Synthesis of Core-Shell Carbon Encapsulated Fe2O3 Composite through a Facile Hydrothermal Approach and Their Application as Anode Materials for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Materials Characterization
2.3. Electrochemical Measurements
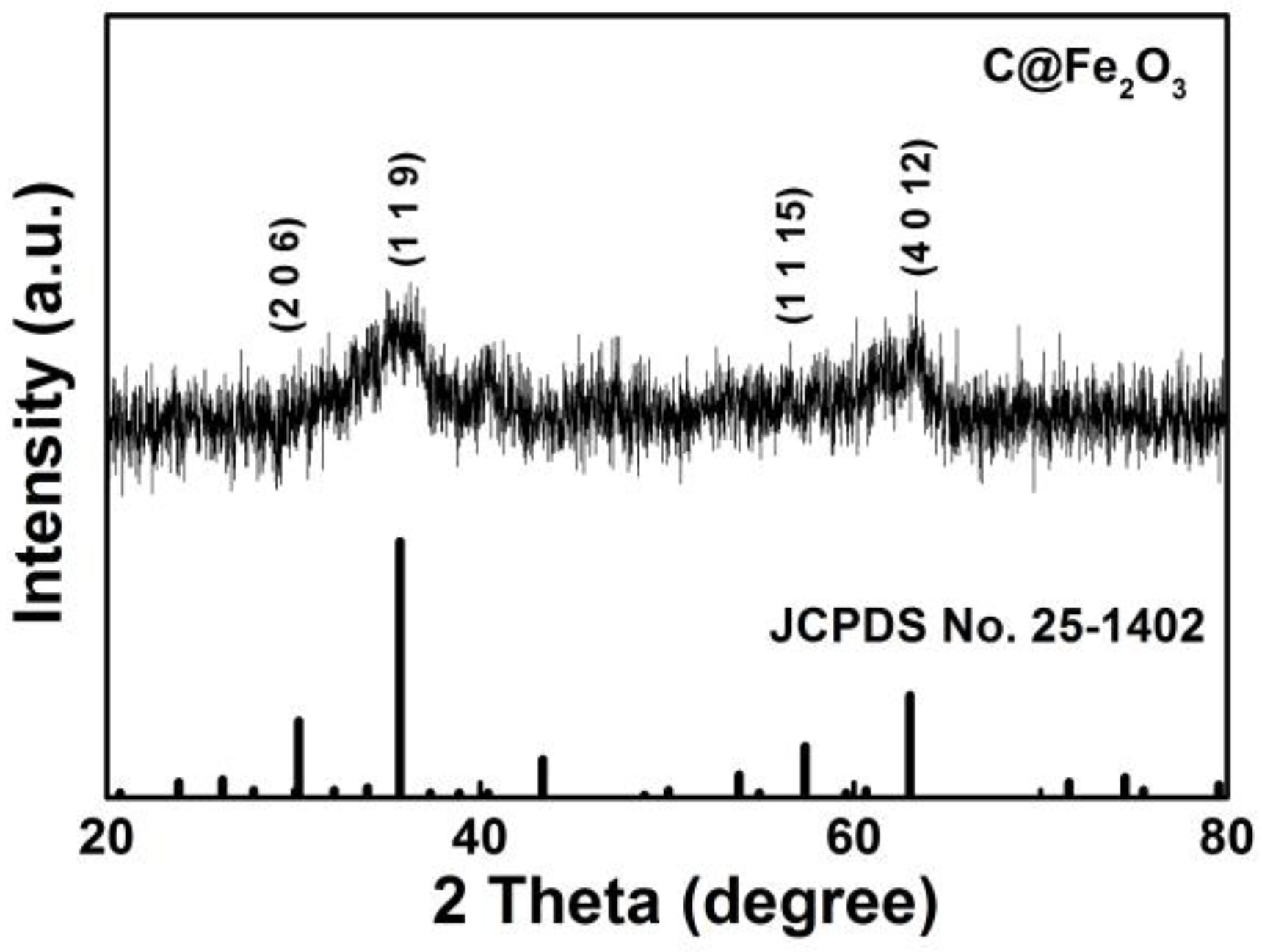
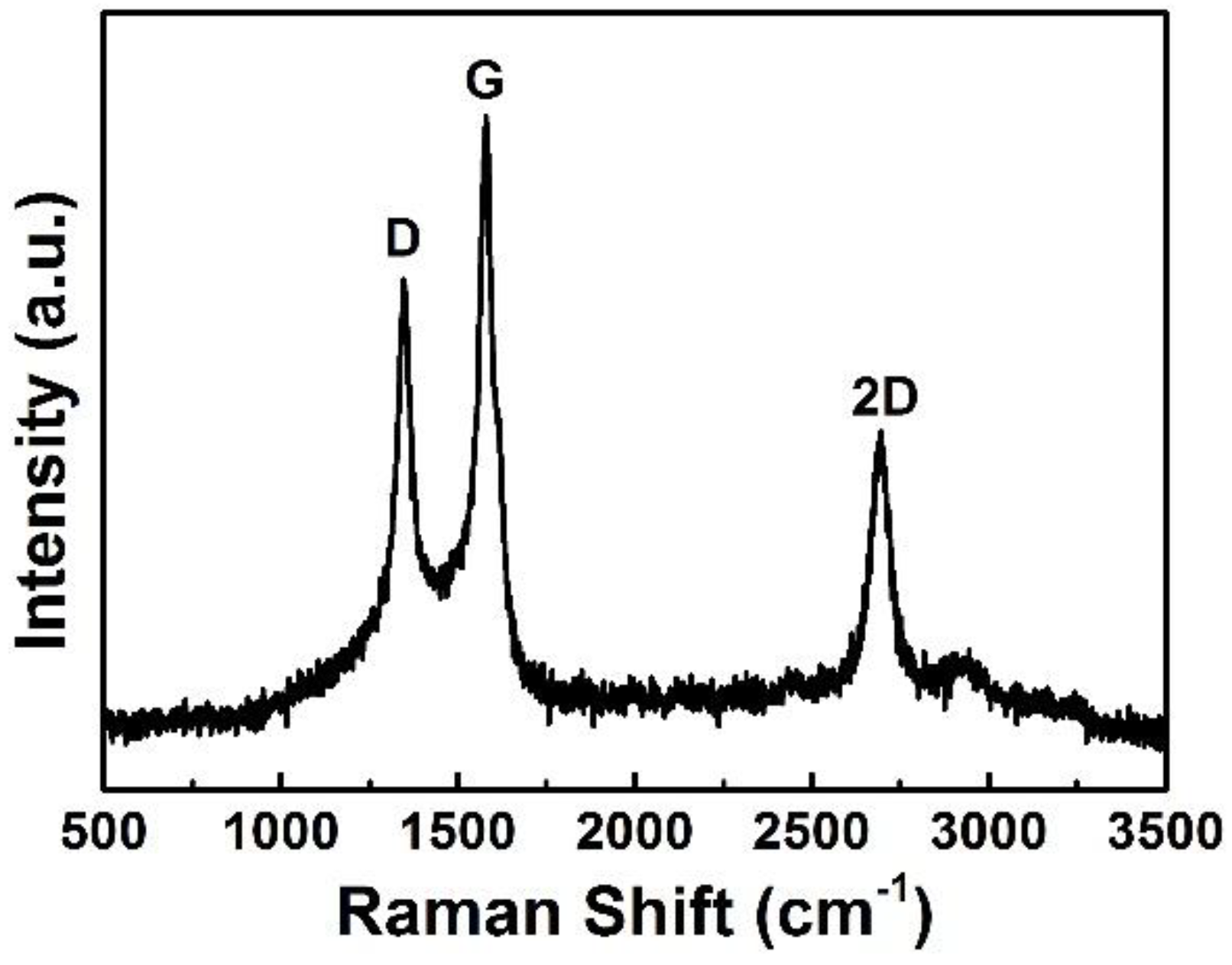
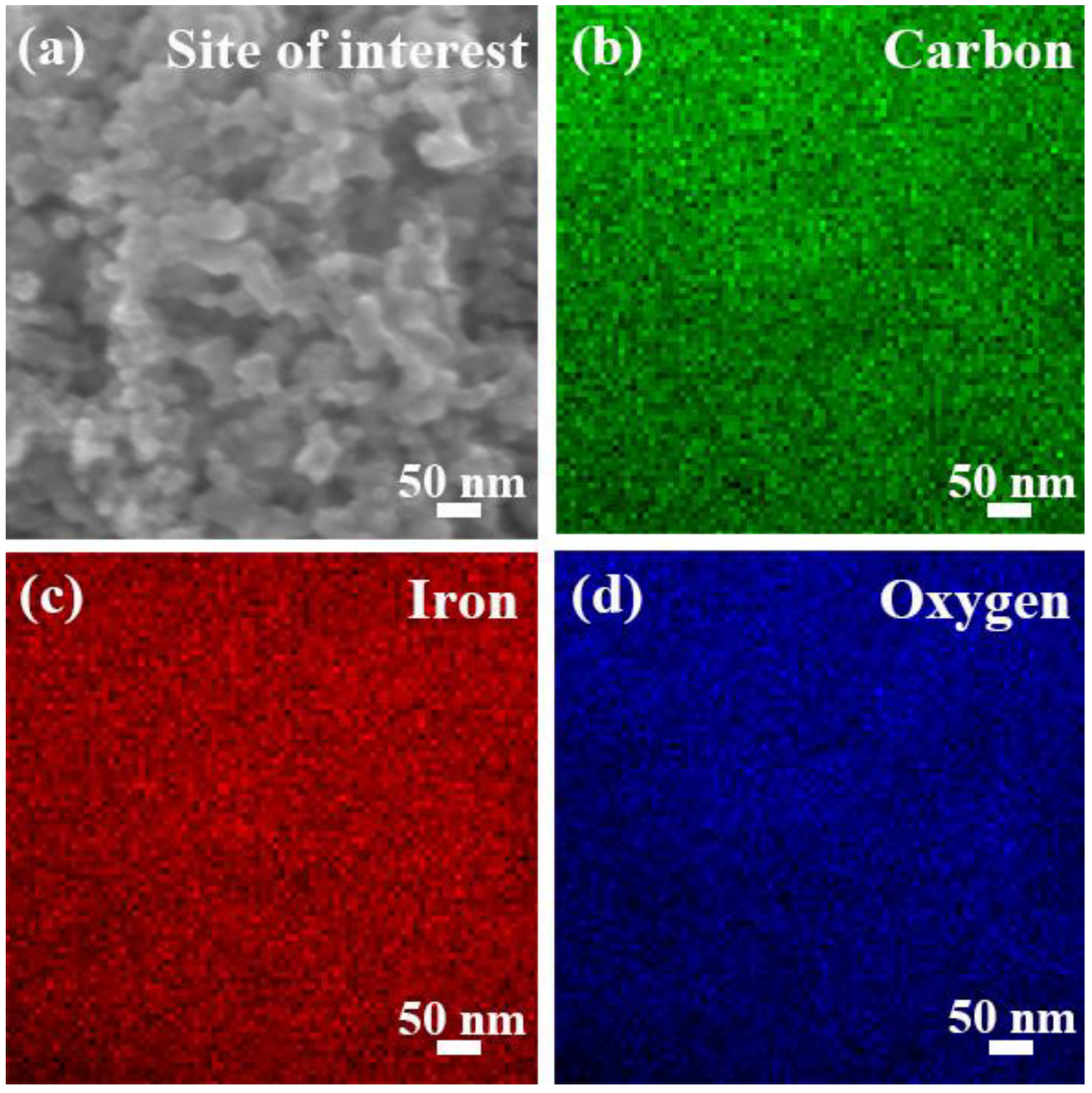
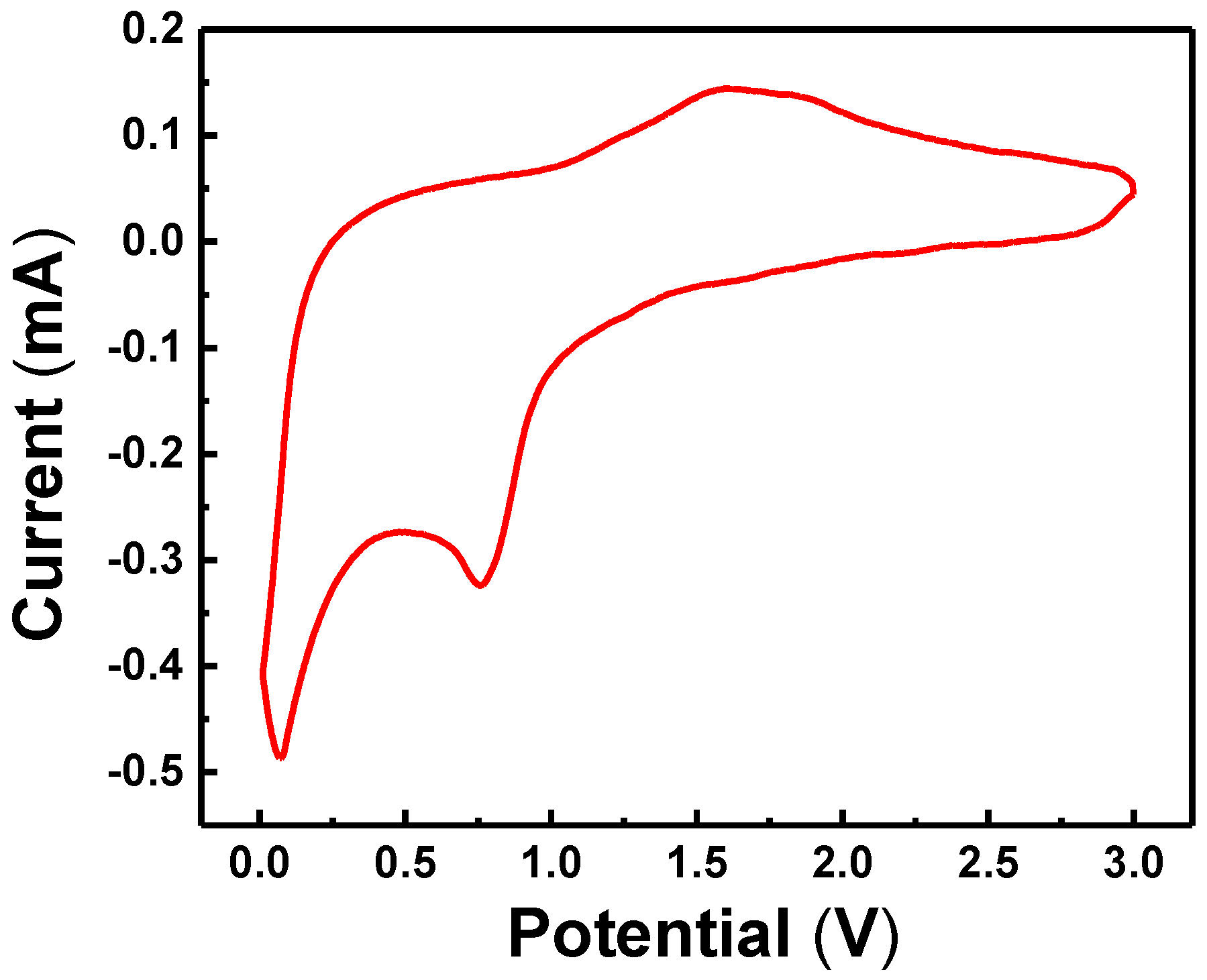
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Firouzi, A.; Qiao, R.M.; Motallebi, S.; Valencia, C.W.; Israel, H.S.; Fujimoto, M.; Wray, L.A.; Chuang, Y.D.; Yang, W.L.; Wessells, C.D. Monovalent manganese based anodes and co-solvent electrolyte for stable low-cost high-rate sodium-ion batteries. Nat. Commun. 2018, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.H.; Xiang, J.M.; Jin, H.F.; Jin, Y.Z.; Wu, L.Z.; Zhang, Y.G.; Mentbayeva, A.; Bakenov, Z. Flexible free-standing Na4Mn9O18/reduced graphene oxide composite film as a cathode for sodium rechargeable hybrid aqueous battery. Electrochim. Acta 2018, 259, 647–654. [Google Scholar] [CrossRef]
- Li, H.P.; Wei, Y.Q.; Zhang, Y.G.; Zhang, C.W.; Wang, G.K.; Zhao, Y.; Yin, F.X.; Bakenov, Z. In situ sol-gel synthesis of ultrafine ZnO nanocrystals anchored on graphene as anode material for lithium-ion batteries. Ceram. Int. 2016, 42, 12371–12377. [Google Scholar] [CrossRef]
- Willis, T.J.; Porter, D.G.; Voneshen, D.J.; Uthayakumar, S.; Demmel, F.; Gutmann, M.J.; Roger, M.; Refson, K.; Goff, J.P. Diffusion mechanism in the sodium-ion battery material sodium cobaltate. Sci. Rep. 2018, 8, 3210. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Yang, Y.C.; Ding, S.S.; Wei, Z.X.; Tang, X.; Li, P.C.; Wang, T.H.; Cao, G.Z.; Zhang, M. S-doped carbon@TiO2 to store Li+/Na+ with high capacity and long life-time. Energy Storage Mater. 2018, 13, 215–222. [Google Scholar] [CrossRef]
- Teng, Y.Q.; Mo, M.S.; Li, Y.; Xue, J.L.; Zhao, H.L. Amorphous carbon-coated ZnO porous nanosheets: Facile fabrication and application in lithium- and sodium-ion batteries. J. Alloys Compd. 2018, 744, 712–720. [Google Scholar] [CrossRef]
- Sultana, I.; Rahman, M.M.; Materi, S.; Ghanooni, A.V.; Glushenkov, A.M.; Chen, Y. K-ion and Na-ion storage performances of Co3O4-Fe2O3 nanoparticle-decorated super P carbon black prepared by a ball milling process. Nanoscale 2017, 9, 3646–3654. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Longoni, G.; Santangelo, S.; Pantò, F.; Stelitano, S.; Frontera, P.; Antonucci, P.; Ruffo, R. Electrochemical characterization of highly abundant, low cost iron (III) oxide as anode material for sodium-ion rechargeable batteries. Electrochim. Acta 2018, 269, 367–377. [Google Scholar] [CrossRef]
- Meng, S.; Zhao, D.L.; Wu, L.L.; Ding, Z.W.; Cheng, X.W.; Hu, T. Fe2O3/nitrogen-doped graphene nanosheet nanocomposites as anode materials for sodium-ion batteries with enhanced electrochemical performance. J. Alloys Compd. 2018, 737, 130–135. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wang, F.F.; Fan, L.Z. Self-standing Na-storage anode of Fe2O3 nanodots encapsulated in porous N-doped carbon nanofibers with ultra-high cyclic stability. Nano Res. 2018, 1, 1–12. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, M.; Zhang, D.; Yuan, T.; Sun, W.; Xu, B.; Yan, M. Transition metal oxides for high performance sodium ion battery anodes. Nano Energy 2014, 5, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.J.; Wang, Y.X.; Chou, S.L.; Li, H.J.; Liu, H.K.; Wang, J.Z. Rapid synthesis ofα-Fe2O3/rGO nanocomposites by microwave autoclave as superior anodes for sodium-ion batteries. J. Power Sources 2015, 280, 107–113. [Google Scholar] [CrossRef]
- Gao, G.; Yu, L.; Wu, H.B.; Lou, X.W. Hierarchical tubular structures constructed by carbon-coated α-Fe2O3 nanorods for highly reversible lithium storage. Small 2014, 10, 1741–1745. [Google Scholar] [CrossRef] [PubMed]
- Li, H.P.; Li, Y.; Zhang, Y.G.; Zhang, C.W. Facile synthesis of carbon-coated Fe3O4 core-shell nanoparticles as anode materials for lithium-ion batteries. J. Nanopart. Res. 2015, 17, 370. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Li, Y.; Li, H.P.; Zhao, Y.; Yin, F.X.; Bakenov, Z. Electrochemical performance of carbon-encapsulated Fe3O4 nanoparticles in lithium-ion batteries: Morphology and particle size effects. Electrochim. Acta 2016, 216, 475–483. [Google Scholar] [CrossRef]
- Jian, Z.L.; Zhao, B.; Liu, P.; Li, F.J.; Zheng, M.B.; Chen, M.W.; Shi, Y.; Zhou, H.S. Fe2O3 nanocrystals anchored onto graphene nanosheets as the anode material for low-cost sodium-ion batteries. Chem. Commun. 2014, 50, 1215–1217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Han, X.; Liu, Y.; Hu, X.; Zhao, Q.; Chen, J. 3D porous γ-Fe2O3@C nanocomposite as high-performance anode material of Na-ion batteries. Adv. Energy Mater. 2015, 5, 1401123–1401130. [Google Scholar] [CrossRef]
- Wu, Z.G.; Zhong, Y.J.; Liu, J.; Wu, J.H.; Guo, X.D.; Zhong, B.H.; Zhang, Z.Y. Subunits controlled synthesis of α-Fe2O3 multi-shelled core-shell microspheres and their effects on lithium/sodium ion battery performances. J. Mater. Chem. A 2015, 3, 10092–10099. [Google Scholar] [CrossRef]
- Philippe, B.; Valvo, M.; Lindgren, F.; Rensmo, H.; Edstrom, K. Investigation of the Electrode/Electrolyte Interface of Fe2O3 Composite Electrodes: Li vs Na Batteries. Chem. Mater. 2014, 26, 5028–5041. [Google Scholar] [CrossRef]
- Wang, S.B.; Wang, W.; Zhan, P.; Jiao, S.Q. Hollow alpha-Fe2O3 nanospheres synthesized using a carbon template as novel anode materials for Na-ion batteries. ChemElectroChem 2014, 1, 1636–1639. [Google Scholar] [CrossRef]
- Valvo, M.; Lindgren, F.; Lafont, U. Towards more sustainable negative electrodes in Na-ion batteries via nanostructured iron oxide. J. Power Sources 2014, 245, 967–978. [Google Scholar] [CrossRef]
- Xu, X.; Cao, R.; Jeong, S.; Cho, J. Spindle-like mesoporous α-Fe2O3 anode material prepared from MOF template for high-rate lithium batteries. Nano Lett. 2012, 12, 4988–4991. [Google Scholar] [CrossRef] [PubMed]
- Su, D.W.; Ahn, H.J.; Wang, G.X. SnO2@graphene nanocomposites as anode materials for Na-ion batteries with superior electrochemical performance. Chem. Commun. 2013, 49, 3131–3133. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, L.W.; Ge, J.B.; Hu, Z.Q.; Sun, H.B.; Sun, H.; Zhang, H.Q.; Zhu, H.M.; Jiao, S.Q. In situ self-assembled FeWO4/graphene mesoporous composites for Li-ion and Na-ion batteries. Chem. Mater. 2014, 26, 3721–3730. [Google Scholar] [CrossRef]
- Liu, X.J.; Chen, T.Q.; Chu, H.P.; Niu, L.Y.; Sun, Z.; Pan, L.K.; Sun, C.Q. Fe2O3-reduced graphene oxide composites synthesized via microwave-assisted method for sodium ion batteries. Electrochim. Acta 2015, 166, 12–16. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Bakenov, Z.; Tan, T.; Huang, J. Synthesis of Core-Shell Carbon Encapsulated Fe2O3 Composite through a Facile Hydrothermal Approach and Their Application as Anode Materials for Sodium-Ion Batteries. Metals 2018, 8, 461. https://doi.org/10.3390/met8060461
Zhang Y, Bakenov Z, Tan T, Huang J. Synthesis of Core-Shell Carbon Encapsulated Fe2O3 Composite through a Facile Hydrothermal Approach and Their Application as Anode Materials for Sodium-Ion Batteries. Metals. 2018; 8(6):461. https://doi.org/10.3390/met8060461
Chicago/Turabian StyleZhang, Yongguang, Zhumabay Bakenov, Taizhe Tan, and Jin Huang. 2018. "Synthesis of Core-Shell Carbon Encapsulated Fe2O3 Composite through a Facile Hydrothermal Approach and Their Application as Anode Materials for Sodium-Ion Batteries" Metals 8, no. 6: 461. https://doi.org/10.3390/met8060461





