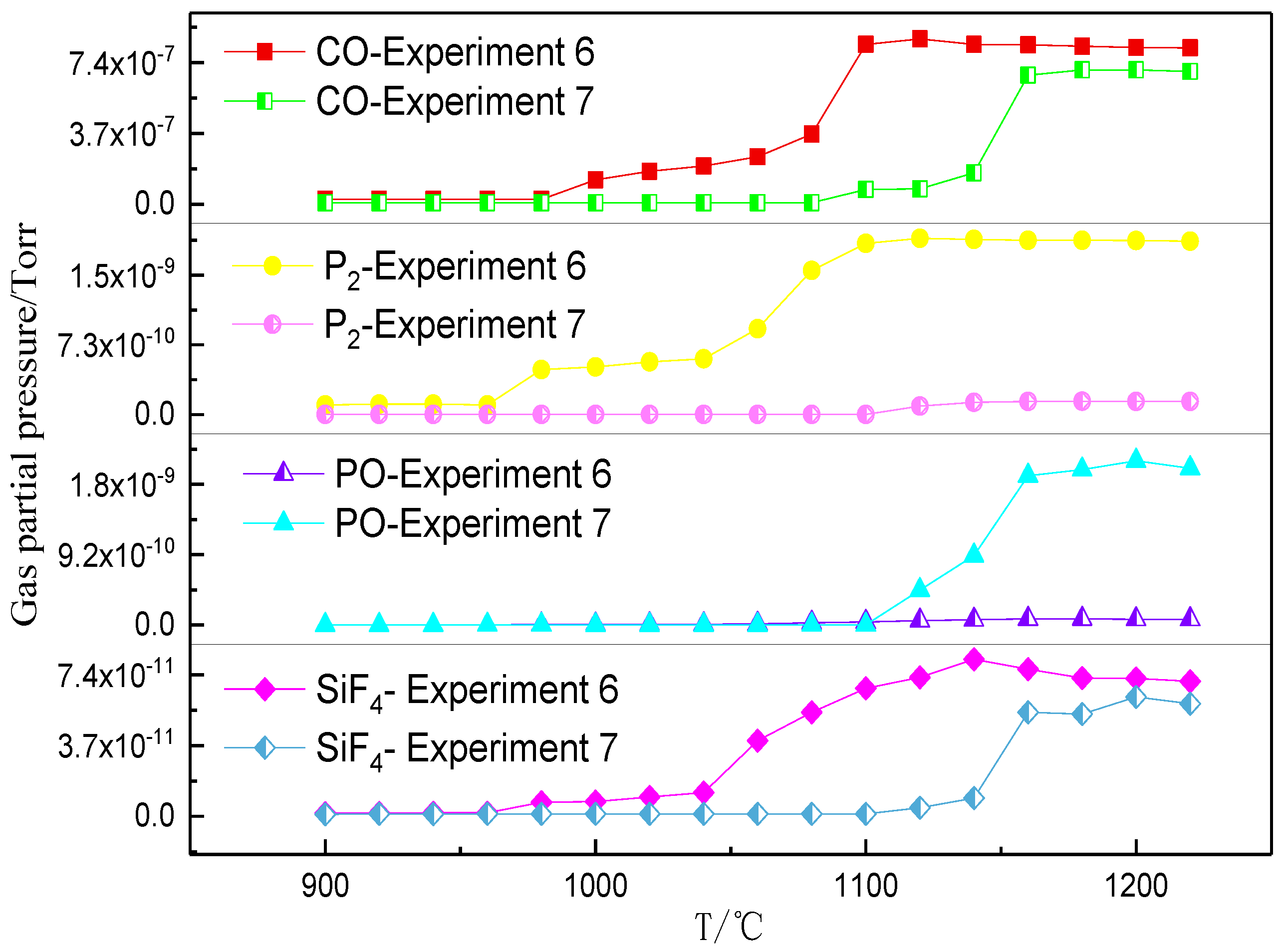
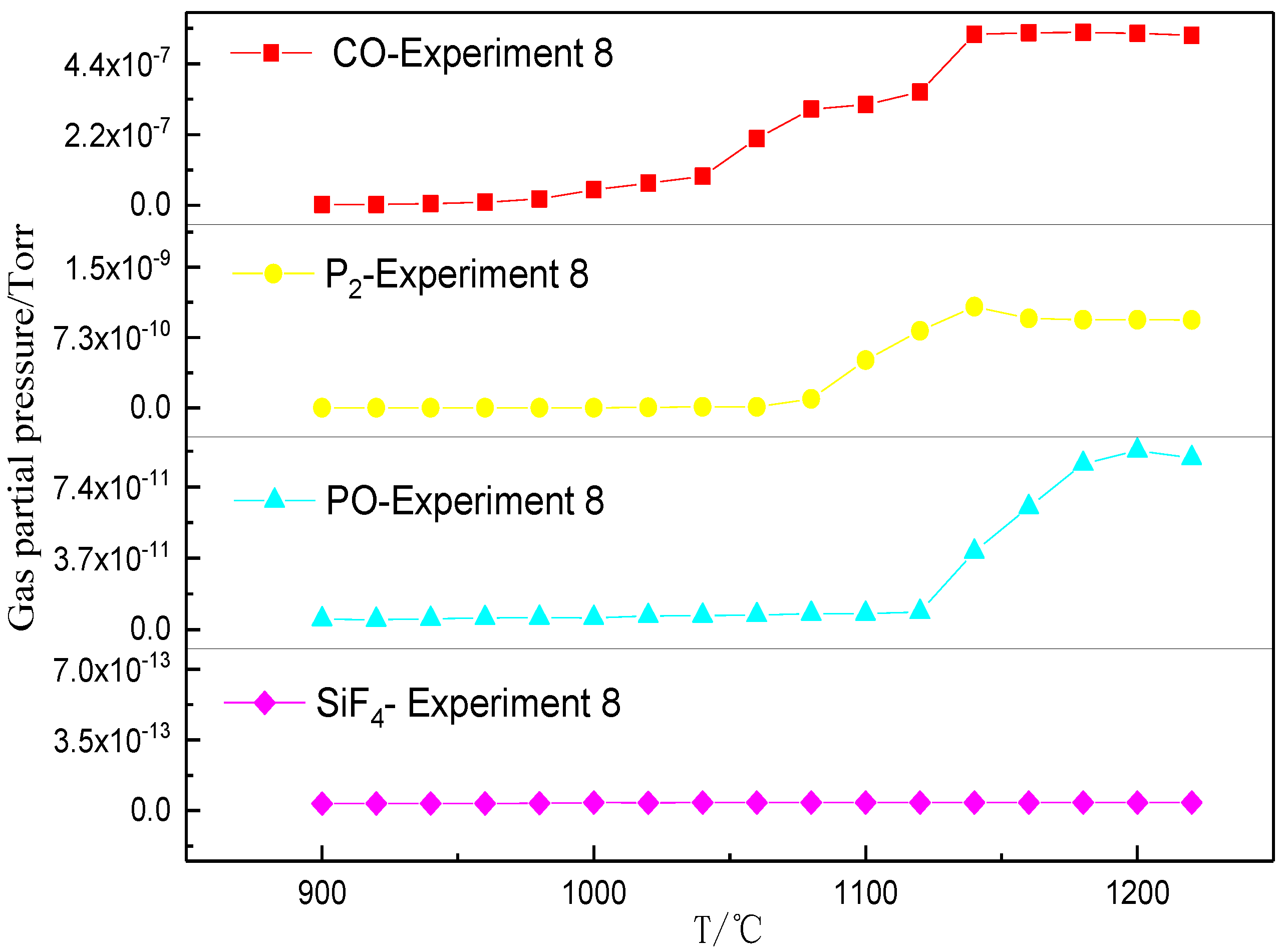
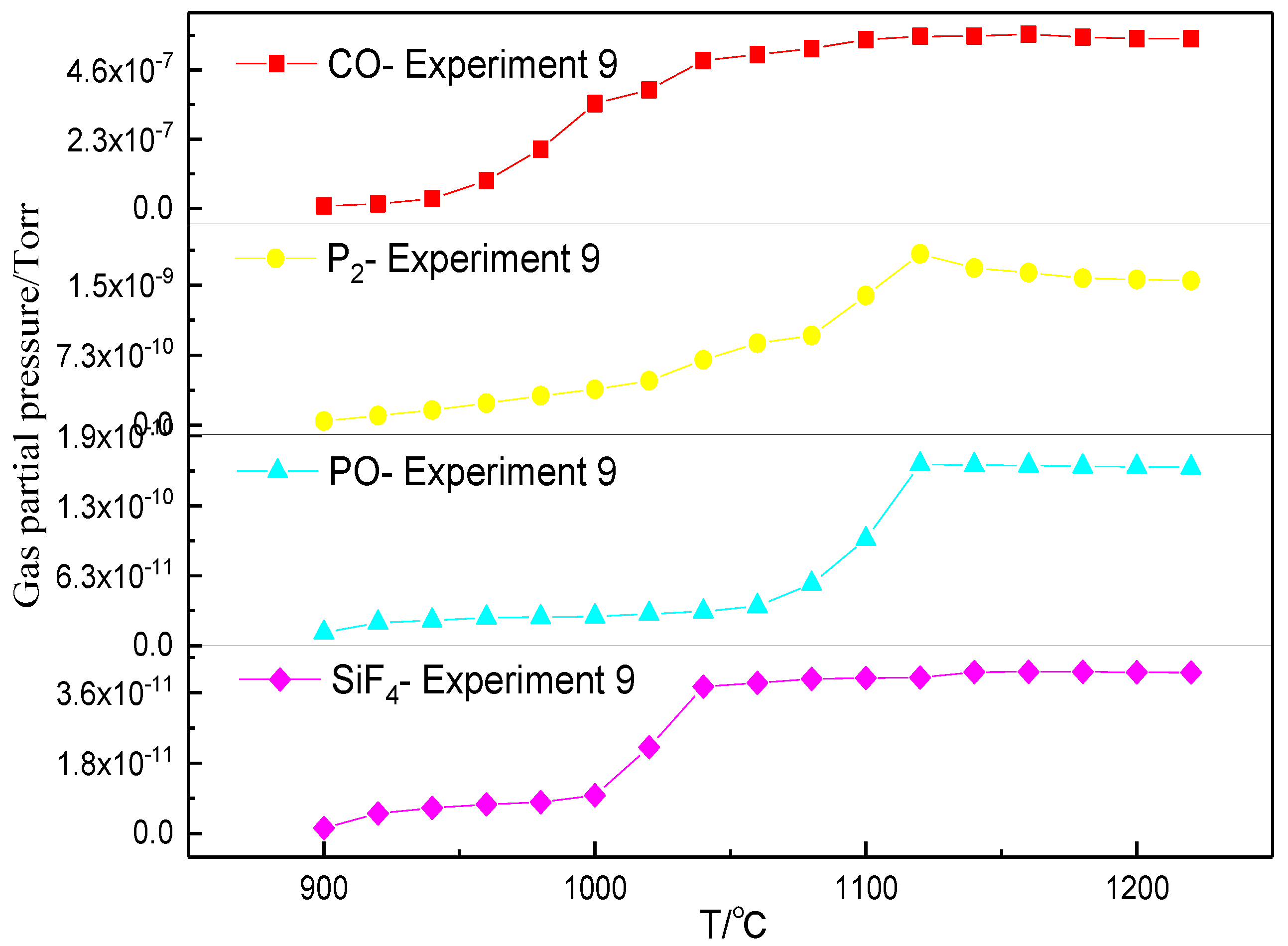
3.2. Phosphorus Vaporization in Carbothermal Reduction under Different Conditions
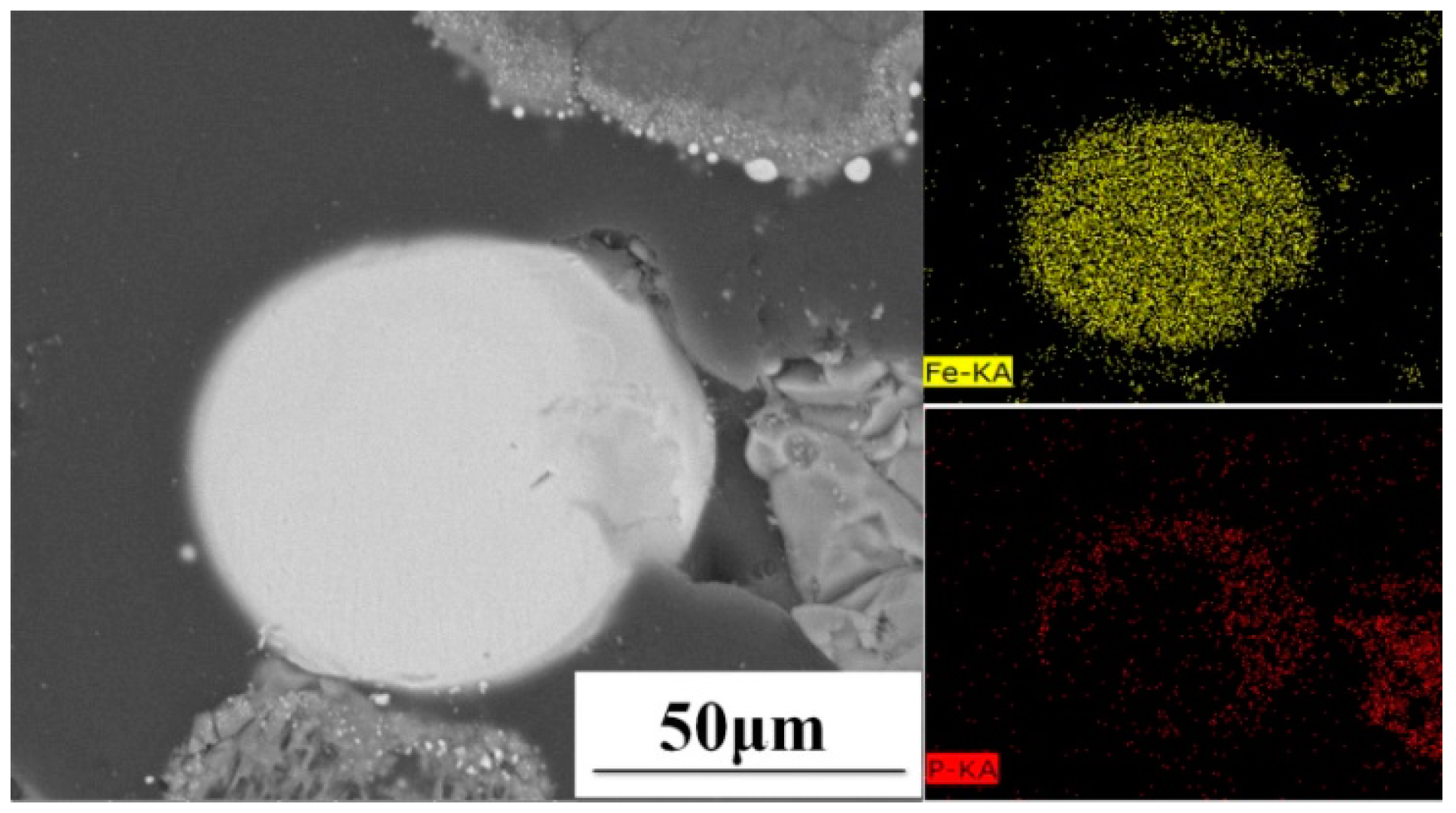
3.3. Effect of Temperature on Dephosphorization Extent and Distribution of Phosphorus in High-Phosphorus Iron Ore
4. Conclusions
- Without the additives’ contribution, the fluoroapatite reduction was difficult to achieve. The theoretical reduction temperature exceeded 1400 °C. When Al2O3 or SiO2 were added, the dephosphorization of fluorapatite was promoted, and the phosphorus-containing gas was generated. The effect of SiO2 was stronger compared to Al2O3. When a sufficient amount of Al2O3 or SiO2 was added, the defluorination reaction of fluorapatite occurred, which accelerated the vaporization dephosphorization of fluorapatite.
- Fluorapatite was reduced when the molar ratio of Al2O3 to Ca10(PO4)6F2 was below 10.3 and the reduction temperature exceeded 1350 °C. Fluorapatite was reduced at 1050 °C when the molar ratio of Al2O3 to Ca10(PO4)6F2 exceeded 10.3. Fluorapatite was reduced at temperatures beyond 1250 °C when the molar ratio of SiO2 to Ca10(PO4)6F2 was below 10.5. Fluorapatite was reduced at 1019 °C when the molar ratio of SiO2 to Ca10(PO4)6F2 exceeded 10.5, which reduced the reduction temperature of fluorapatite. These results indicated that it was possible for gasification dephosphorization to occur at lower temperatures.
- The amount of carbonhad a significant effect on the gas-phase products of phosphorus. When a sufficient amount of carbon was added, the phosphorus-containing gas was primarily produced as P2, and the dephosphorization temperature was low. When inadequate amounts of carbon were present, the phosphorus-containing gas primarily produced PO, and the dephosphorization temperature was high.
- The dephosphorization ratio was very low within the temperature range of 800–1000 °C. When the temperature was in the range of 1000–1100 °C, the dephosphorization ratio rapidly increased. Beyond 1100 °C, the dephosphorization ratio was essentially unchanged or even slightly decreased. The temperature range of 1000–1100 °C was the optimum for gasification dephosphorization.
- During the carbothermal reduction process of high-phosphorus iron ore, the most effective means of dephosphorization was to volatilize phosphorus as a gas, which could prevent phosphorus from entering the iron in the subsequent process. This paper provides a theoretical basis for the dephosphorization of high-phosphorus iron ore by vaporization and provides new insights for the utilization of high-phosphorus iron ore.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Baioumy, H.; Omran, M.; Fabritius, T. Mineralogy, geochemistry and the origin of high-phosphorus oolitic iron ores of Aswan, Egypt. Ore Geol. Rev. 2017, 80, 185–199. [Google Scholar] [CrossRef]
- Zhu, D.; Guo, Z.; Xue, Q.G.; Zhang, F. Synchronous upgrading iron and phosphorus removal from high phosphorus oolitic hematite ore by high temperature flash reduction. Metals 2016, 6, 123. [Google Scholar] [CrossRef]
- Matinde, E.; Hino, M. Dephosphorization treatment of high phosphorus iron ore by pre-reduction, air jet milling and screening methods. Int. ISIJ 2011, 51, 544–551. [Google Scholar] [CrossRef]
- Huang, D.B.; Zong, Y.B.; Wei, R.F.; Gao, W.; Liu, X.M. Direct reduction of high phosphorus oolitic hematite ore based on biomass pyrolysis. J. Iron Steel Res. Int. 2016, 23, 874–883. [Google Scholar] [CrossRef]
- Sun, Y.S.; Han, Y.X.; Gao, P.; Wang, Z.H.; Ren, D.Z. Recovery of iron from high phosphorus oolitic iron ore using coal-based reduction followed by magnetic separation. Int. J. Miner. Metall. Mater. 2013, 20, 411–419. [Google Scholar] [CrossRef]
- Lei, Y.; Li, Y.; Chen, W. Microwave carbothermic reduction of oolitic hematite. Int. ISIJ 2017, 57, 791–794. [Google Scholar] [CrossRef]
- Matinde, E.; Hino, M. Dephosphorization treatment of high phosphorus iron ore by pre-reduction, mechanical crushing and screening methods. Int. ISIJ 2011, 51, 220–227. [Google Scholar] [CrossRef]
- Yu, W.; Tang, Q.Y.; Chen, J.A.; Sun, T.C. T Thermodynamic analysis of the carbothermic reduction of a high-phosphorusoolitic iron ore by FactSage. Int. J. Miner. Metall. Mater. 2016, 23, 1126–1132. [Google Scholar] [CrossRef]
- Mu, J.; Leder, F.; Park, W.C. Reduction of phosphate ores by carbon: Part 1 process variables for design of rotary kiln system. Metall. Trans. B 1986, 17, 861–868. [Google Scholar] [CrossRef]
- Mu, J.; Leder, F.; Park, W.C. Reduction of phosphate ores by carbon: Part 2 rate limiting steps. Metall. Trans. B 1986, 17, 869–877. [Google Scholar] [CrossRef]
- L’vov, B.V. Mechanism of carbothermal reduction of iron, cobalt, nickel and copper oxides. Thermochim. Acta 2000, 360, 109–120. [Google Scholar] [CrossRef]
- Jiang, L.K.; Qiu, L.Y.; Liang, B. Solid reaction mechanism for the thermal reduction of fluorapatite by carbon. J. Chengdu Univ. Sci. Technol. 1995, 5, 1–7. [Google Scholar]
- Tang, X.L.; Zhang, Z.T.; Guo, M. Viscosities behavior of CaO-SiO2-MgO-alumina slag with low mass ratio of CaO to SiO2 and wide range of alumina content. J. Iron Steel Res. Int. 2011, 18, 1–6. [Google Scholar] [CrossRef]
- Williams, Q.; Knittle, E. Infrared and Raman spectra of Ca5(PO4)3F2-fluorapatite at high pressures: Compression-induced changes in phosphate site and Davydov splittings. Phys. Chem. Solids 1996, 57, 417–422. [Google Scholar] [CrossRef]
- Ning, X.Y.; Xue, Q.G.; Wang, G. Mechanism of direct reduction carbon and melting separation for carbon-bearing pellets. Chin. J. Eng. 2014, 36, 1166–1173. [Google Scholar]
- Liu, Y.C.; Li, Q.X.; Liu, Y.C. Preparation of phosphorus by carbothermal reduction mechanism in vacuum. Adv. Mater. Res. 2012, 49, 268–274. [Google Scholar] [CrossRef]
- Cheng, C.; Xue, Q.G.; Wang, G.; Zhang, Y.Y.; Wang, J.S. Phosphorus migration during direct reduction of coal composite high-phosphorus iron ore pellets. Metall. Mater. Trans. B 2016, 47, 154–163. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Xue, Q.G.; Wang, G.; Wang, J.S. Intermittent microscopic observation of structure change and mineral reactions of high phosphorus oolitic hematite in carbothermic reduction. ISIJ Int. 2017, 57, 1149–1155. [Google Scholar] [CrossRef]
- Cheng, C.; Xue, Q.G.; Zhang, Y.Y. Dynamic migration process and mechanism of phosphorus permeating into metallic iron with carburizing in coal-based direct reduction. ISIJ Int. 2015, 55, 2576–2581. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P. FactSage thermochemical software and databases, 2010–2016. Calphad-Comput. Coupling Phase Diagr. Thermochem. 2016, 54, 35–53. [Google Scholar] [CrossRef]



| Composition | Fe2O3 | Ca10(PO4)6F2 | SiO2 | Al2O3 | CaO | MgO |
|---|---|---|---|---|---|---|
| Chemical composition of raw ore | 73.46 | 6.23 | 7.77 | 5.07 | 0.79 | 0.74 |
| Experiment No. | Ca10(PO4)6F2 | Al2O3 | SiO2 | Fe2O3 | C |
|---|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 0 | 15 |
| 2 | 1 | 9 | 0 | 0 | 15 |
| 3 | 3 | 31 | 0 | 0 | 45 |
| 4 | 1 | 0 | 9 | 0 | 15 |
| 5 | 2 | 0 | 21 | 0 | 30 |
| 6 | 2 | 20 | 41 | 0 | 30 |
| 7 | 2 | 20 | 41 | 0 | 18 |
| 8 | 1 | 0 | 0 | 1 | 18 |
| 9 | 2 | 20 | 41 | 2 | 36 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xue, Q.; Wang, G.; Wang, J. Phosphorus-Containing Mineral Evolution and Thermodynamics of Phosphorus Vaporization during Carbothermal Reduction of High-Phosphorus Iron Ore. Metals 2018, 8, 451. https://doi.org/10.3390/met8060451
Zhang Y, Xue Q, Wang G, Wang J. Phosphorus-Containing Mineral Evolution and Thermodynamics of Phosphorus Vaporization during Carbothermal Reduction of High-Phosphorus Iron Ore. Metals. 2018; 8(6):451. https://doi.org/10.3390/met8060451
Chicago/Turabian StyleZhang, Yuanyuan, Qingguo Xue, Guang Wang, and Jingsong Wang. 2018. "Phosphorus-Containing Mineral Evolution and Thermodynamics of Phosphorus Vaporization during Carbothermal Reduction of High-Phosphorus Iron Ore" Metals 8, no. 6: 451. https://doi.org/10.3390/met8060451




