Electrochemical Behavior of Biodegradable FeMnSi–MgCa Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
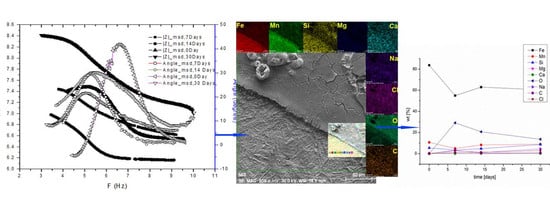
3.1. Surface Characterization
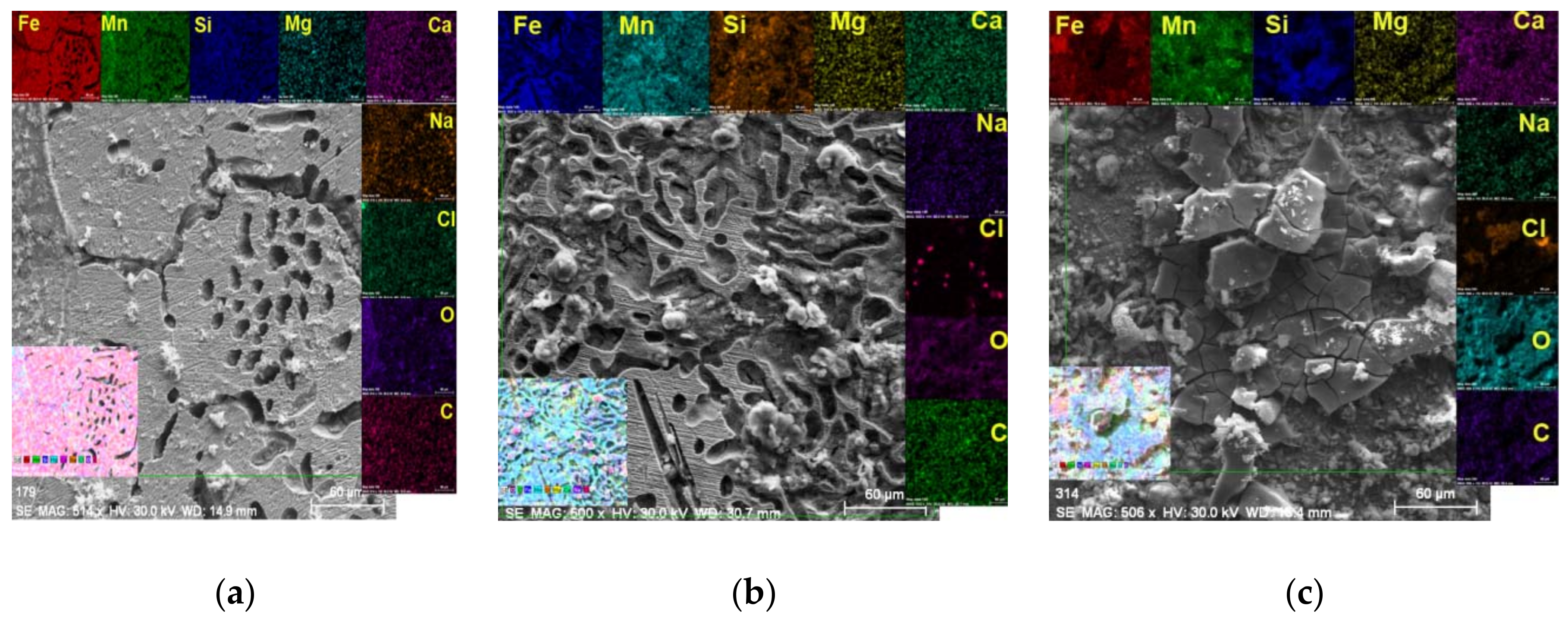
3.2. Surface Chemical Characterization
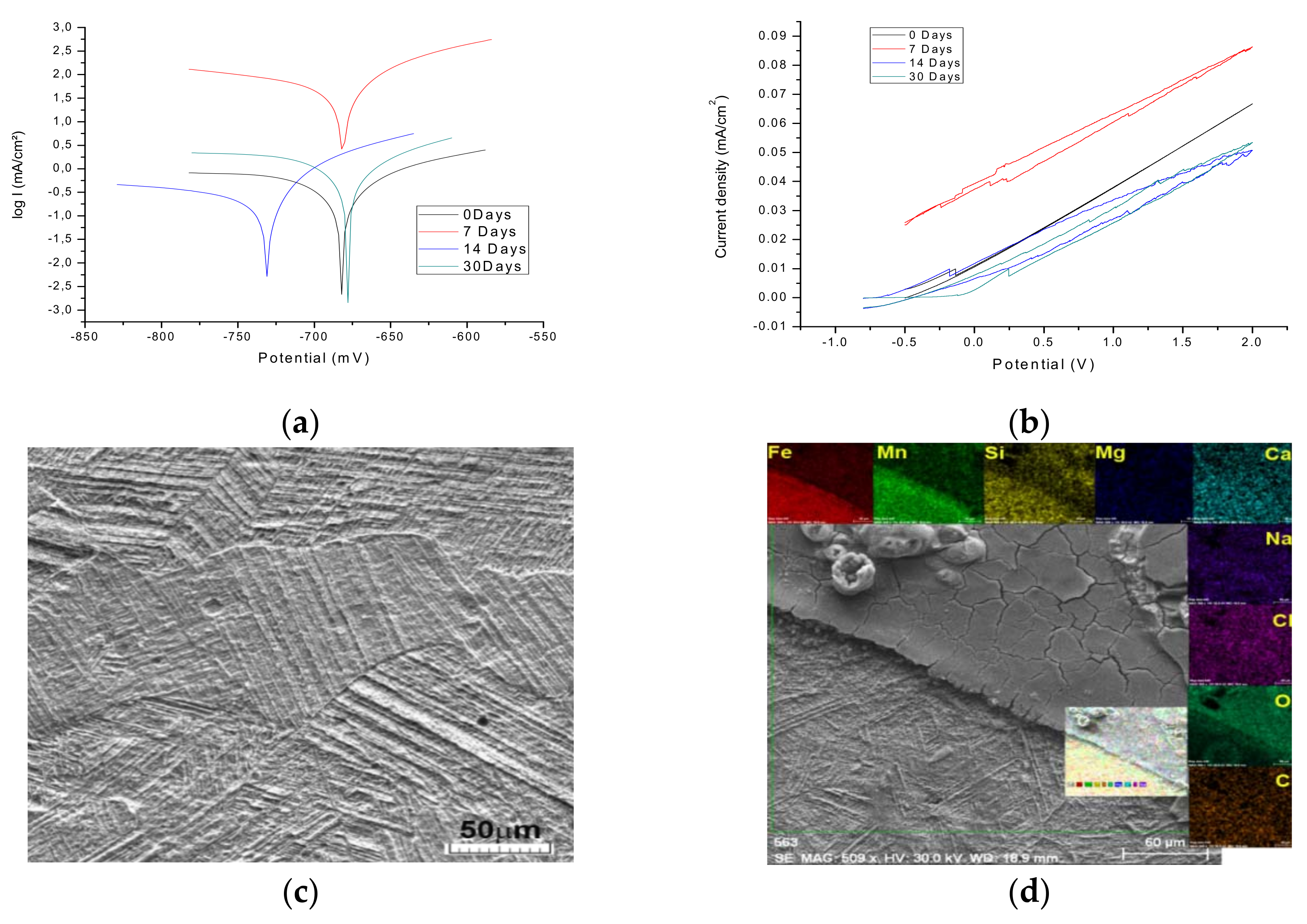
3.3. Surface Electrochemical Characterization
4. Conclusions
- We demonstrated that micro-alloying the FeMnSi alloy with Ca and Mg elements resulted in obtaining an α-phase microstructure in a melted state with finely distributed Ca and Mg elements.
- By adding small quantities of Ca and Mg, the corrosion rate in SBF can be increased due to the formation of fine precipitates and also by changing the mechanical behavior and initial corrosion mechanism of the alloy. Further work in in vivo tests of this new material is proposed to evaluate the behavior of the material in real conditions in short, medium, or long periods.
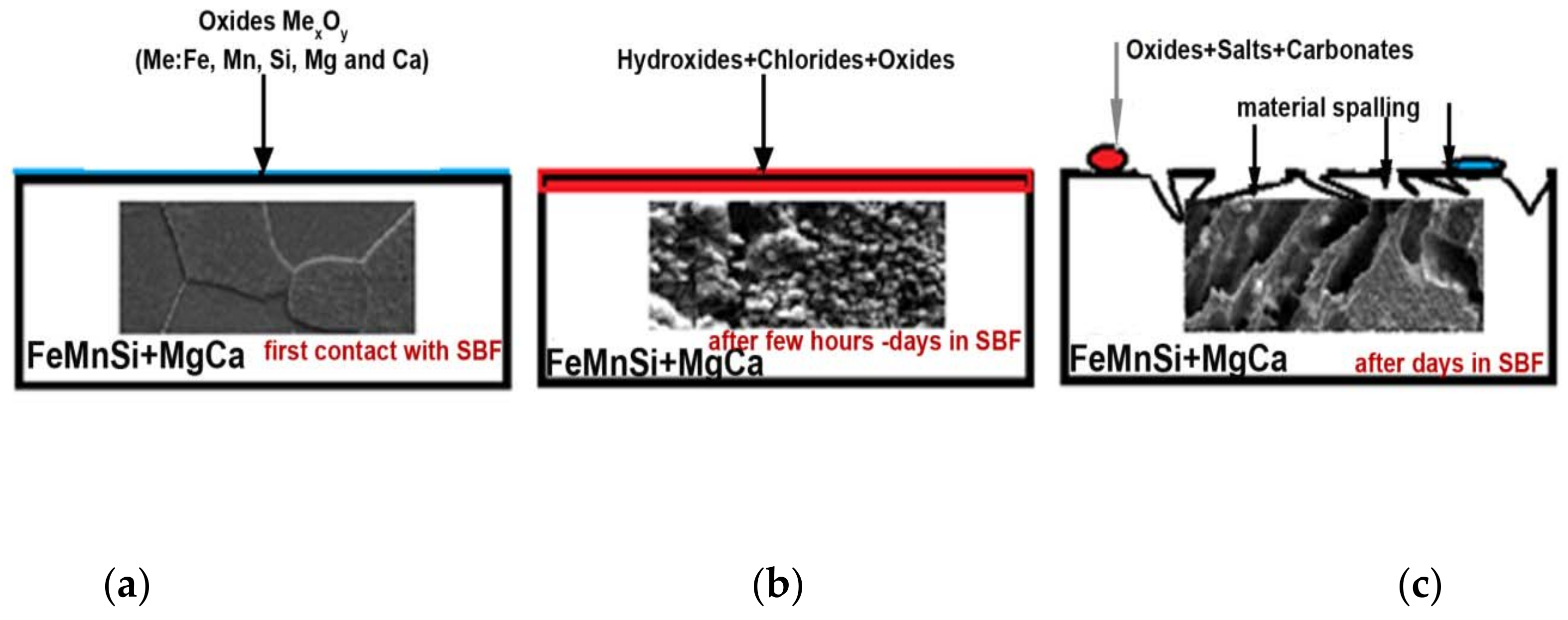
- When introducing and maintaining the FeMnSi–MgCa alloy in artificial blood plasma (SBF), the corrosion process starts from the initial moment and continues throughout the immersion period. Initially, corrosion products (possibly iron and manganese oxides) are adsorbed as microparticles on the surface of the alloy; over time, a compact layer is formed on the surface, which allows the transfer of diffusion loads. After a longer period in the solution, the compact layer deteriorates (becomes porous or cracked) and continues the corrosion in the form of crevasses.
Author Contributions
Funding
Conflicts of Interest
References
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloy. Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Kafri, A.; Shira, O.; Goldman, J.; Drelich, J.; Aghion, E. The Suitability of Zn-1.3%Fe Alloy as a Biodegradable Implant Material. Metals 2018, 8, 153. [Google Scholar] [CrossRef]
- Moravej, M.; Purnama, A.; Fiset, M.; Couet, J.; Mantovani, D. Electroformed pure iron as a new biomaterial for degradable stents: In vitro degradation and preliminary cell viability studies. Acta Biomater. 2010, 6, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Schinhammer, M.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.J. Design strategy for biodegradable Fe-based alloys for medical applications. Acta Biomater. 2010, 6, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, Y.; Liu, J.; Jiang, C.; Li, Y. In vitro corrosion properties and cytocompatibility of Fe-Ga alloys as potential biodegradable metallic materials. Mater. Sci. Eng. C 2017, 71, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, K.; Adil, T.; Melih, U.; Baver, A.; Ferhat, G. Fixation of medial malleolar fractures with magnesium bioabsorbable headless compression screws: Short-term clinical and radiological outcomes in eleven patients. Arch. Orthop. Trauma Surg. 2018, 1–7. [Google Scholar] [CrossRef]
- Mostavan, A.; Paternoster, C.; Tolouei, R.; Ghali, E.; Dubé, D.; Mantovani, D. Effect of electrolyte composition and deposition current for Fe/Fe-P electroformed bilayers for biodegradable metallic medical applications. Mater. Sci. Eng. C 2017, 70, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Obayi, C.S.; Tolouei, R.; Paternoster, C.; Turgeon, S.; Okorie, B.A.; Obikwelu, D.O.; Cassar, G.; Buhagiar, J.; Mantovan, D. Influence of cross-rolling on the micro-texture and biodegradation of pure iron as biodegradable material for medical implants. Acta Biomater. 2015, 17, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Im, S.H.; Jung, Y.; Kim, S.H. Current status and future direction of biodegradable metallic and polymeric vascular scaffolds for next-generation stents. Acta Biomater. 2017, 60, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Stukenborg-Colsman, C.; Waizy, H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: Short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Online 2013, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, M.U.; Saqlain, S.A. Dissolution behavior of bioactive glass ceramics with different CaO/MgO ratios in SBF-K9 and r-SBF. Prog. Nat. Sci.-Mater. 2014, 24, 354–363. [Google Scholar] [CrossRef]
- Armarego, W.L.F. Chapter 4—Purification of Inorganic and Metal-Organic Chemicals in Purification of Laboratory Chemicals; Butterworth-Heinemann: Oxford, UK, 2017; pp. 635–770. ISBN 9780128054574. [Google Scholar]
- Shi, S.Q.; Wen, C.; Kaiwen, L.; Changlei, X.; Dongmao, Z. Phase transitions of carbon-encapsulated iron oxide nanoparticles during the carbonization of cellulose at various pyrolysis temperatures. J. Anal. Appl. Pyrol. 2015, 115, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Daeho, H.; Da-Tren, C.; Velikokhatnyi, O.I.; Abhijit, R.; Boeun, L.; Swink, I.; Issaev, I.; Kuhn, H.A.; Kumta, P.N. Binder-jetting 3D printing and alloy development of new biodegradable Fe-Mn-Ca/Mg alloys. Acta Biomater. 2016, 45, 375–386. [Google Scholar] [CrossRef]
- Gu, X.N.; Zhou, W.R.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Zhong, S.P.; Xi, T.F.; Chen, L.J. Corrosion fatigue behaviors of two biomedical Mg alloys-AZ91D and WE43-In simulated body fluid. Acta Biomater. 2010, 6, 4605–4613. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, J.; Bolat, G.; Cimpoesu, N.; Trinca, L.C.; Mareci, D.; Souto, M.R. Electrochemical characterization of pulsed layer deposited hydroxyapatite-zirconia layers on Ti-21Nb-15Ta-6Zr alloy for biomedical application. Appl. Surf. Sci. 2016, 385, 368–378. [Google Scholar] [CrossRef]
- Hufenbach, J.; Wendrock, H.; Kochta, F.; Kühn, U.; Gebert, A. Novel biodegradable Fe-Mn-C-S alloy with superior mechanical and corrosion properties. Mater. Lett. 2017, 186, 330–333. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.F.; Liquan, R. In vitro investigation of Fe30Mn6Si shape memory alloy as potential biodegradable metallic material. Mater. Lett. 2011, 65, 540–543. [Google Scholar] [CrossRef]
- Cimpoeșu, N.; Trincă, L.C.; Dascălu, G.; Stanciu, S.; Gurlui, S.O.; Mareci, D. Electrochemical Characterization of a New Biodegradable FeMnSi Alloy Coated with Hydroxyapatite-Zirconia by PLD Technique. J. Chem. 2016, 2016. [Google Scholar] [CrossRef]
- Schinhammer, M.; Steiger, P.; Moszner, F.; Löffler, J.F.; Uggowitzer, P.J. Degradation performance of biodegradable Fe–Mn–C(–Pd) alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, S.; Ursanu, A.; Trincă, L.C.; Trofin, A.E.; Solcan, C.; Munteanu, C.; Cimpoesu, N.; Acatrinei, D.; Sindilar, E.V.; Stanciu, T.; et al. Study on the biodegradability of FeMnSi alloy. Environ. Eng. Manag. J. 2016, 15, 973–980. [Google Scholar]
- Liu, Y.; Liu, D.; Zhao, Y.; Chen, M. Corrosion degradation behavior of Mg−Ca alloy with high Ca content. in SBF. Trans. Nonferr. Met. Soc. China 2015, 25, 3339–3347. [Google Scholar] [CrossRef]
- Chelariu, R.; Suditu, G.D.; Mareci, D.; Bolat, G.; Cimpoesu, N.; Leon, F.; Curteanu, S. Prediction of Corrosion Resistance of Some Dental Metallic Materials with an Adaptive Regression Model. JOM 2015, 67, 767–774. [Google Scholar] [CrossRef]
- Asgari, M.; Hang, R.; Wang, C.; Yu, Z.; Li, Z.; Xiao, Y. Biodegradable MetallicWires in Dental and Orthopedic Applications: A Review. Metals 2018, 8, 212. [Google Scholar] [CrossRef]
- Hermawan, H.; Mantovani, D. Process of prototyping coronary stents from biodegradable Fe–Mn Alloys. Acta Biomater. 2013, 9, 8585–8592. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Xi, T.; Zheng, Y. A review on in vitro corrosion performance test of biodegradable metallic materials. Trans. Nonferr. Met. Soc. China 2013, 23, 2283–2293. [Google Scholar] [CrossRef]





| Chemical Composition (Ions) (mmol/dm3) | Na+ | K+ | Mg2+ | Ca2+ | Cl− | HCO3− | HPO42− | SO42− |
|---|---|---|---|---|---|---|---|---|
| Simulated body fluid | 142 | 5 | 1.5 | 2.5 | 147.8 | 4.2 | 1 | 0.5 |
| Human blood plasma | 142 | 5 | 1.5 | 2.5 | 103 | 27 | 1 | 0.5 |
| Sample/Dimension | Length (mm) | Width (mm) | Thickness (mm) | Total Area (mm2) | Initial Weight (g) | Weight after Immersion (g) | Weight after Immersion + Sonication (g) |
|---|---|---|---|---|---|---|---|
| 7 days | 10.1 | 6.94 | 4.54 | 294.91 | 2.397 | 2.380 | 2.3706 |
| 14 days | 10.25 | 6.98 | 4.54 | 299.54 | 2.426 | 2.401 | 2.372 |
| 30 days | 10.03 | 6.83 | 4.54 | 290.1 | 2.310 | 2.279 | 2.265 |
| Parameter | 0 Days | 7 Days | 14 Days | 30 Days |
|---|---|---|---|---|
| OCP (mV) | −753 | −713 | −679 | −751 |
| Ecor (Ej=0) (mV) | −682 | −681 | −727 | −677.9 |
| Rp (ohm · cm2) | 49.01 | 383.31 | 17.71 | 19.14 |
| bamV | 168 | 82 | 158 | 164.5 |
| bcmV | −1340 | −280 | −451 | −1116.3 |
| jcorr (mA/cm2) | 0.695 | 0.0571 | 2.022 | 1.7740 |
| vcor (mm/year) | 8.126 | 0.668 | 23.640 | 20.74 |
| Rp = (dE/dj)E (Ω · cm2) | 47.98 | 340.49 | 17.87 | 18.60 |
| Parameters | Rs (ohm · cm2) | Q1 (S · sn/cm2) | n1 | R1 (kohm · cm2) | Q2, S · sn/cm2 | n2 | L (Henry · cm2) | W (S · s½ · cm2) | R2 (kohm · cm2) | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 Days | 444.6 | 12.1210−6 | 0.694 | 3.863 | - | - | 108.2 | - | 1.096 | 5.70 10−4 |
| 7 Days | 712.8 | 30.1 10−6 | 0.721 | 3.409 | - | - | - | 2.21 10−4 | - | 1.86 10−4 |
| 14 Days | 587.4 | 30.6 10−6 | 0.730 | 2.651 | 2.21 10−4 | 0.799 | - | - | 1.695 | 1.86 10−4 |
| 30 Days | 447.8 | 7.81 10−5 | 0.435 | 1.007 103 | 2.24 10−5 | 0.797 | - | - | 3.239 × 103 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimpoeşu, N.; Săndulache, F.; Istrate, B.; Cimpoeşu, R.; Zegan, G. Electrochemical Behavior of Biodegradable FeMnSi–MgCa Alloy. Metals 2018, 8, 541. https://doi.org/10.3390/met8070541
Cimpoeşu N, Săndulache F, Istrate B, Cimpoeşu R, Zegan G. Electrochemical Behavior of Biodegradable FeMnSi–MgCa Alloy. Metals. 2018; 8(7):541. https://doi.org/10.3390/met8070541
Chicago/Turabian StyleCimpoeşu, Nicanor, Florin Săndulache, Bogdan Istrate, Ramona Cimpoeşu, and Georgeta Zegan. 2018. "Electrochemical Behavior of Biodegradable FeMnSi–MgCa Alloy" Metals 8, no. 7: 541. https://doi.org/10.3390/met8070541