Effects of Vanadium on Microstructure and Wear Resistance of High Chromium Cast Iron Hardfacing Layer by Electroslag Surfacing
Abstract
:1. Introduction
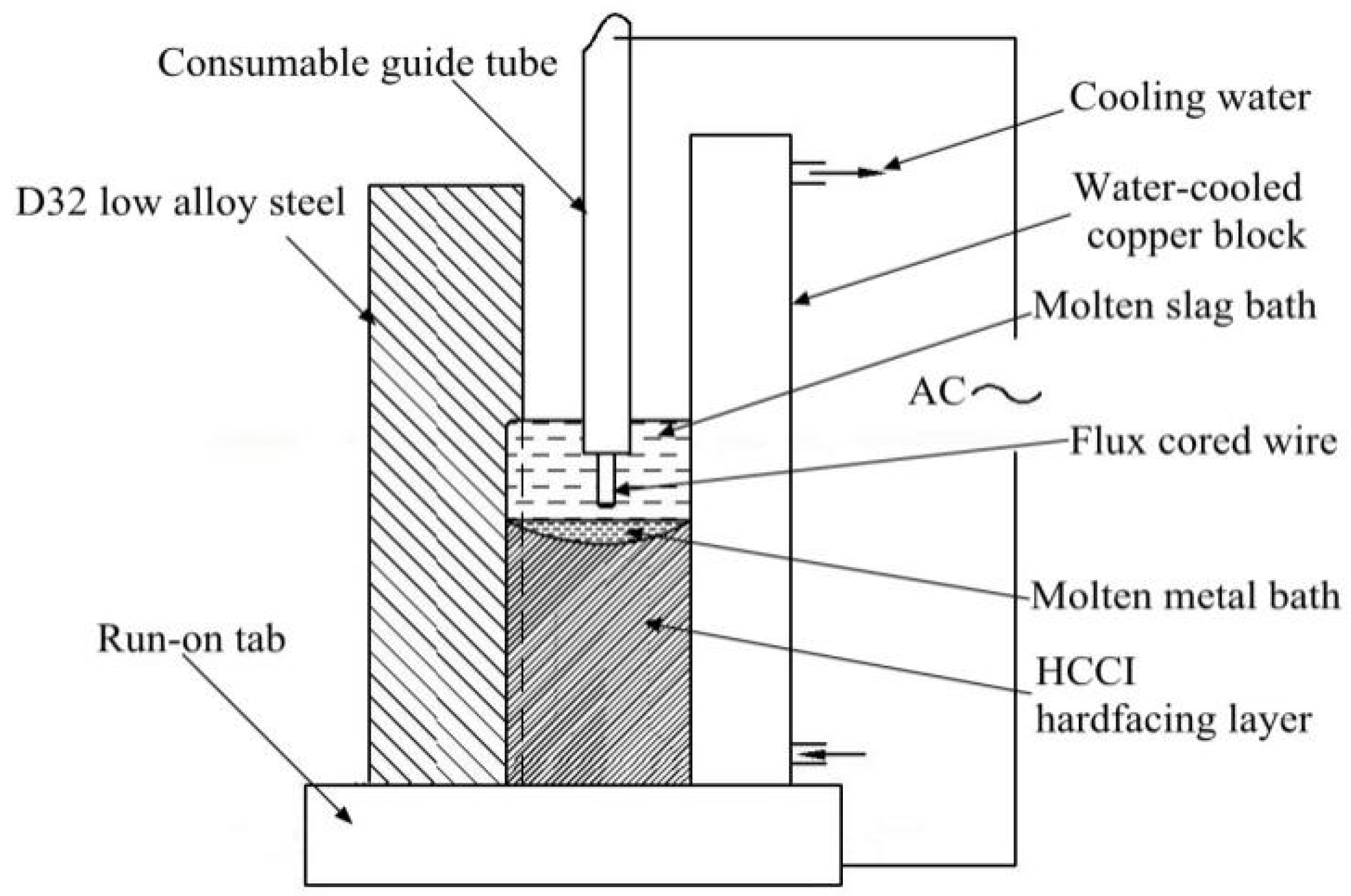
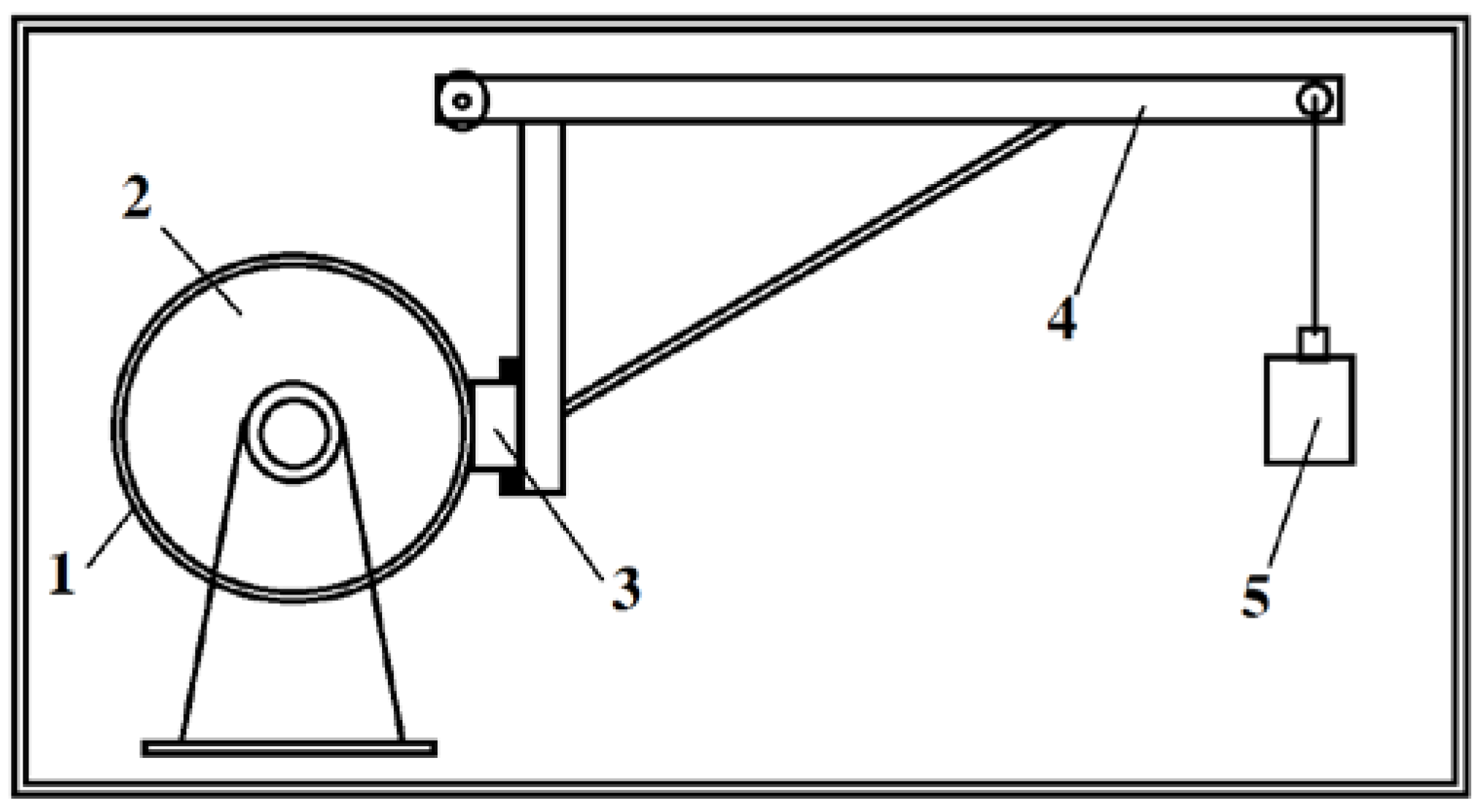
2. Materials and Methods
3. Results and Discussion
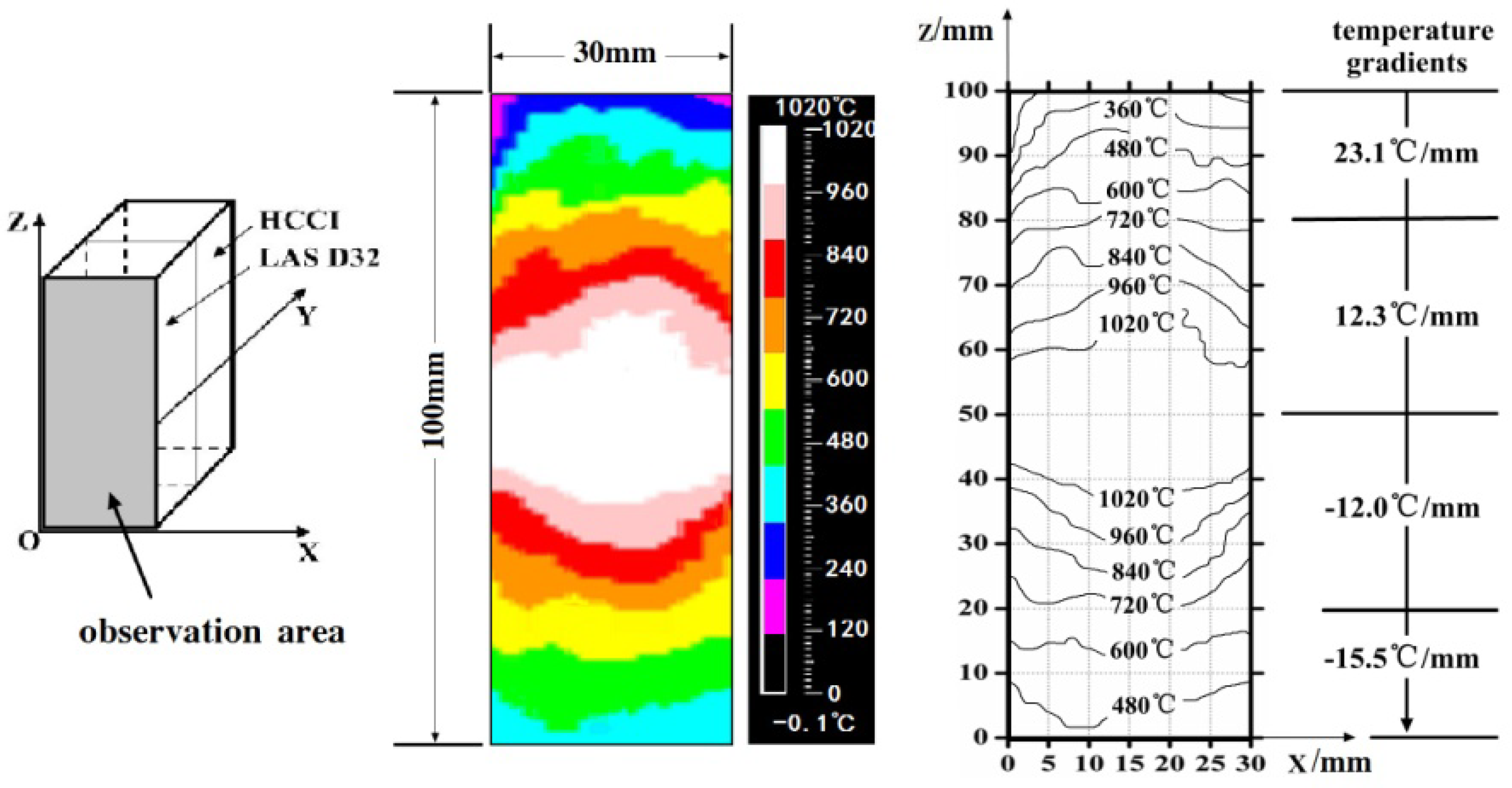
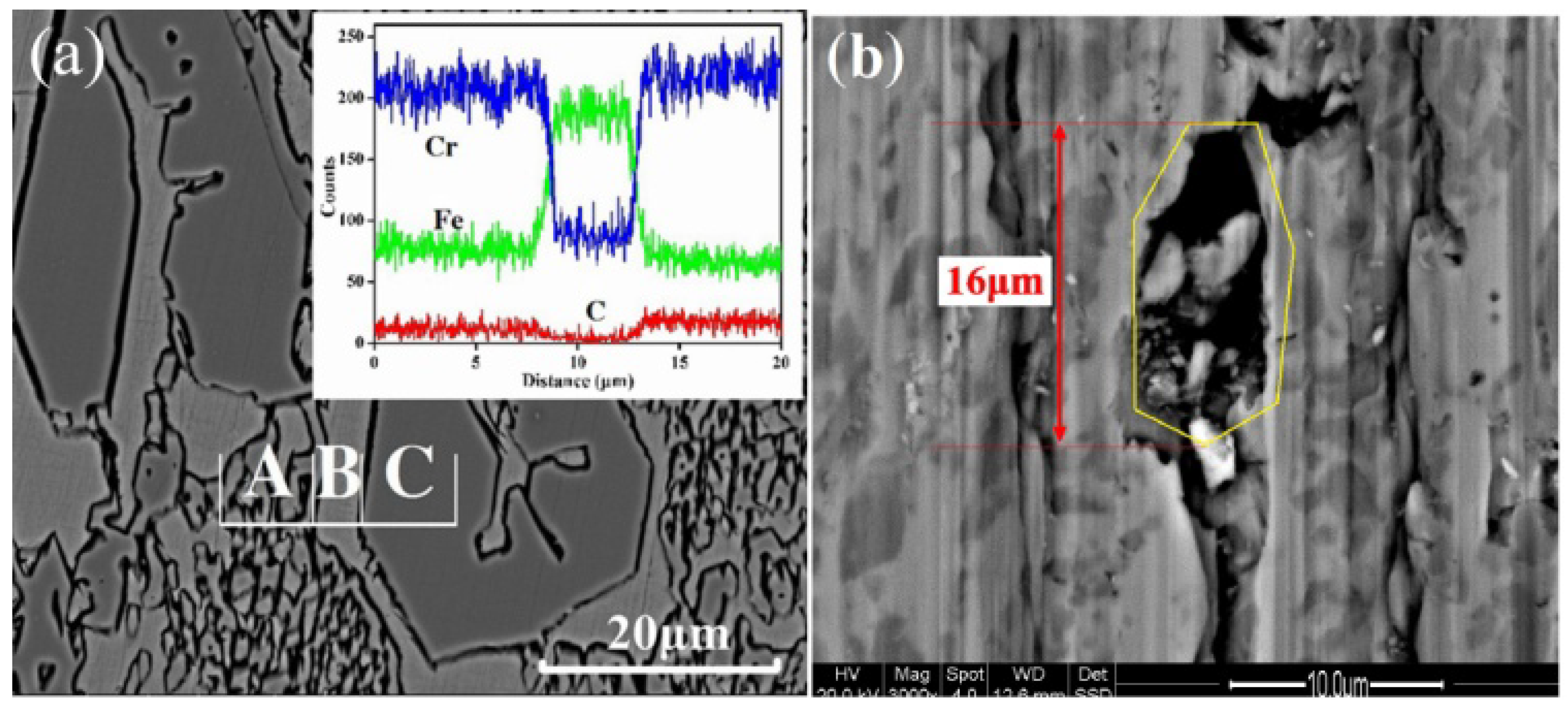
3.1. Interface of the HCCI Electroslag Hardfacing Layer and Low Alloy Steel
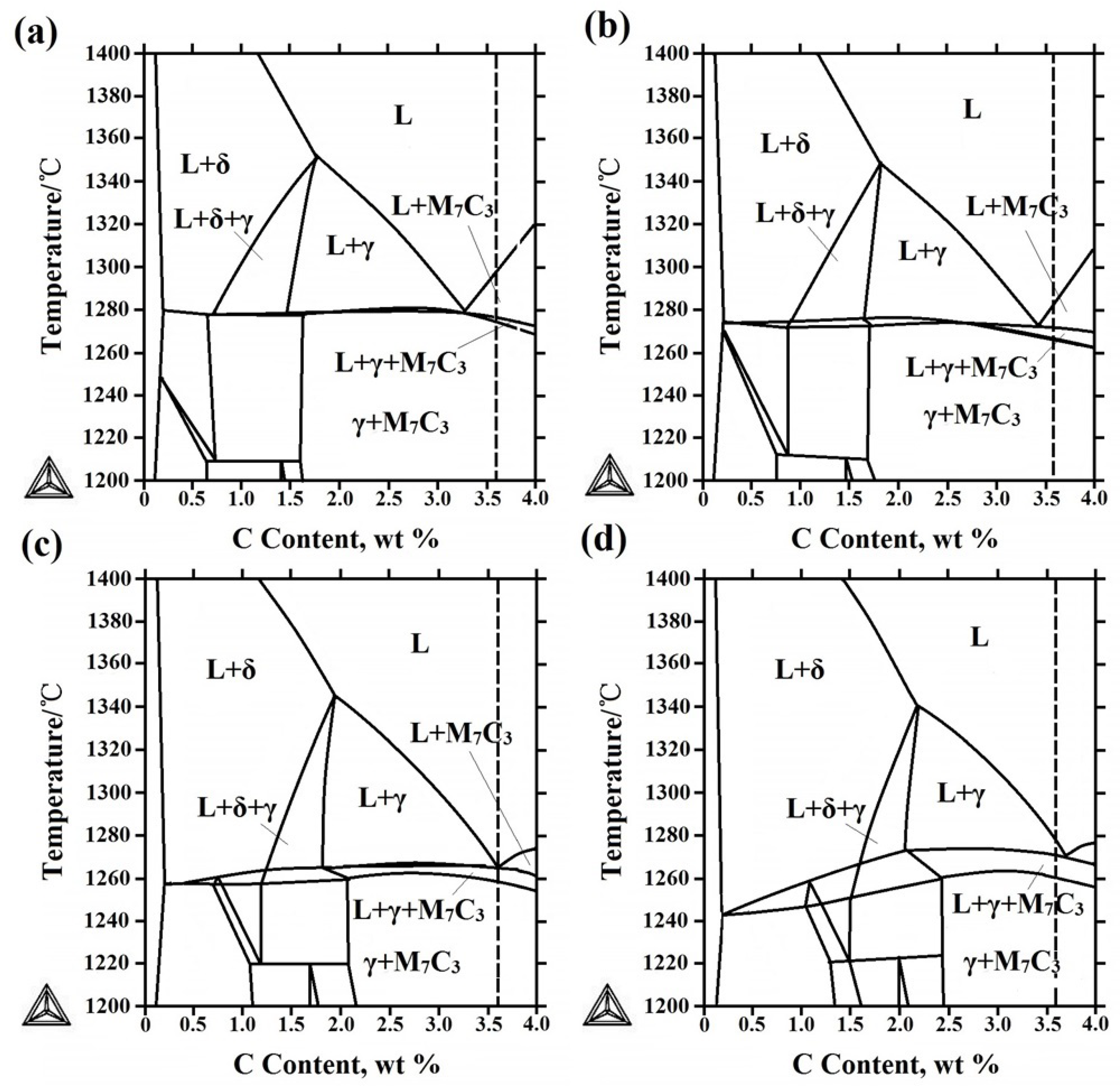
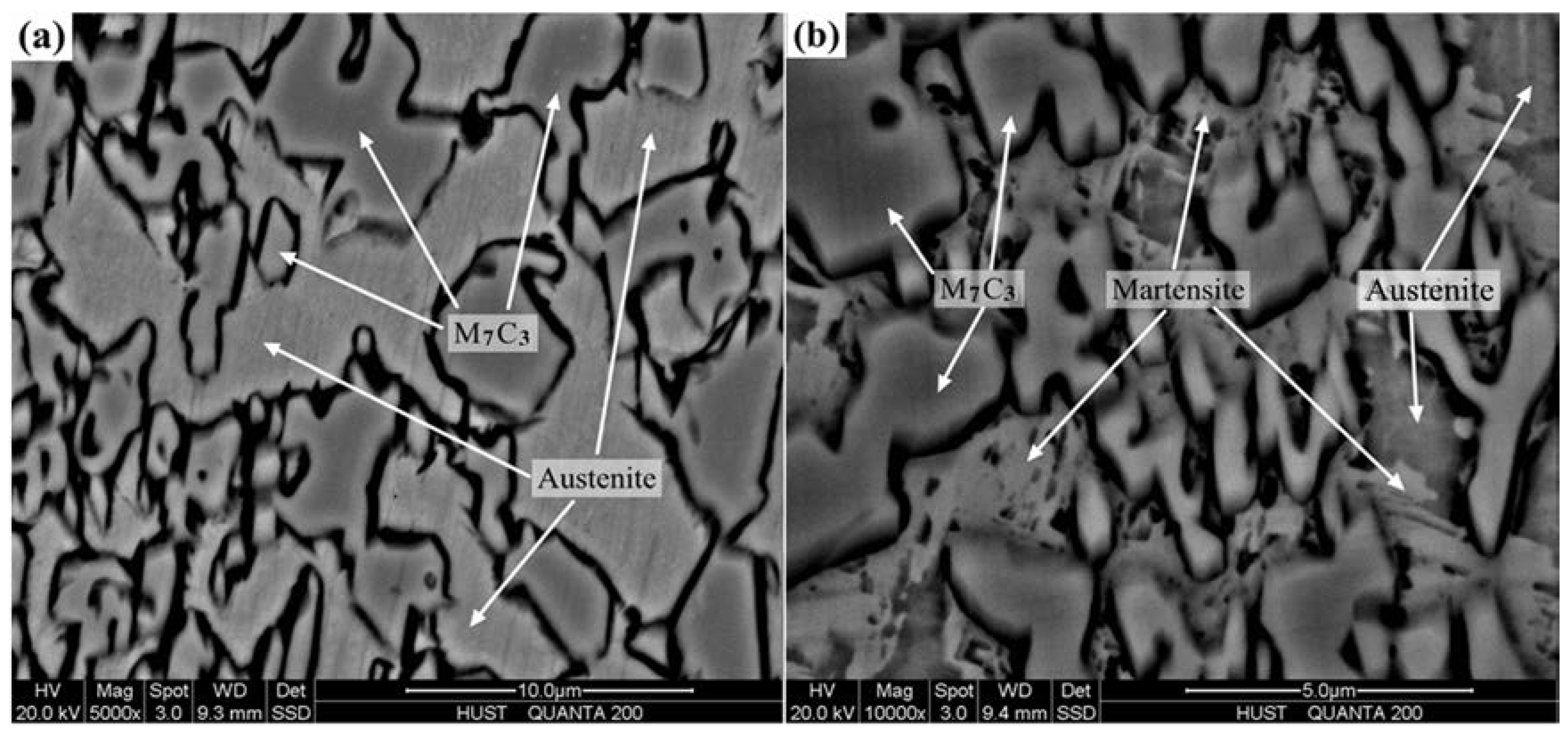
3.2. Microstructure of V Alloyed HCCI Hardfacing Layers
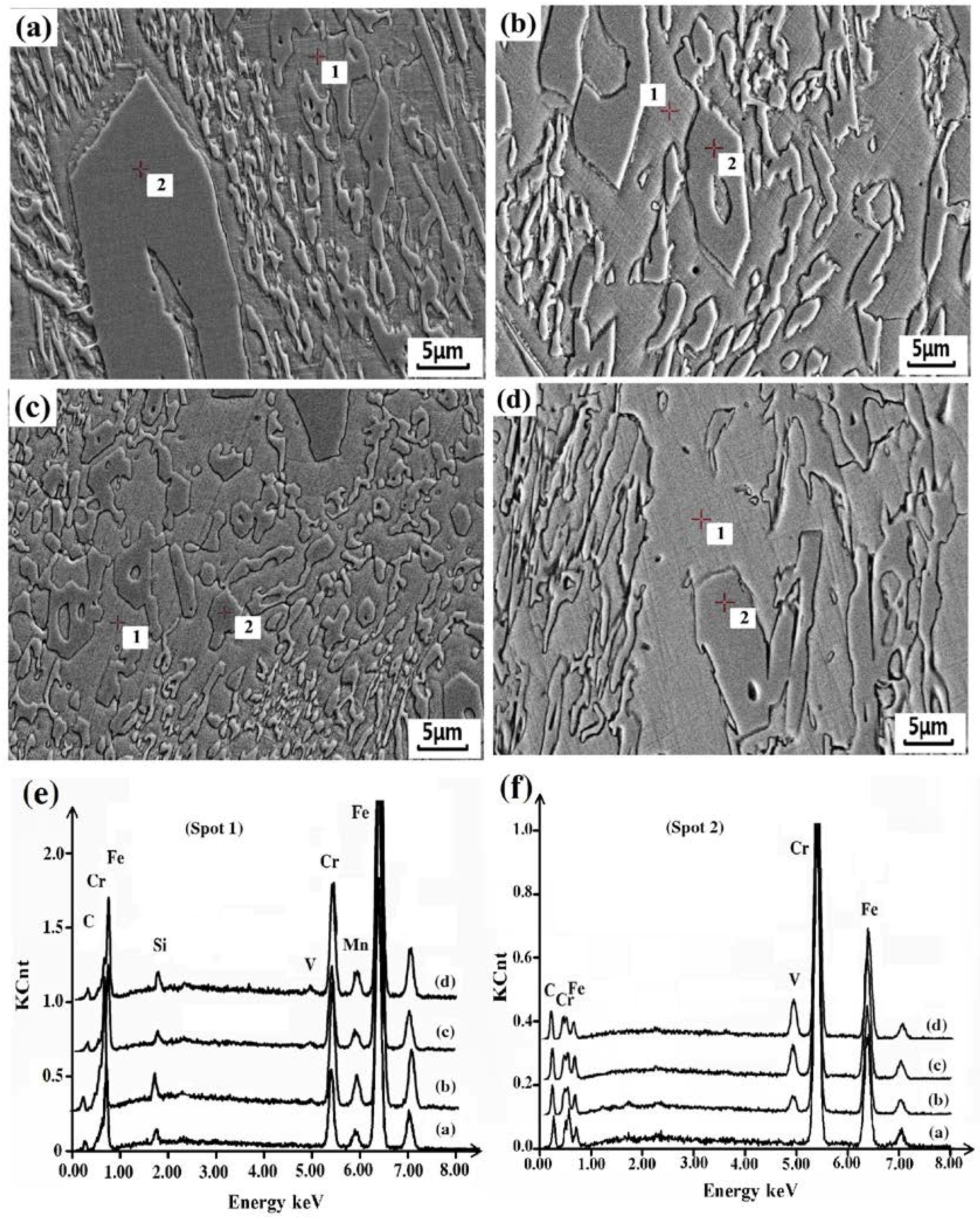
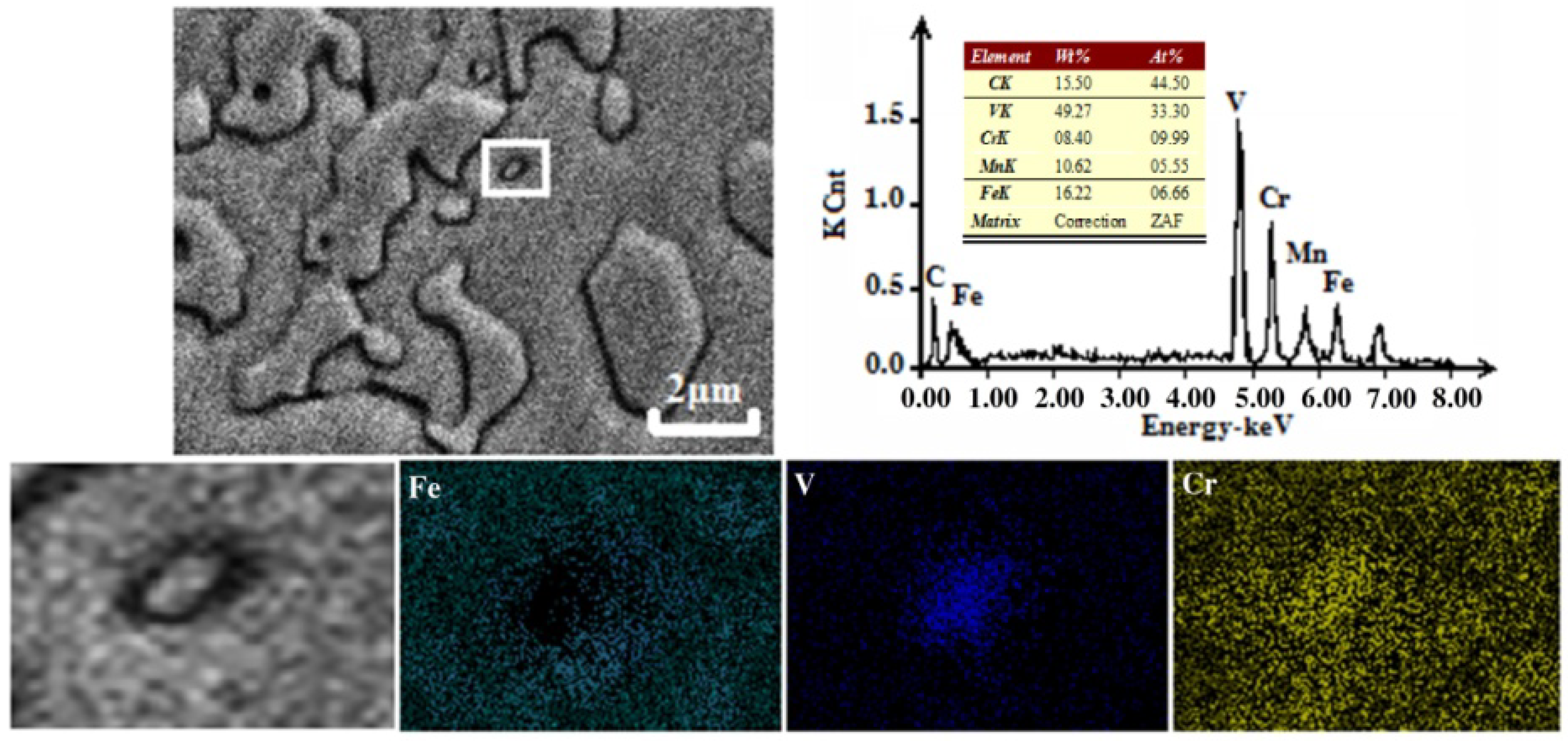
3.3. Effect of V on Carbides in HCCI Hardfacing Layers
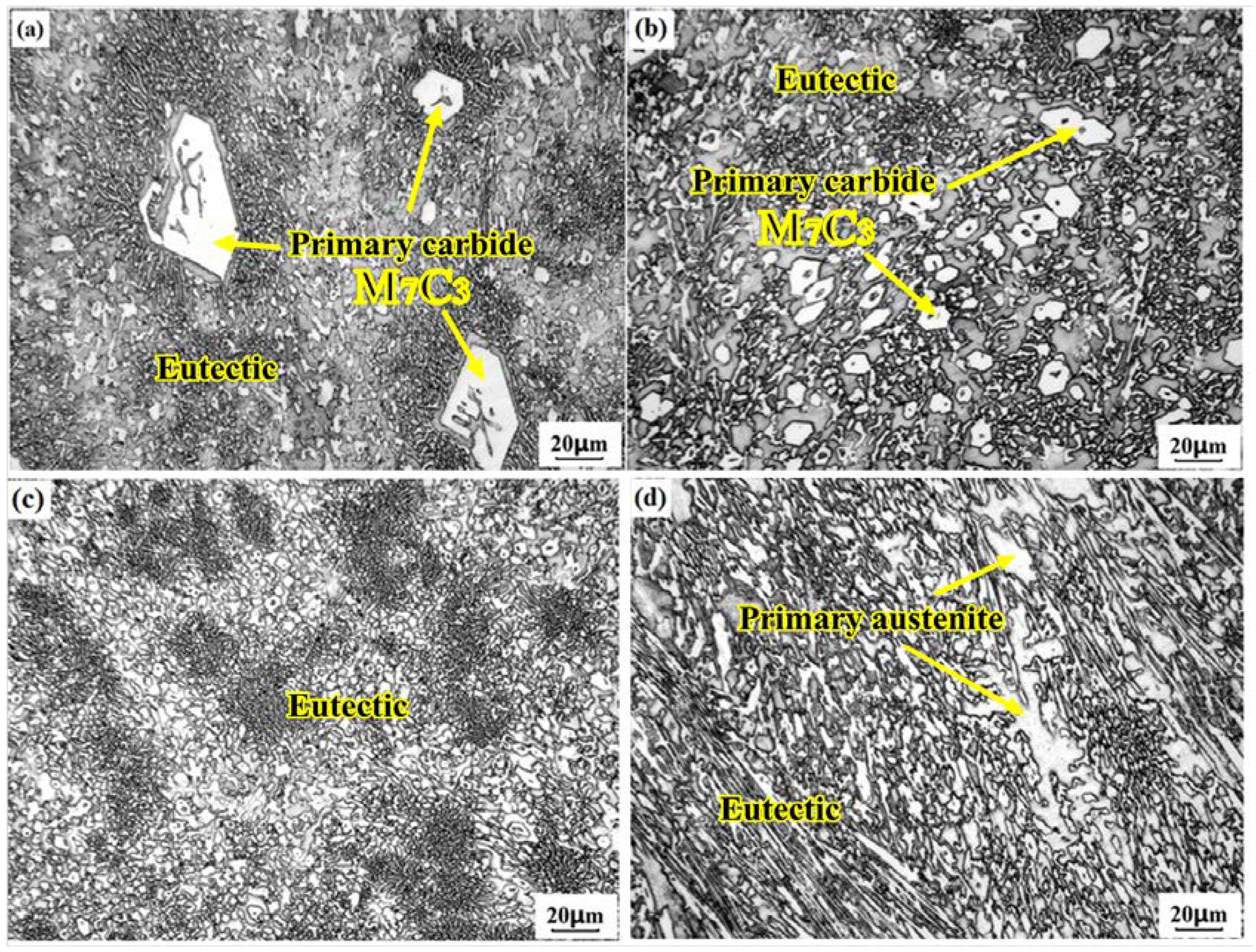
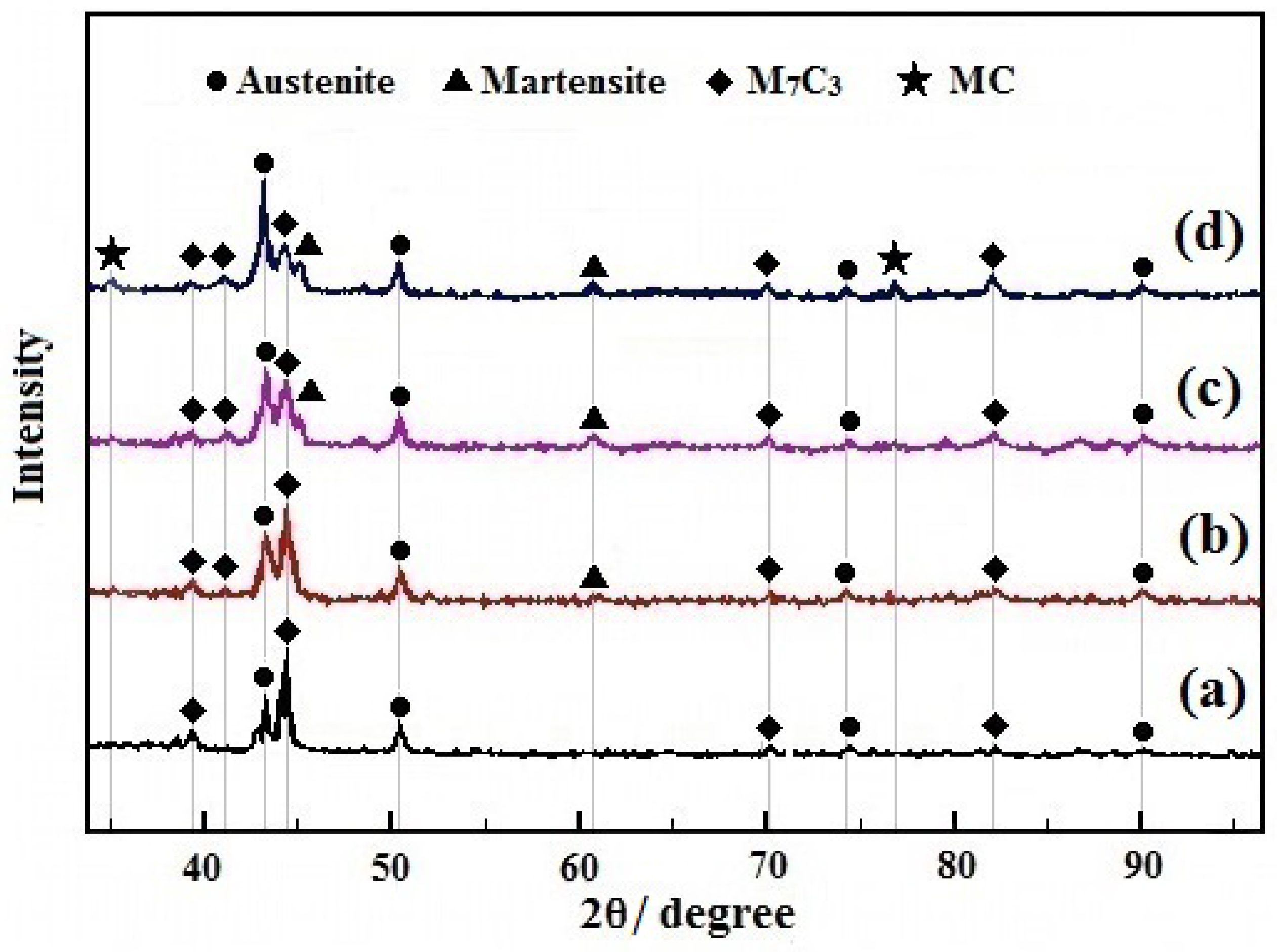
3.3.1. Carbide Types
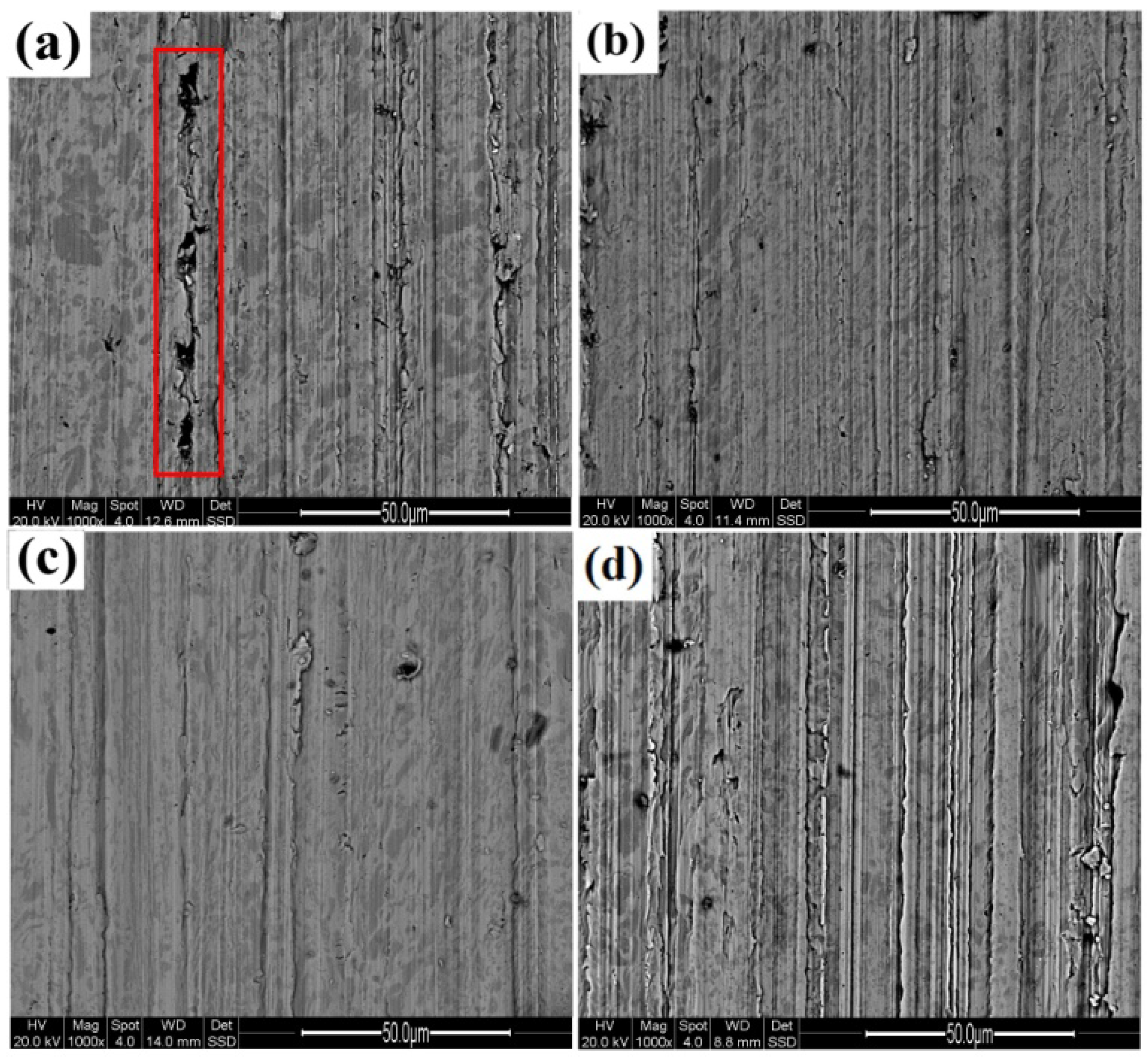
3.3.2. Area Fraction and Size of M7C3 Carbides
3.4. Hardness and Wear Resistance of V Alloyed HCCI Electroslag Hardfacing Layers
3.4.1. Hardness
3.4.2. Wear Resistance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, T.; Maria, S.; Dwivedi, D.K. Abrasive wear behavior of Fe-30Cr-3.6C overlays deposited on mild steel. ISIJ Int. 2005, 45, 1322–1325. [Google Scholar] [CrossRef]
- Correa, E.O.; Alcântara, N.G.; Tecco, D.G.; Kumar, R.V. The relationship between the microstructure and abrasive resistance of a hardfacing layer in the Fe-Cr-C-Nb-V system. Metall. Mater. Trans. 2007, 38, 1671–1680. [Google Scholar] [CrossRef]
- Zahiri, R.; Sundaramoorthy, R.; Patrick, L.; Subramanian, C. Hardfacing using ferro-alloy powder mixtures by submerged arc welding. Surf. Coat. Technol. 2014, 260, 220–229. [Google Scholar] [CrossRef]
- Tang, X.H.; Chung, R.; Pang, C.J.; Li, D.Y.; Hinckley, B.; Dolman, K. Microstructure of high (45 wt %) chromium cast irons and their resistances to wear and corrosion. Wear 2011, 271, 1426–1431. [Google Scholar] [CrossRef]
- Wang, H.; Yu, S.F. Influence of heat treatment on microstructure and sliding wear resistance of high chromium cast iron electroslag hardfacing layer. Surf. Coat. Technol. 2017, 319, 182–190. [Google Scholar] [CrossRef]
- Zhou, Q.; Su, J. Chromium Family of Wear Resistant Cast Iron; Xi’an Jiaotong University Press: Xi’an, China, 1986; pp. 111–112. [Google Scholar]
- Fulcher, J.K.; Kosel, T.H.; Fiore, N.F. The effect of carbide volume fraction on the low stress abrasion resistance of high Cr-Mo white cast irons. Wear 1983, 84, 313–325. [Google Scholar] [CrossRef]
- Sapate, S.; Rama Rao, A. Erosive wear behavior of weld hardfacing high chromium cast irons: Effect of erodent particles. Tribol. Int. 2006, 39, 206–212. [Google Scholar] [CrossRef]
- Zhi, X.H.; Liu, J.Z.; Xing, J.D.; Ma, S. Effect of cerium modification on microstructure and properties of hypereutectic high chromium cast iron. Mater. Sci. Eng. A 2014, 603, 98–103. [Google Scholar] [CrossRef]
- Wang, Y.; Gou, J.F.; Chu, R.Q.; Zhen, D.; Liu, S. The effect of nano-additives containing rare earth oxides on sliding wear behavior of high chromium cast iron hardfacing layers. Tribol. Int. 2016, 103, 102–112. [Google Scholar] [CrossRef]
- Zhi, X.H.; Xing, J.D.; Fu, H.G.; Xiao, B. Effect of niobium on the as-cast microstructure of hypereutectic high chromium cast iron. Mater. Lett. 2008, 62, 857–860. [Google Scholar] [CrossRef]
- Wu, X.J.; Xing, J.D.; Fu, H.G.; Zhi, X. Effect of titanium on the morphology of primary M7C3 carbides in hypereutectic high chromium white iron. Mater. Sci. Eng. A 2007, 457, 180–185. [Google Scholar] [CrossRef]
- Filipovic, M.; Kamberovic, Z.; Korac, M.; Gavrilovski, M. Microstructure and mechanical properties of Fe-Cr-C-Nb white cast irons. Mater. Des. 2013, 47, 41–48. [Google Scholar] [CrossRef]
- Filipovic, M.; Romhanji, E.; Kamberovic, Z.; Korac, M. Matrix microstructure and its micro-analysis of constituent phases in as-cast Fe-Cr-C-V alloys. Mater. Trans. 2009, 50, 2488–2492. [Google Scholar] [CrossRef]
- Mirjana, M. Filipović, Iron-chromium-carbon-vanadium white cast irons: the microstructure and properties. Hem. Ind. 2014, 68, 413–427. [Google Scholar] [CrossRef]
- Wu, L.L.; Xiao, F.R.; Wang, Y.C.; Liang, C.B.; Sun, G.P.; Liao, B. Effect of vanadium on microstructure and wear resistance of Ni-Cr alloyed cast iron. Int. J. Cast Met. Res. 2013, 26, 176–183. [Google Scholar] [CrossRef]
- Arnoldo, B.J. Microstructure of vanadium-, niobium-and titanium-alloyed high-chromium white cast irons. Int. J. Cast Met. Res. 2001, 13, 343–361. [Google Scholar] [CrossRef]
- Waleed, E.; Rashad, R.; Sayed, E.; Saied, E. Influence of Vanadium and Boron Additions on the Microstructure, Fracture Toughness, and Abrasion Resistance of Martensite-Carbide Composite Cast Steel. Adv. Mater. Sci. Eng. 2016, 2016, 1203756. [Google Scholar] [CrossRef]
- Bedolla-Jacuinde, A.; Guerra, F.V.; Mejía, I.; Zuno-Silva, J.; Rainforth, M. Abrasive wear of V-Nb-Ti alloyed high-chromium white irons. Wear 2015, 332–333, 1006–1011. [Google Scholar] [CrossRef]
- Qi, X.; Jia, Z.; Yang, Q.; Yang, Y. Effects of vanadium additive on structure property and tribological performance of high chromium cast iron hardfacing metal. Sur. Coat. Technol. 2011, 205, 5510–5514. [Google Scholar] [CrossRef]
- Cemil, C. An investigation of the wear behaviors of white cast irons under different compositions. Mater. Des. 2006, 27, 437–445. [Google Scholar] [CrossRef]
- Radulovic, M.; Fiset, M.; Peev, K. The influence of vanadium on fracture toughness and abrasion resistance in high chromium white cast irons. J. Mater. Sci. 1994, 29, 5085–5094. [Google Scholar] [CrossRef]
- Ogi, K.; Matsubara, Y.; Matsuda, K. Eutectic solidification of high chromium cast iron-eutectic mechanism of eutectic growth. AFS Trans. 1982, 89, 197–204. [Google Scholar]
- Liu, X.Y.; Piao, Z.M.; Wang, X.H.; Zhao, J.; Cao, Z.Q.; Li, H.; Lai, C.B.; Wu, B.; Xu, H.Q.; Huang, Z.Y.; et al. GB 712-2011, Ship and Ocean Engineering Structural Steel; Standards Press of China: Beijing, China, 2011. [Google Scholar]
- De Mello, J.D.B.; Durand-Charre, M.; Mathia, T. Abrasion mechanisms of white cast iron II: Influence of the metallurgical structure of V-Cr white cast irons. Mater. Sci. Eng. 1986, 78, 127–134. [Google Scholar] [CrossRef]
- Liang, Y.J.; Che, Y.C. Inorganic Thermodynamic Data Manual; North East University Press: Shen Yang, China, 1993; pp. 508–510. ISBN 7-81006-532-7. [Google Scholar]
- Radzikowska, J.M. Metallography and microstructures of cast iron. In ASM Handbooks: Metallography and Microstructures; ASM International: Novelty, OH, USA, 2004; pp. 565–587. [Google Scholar]
- Wu, H.Q.; Sasaguri, N.; Matsubara, Y.; Hashimoto, M. Solidification of Multi-Alloyed White Cast Iron: Type and Morphology of Carbides. Trans. Am. Fish. Soc. 1996, 140, 103–108. [Google Scholar]
- Hao, F.; Li, D.; Dan, T.; Ren, X.; Liao, B.; Yang, Q. Effect of rare earth oxides on the morphology of carbides in hardfacing metal of high chromium cast iron. J. Rare Earth 2011, 29, 168–172. [Google Scholar] [CrossRef]





| Materials | C | Si | Mn | P | S | Cu | Cr | Ni | Nb | V | Ti | Mo | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D32 | 0.13 | 0.22 | 1.30 | 0.01 | 0.01 | 0.32 | 0.20 | 0.39 | 0.04 | 0.08 | 0.02 | 0.05 | 0.02 | Bal. |
| Hardfacing | C | Cr | V | Si | Mn | Fe |
|---|---|---|---|---|---|---|
| 1# | 3.62 | 19.48 | -- | 0.86 | 2.31 | Bal. |
| 2# | 3.59 | 20.19 | 0.83 | 0.68 | 2.25 | Bal. |
| 3# | 3.58 | 19.40 | 1.50 | 0.68 | 2.06 | Bal. |
| 4# | 3.56 | 19.57 | 2.32 | 0.64 | 1.91 | Bal. |
| Hardfacing Layer | M7C3 | MC |
|---|---|---|
| 3.6C-20Cr-Fe | (Cr4.7Fe2.3)C3 | -- |
| 3.6C-20Cr-Fe-0.83V | (Cr4.6Fe2.2V0.2)C3 | -- |
| 3.6C-20Cr-Fe-1.50V | (Cr4.5Fe2.1V0.4)C3 | -- |
| 3.6C-20Cr-Fe-2.32V | (Cr4.4Fe2.1 V0.5)C3 | (Cr0.23V0.77)C |
| Alloying Elements | C | Cr | Si | Mn | V |
|---|---|---|---|---|---|
| 0.14 | −0.024 | 0.08 | −0.012 | −0.077 | |
| −0.34 | 0.042 | 0.015 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yu, S.F.; Khan, A.R.; Huang, A.G. Effects of Vanadium on Microstructure and Wear Resistance of High Chromium Cast Iron Hardfacing Layer by Electroslag Surfacing. Metals 2018, 8, 458. https://doi.org/10.3390/met8060458
Wang H, Yu SF, Khan AR, Huang AG. Effects of Vanadium on Microstructure and Wear Resistance of High Chromium Cast Iron Hardfacing Layer by Electroslag Surfacing. Metals. 2018; 8(6):458. https://doi.org/10.3390/met8060458
Chicago/Turabian StyleWang, Hao, Sheng Fu Yu, Adnan Raza Khan, and An Guo Huang. 2018. "Effects of Vanadium on Microstructure and Wear Resistance of High Chromium Cast Iron Hardfacing Layer by Electroslag Surfacing" Metals 8, no. 6: 458. https://doi.org/10.3390/met8060458




