A New Method to Recycle Stainless–Steel Duplex UNS S31803 Chips
Abstract
:1. Introduction
2. Materials and Methods
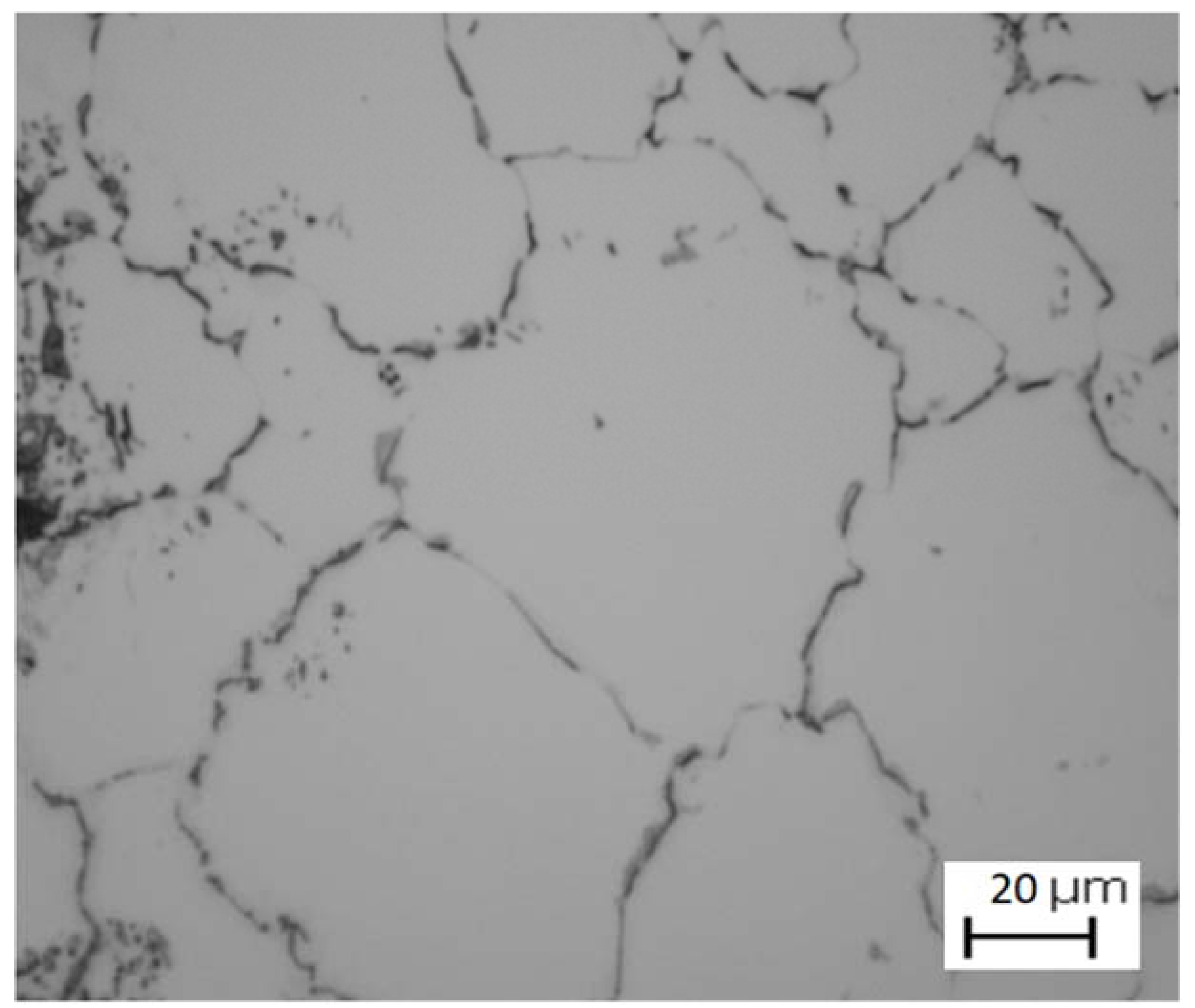
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tranchida, G.; Clesi, M.; Di Franco, F.; Di Quarto, F.; Santamaria, M. Electronic properties and corrosion resistance of passive films on austenitic and duplex stainless–steels. Electrochim. Acta 2018, 273, 412–423. [Google Scholar] [CrossRef]
- Sheikhzadeh, M.; Sanjabi, S. Structural characterization of stainless–steel/TiC nanocomposites produced by high-energy ball-milling method at different milling times. Mater. Des. 2012, 39, 366–372. [Google Scholar] [CrossRef]
- Shashanka, R.; Chaira, D. Optimization of milling parameters for the synthesis of nano-structured duplex and ferritic stainless–steel powders by high energy planetary milling. Powder Technol. 2015, 278, 35–45. [Google Scholar] [CrossRef]
- Gunn, R.N. Duplex Stainless–Steels: Microstructures, Properties and Applications, 2nd ed.; Woodlhead Publishing: Cambridge, UK, 2003. [Google Scholar]
- Fréchard, S.; Martin, F.; Clément, C.; Cousty, J. AFM and EBSD combined studies of plastic deformation in a duplex stainless–steel. Mater. Sci. Eng. A. 2006, 418, 312–319. [Google Scholar] [CrossRef]
- Cabrera, J.M.; Mateo, A.; Llanes, L.; Prado, J.M.; Anglada, M. Hot deformation of duplex stain-less steels. J. Mater. Process. Technol. 2003, 143, 321–323. [Google Scholar] [CrossRef]
- Brytan, Z.; Dobrzánski, L.A.; Actis Grande, M.; ROSSO, M. The influence of sintering time on the properties of PM duplex stainless–steel. J. Achiev. Mater. Manuf. Eng. 2009, 37, 387–396. [Google Scholar]
- Grupta, S.; Shashanka, R.; Chaira, D. Synthesis of nano-structured duplex and ferritic stainless–steel powders by planetary milling: An experimental and simulation study. IOP Conf. Ser. Mater. Sci. Eng. 2015, 75, 01203. [Google Scholar]
- Tański, T.; Labisz, K.; Brytan, Z. Fatigue behaviour of sintered duplex stainless–steel. Procedia Eng. 2014, 74, 421–428. [Google Scholar] [CrossRef]
- Dobrzánski, L.A.; Brytan, L.A.; Actis Grande, Z.M.; Rosso, M. Corrosion resistance of sintered duplex stainless–steels in the salt fog spray test. J. Mater. Process. Technol. 2007, 192, 443–448. [Google Scholar] [CrossRef]
- Shashanka, R.; Chaira, R.D. Development of nano-structured duplex and ferritic stainless–steels by pulverisette planetary milling followed by pressureless sintering. Mater. Character. 2015, 99, 220–229. [Google Scholar]
- Garcia, C.; Martin, F.; Blanco, Y. Effect of sintering cooling rate on corrosion resistance of powder metallurgy austenitic, ferritic and duplex stainless–steels sintered in nitrogen. Corros. Sci. 2012, 61, 45–52. [Google Scholar] [CrossRef]
- Gronnostajski, J.; Chmura, W.; Gronostajski, Z. Phases created during diffusion bonding of aluminum and aluminum bronze chips. J. Achiev. Mater. Manuf. Eng. 2006, 19, 32–37. [Google Scholar]
- Canakci, A.; Varol, T. A novel method for the production of metal powders without conventional atomization process. J. Clean. Prod. 2015, 99, 312–319. [Google Scholar] [CrossRef]
- Rane, K.K.; Date, P.P. Sustainable Recycling of Ferrous Metallic Scrap Using Powder Metallurgy Process. J. Sustain. Metall. 2017, 3, 251–264. [Google Scholar] [CrossRef]
- Fogagnolo, J.B.; Velasco, F.; Robert, M.H.; Torralba, J.M. Effect of mechanical alloying on the morphology, microstructure and properties of aluminium matrix composite powders. Mater. Sci. Eng. A 2003, 342, 131–143. [Google Scholar] [CrossRef]
- Costa, C.E.; Zapata, W.C.; Parucker, M.L. Characterization of casting iron powder from recycled swarf. J. Mater. Process. Technol. 2003, 143–144, 138–143. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Afkham, Y.; Khosroshahi, R.A.; Kheirifard, R.; Mousavian, R.T.; Brabazon, D. Microstructure and morphological study of ballmilled metal matrix nanocomposites. Phys. Met. Metallogr. 2017, 118, 749–758. [Google Scholar] [CrossRef]
- Ehteshamzadeh, M.A.; Mousavian, R.T. Fabrication of an r-Al2Ti intermetallic matrix composite reinforced with α-Al2O3 ceramic by discontinuous mechanical milling for thermite reaction. Int. J. Miner. Metall. Mater. 2014, 21, 1037–1043. [Google Scholar]
- Afkham, Y.; Khosroshahi, R.A.; Mousavian, R.T.; Brabazon, D.; Kheirifard, R. Microstructural characterization of ball-milled metal matrix nanocomposites (Cr, Ni, Ti)-25 wt % (Al2O3np, SiCnp). Particulate Sci. Technol. 2018, 36, 72–83. [Google Scholar] [CrossRef]
- Mendonça, C.S.P.; Dias, A.N.O.; Melo, M.L.N.M.; Ribeiro, V.A.S.; Da Silva, M.R.; Oliveira, A.F.; Silva, G. Evaluation of high-energy milling efficiency in stainless–steel with addition of vanadium carbides. Int. J. Adv. Manuf. Technol. 2018, 95, 3093–3099. [Google Scholar] [CrossRef]
- Baldo, S.; Mészáros, I. Effect of cold rolling on microstructure and magnetic properties in a metastable lean duplex stainless–steel. J. Mater. Sci. 2010, 45, 5339–5534. [Google Scholar] [CrossRef]
- Tavares, S.S.M.; Silva, M.R.; Pardal, J.M.; Abreu, H.F.H.; Gomes, A.M. Microstructural changes produced by plastic deformation in the UNS S31803 duplex stainless–steel. J. Mater. Technol. 2006, 180, 318–322. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Wang, Y.; Yu, H.; Zhang, C. Fabrication of nanostructure in inner-surface of AISI 304 stainless–steel pipe with surface plastic deformation. J. Mater. Sci. Technol. 2018, in press. [Google Scholar] [CrossRef]
- Oleszak, D.; Grabias, A.; Pekala, M.; Swiderska-Sroda, A.; Kulik, T. Evolution of structure in austenitic steel powders during ball milling and subsequent sintering. J. Alloy. Compd. 2007, 434–435, 340–343. [Google Scholar] [CrossRef]
- Trueman, A.R.; Schweinsberg, D.P.; Hope, G.A. A study of the effect of cobalt additions on the corrosion of tungsten carbide/carbon steel metal matrix composites. Corros. Sci. 1999, 41, 1377–1389. [Google Scholar] [CrossRef]
- Pramanik, A.; Basak, A.K.; Dong, Y.; Shankar, S.; Littlefair, G. Milling of nanoparticles reinforced Al-based metal matrix composites. J. Compos. Sci. 2018, 2, 13. [Google Scholar] [CrossRef]
- Vijaya, M.; Srinivas, K. Development and Mechanical Properties of SiC Reinforced Aluminium Metal Matrix Composites. J. Recent Act. Prod. 2018, 3, 1–9. [Google Scholar]
- Lynch, C.T.; Kershaw, J.P. Metal Matrix Composites; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Śusniak, M.; Karwan-baczewska, J.; Dutiewicz, J.; Actis Grange, M.; Rosso, M. Structure investigation of ball milled composite powder based on AlSi5Cu2 alloy chips modified by SiC particles. Arch. Metall. Mater. 2013, 58, 437–441. [Google Scholar]
- Torralba, J.M.; Da Costa, C.E.; Velasco, F. P/M aluminum matrix composites: An overview. J. Mater. Process. Technol. 2013, 133, 203–206. [Google Scholar] [CrossRef]
- Śusniak, M.; Karwan-Baczewska, J.; Dutkiewicz, J.; Actis Grande, M.; Rosso, M. An experimental study of aluminum alloy matrix composite reinforced sic made by hot pressing method. Arch. Metall. Mater. 2015, 60, 1523–1527. [Google Scholar] [CrossRef]
- Gubernat, A.; Zych, L. The isothermal sintering of the single-phase non stoichiometric niobium carbide (NbC1−x) and tantalum carbide (TaC1−x). J. Eur. Ceram. Soc. 2014, 34, 2885–2894. [Google Scholar] [CrossRef]
- Kosolapova, T.Y. Carbides Properties, Production and Applications; Springer Publishing: New York, NY, USA, 1971. [Google Scholar]
- Kuffner, B.H.B.; Diogo, W.S.; Amancio, D.A.; Rodrigues, G.; Silva, G. Evaluation of the milling efficiency increase of AISI 52100 steel using niobium carbide addition through high energy ball milling. Rem: Revista Escola de Minas 2015, 68, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.N.O.; Oliveira, L.A.; Mendonça, C.S.P.; Junqueira, M.M.; Melo, M.L.N.M.; Silva, G. Comparative analysis of niobium and vanadium carbide efficiency in the high energy mechanical milling of aluminum bronze alloy. REM Int. Eng. J. 2018, 71, 59–65. [Google Scholar] [CrossRef] [Green Version]
- ASTM C20-00. Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water; ASTM International: West Conshohocken, PA, USA, 2015.
- Lin, J.T.; Bhattacharyya, D.; Ferguson, W.G. Chip formation in the machining of SiC-particle-reinforced aluminium-matrix composites. Compos. Sci. Technol. 1998, 58, 285–291. [Google Scholar] [CrossRef]
- Mendonça, C.S.P.; Oliveira, A.F.; Oliveira, L.A.; da Silva, M.R.; Melo, M.L.N.M.; Gilbert, S. Structural and Magnetic Properties of Duplex Stainless–steel (UNS S31803) Powders Obtained by high Energy Milling of Chips with Additions of NbC. Mater. Res. 2017, 20, 1–5. [Google Scholar] [CrossRef]
- Abdoli, H.; Asgharzadeh, H.; Salahi, E. Sintering behavior of Al–AlN-nanostructured composite powder synthesized by high-energy ball milling. J. Alloy. Compd. 2009, 473, 116–122. [Google Scholar] [CrossRef]
- Varol, T.; Canakci, A. Effect of weight percentage and particle size of B 4 C reinforcement on physical and mechanical properties of powder metallurgy Al2024-B 4 C composites. Met. Mater. Int. 2013, 19, 1227–1234. [Google Scholar] [CrossRef]
- Nowosielski, R.; Pilarczyk, W. Structure and properties of Fe-6.67% C alloy obtained by mechanical alloying. J. Mater. Process. Technol. 2005, 162, 373–378. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Pearson: London, UK, 2013. [Google Scholar]
- Di Schino, A.; Barteri, M.; Kenny, J.M. Development of ultra-fine grain structure by martensitic reversion in stainless–steel. J. Mater. Sci. Lett. 2002, 21, 751–753. [Google Scholar] [CrossRef]
- Rajasekhara, S.; Karjalainen, L.P.; Kyröläinen, A.; Ferreira, P.J. Microstructure evolution in nano/submicron grained AISI 301LN stainless–steel. Mater. Sci. Eng. A 2010, 527, 1986–1996. [Google Scholar] [CrossRef]
- Shakhova, I.; Dudko, V.; Belyakov, A.; Tsuzaki, K.; Kaibyshev, R. Effect of large strain cold rolling and subsequent annealing on microstructure and mechanical properties of an austenitic stainless–steel. Mater. Sci. Eng. A 2012, 545, 176–186. [Google Scholar] [CrossRef]
- Shirdel, M.; Mirzadeh, H.; Parsa, M.H. Nano/ultrafine grained austenitic stainless–steel through the formation and reversion of deformation-induced martensite: Mechanisms, microstructures, mechanical properties, and TRIP effect. Mater. Charact. 2005, 103, 150–161. [Google Scholar] [CrossRef]
- Moallemi, M.; Zarei-hanzaki, A.; Baghbadorani, H.S. Evolution of microstructure and mechanical properties in a cold deformed nitrogen bearing TRIP-assisted duplex stainless–steel after reversion annealing. Mater. Sci. Eng. A 2017, 683, 83–89. [Google Scholar] [CrossRef]
- Herrera, C.; Plaut, R.L.; Padilha, A.F. Microstructural Refinement during Annealing of Plastically Deformed Austenitic Stainless–steels. Mater. Sci. Forum 2007, 550, 423–428. [Google Scholar] [CrossRef]
- Fargas, G.; Akdut, N.; Anglada, M.; Mateo, A. Microstructural evolution during industrial rolling of a duplex stainless–steel. ISIJ Int. 2008, 48, 1596–1602. [Google Scholar] [CrossRef]
- Malta, P.O.; Dias, F.L.; Souza, A.C.M.; Santos, D.B. Microstructure and texture evolution of duplex stainless–steels with different molybdenum contents. Mater. Charact. 2018, 142, 406–421. [Google Scholar] [CrossRef]
- Badji, R.; Bacroix, B.; Bouabdallah, M. Texture, microstructure and anisotropic properties in annealed 2205 duplex stainless–steel welds. Mater. Charact. 2011, 62, 833–843. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Muthupandi, V.; Jayachitra, R. Influence of heat treatment on the microstructure, ultrasonic attenuation and hardness of SAF 2205 duplex stainless–steel. Mater. Sci. Eng. A 2011, 529, 447–451. [Google Scholar] [CrossRef]
- Gholami, M.; Hoseinpoor, M.; Moayed, M.H. A statistical study on the effect of annealing temperature on pitting corrosion resistance of 2205 duplex stainless–steel. Corros. Sci. 2015, 94, 156–164. [Google Scholar] [CrossRef]
- Padilha, A.F.; Aguiar, D.J.M.; Plaut, R.L. Duplex Stainless–steels: A Dozen of Significant Phase Transformations. Defect Diffus. Forum 2012, 322, 163–174. [Google Scholar] [CrossRef]
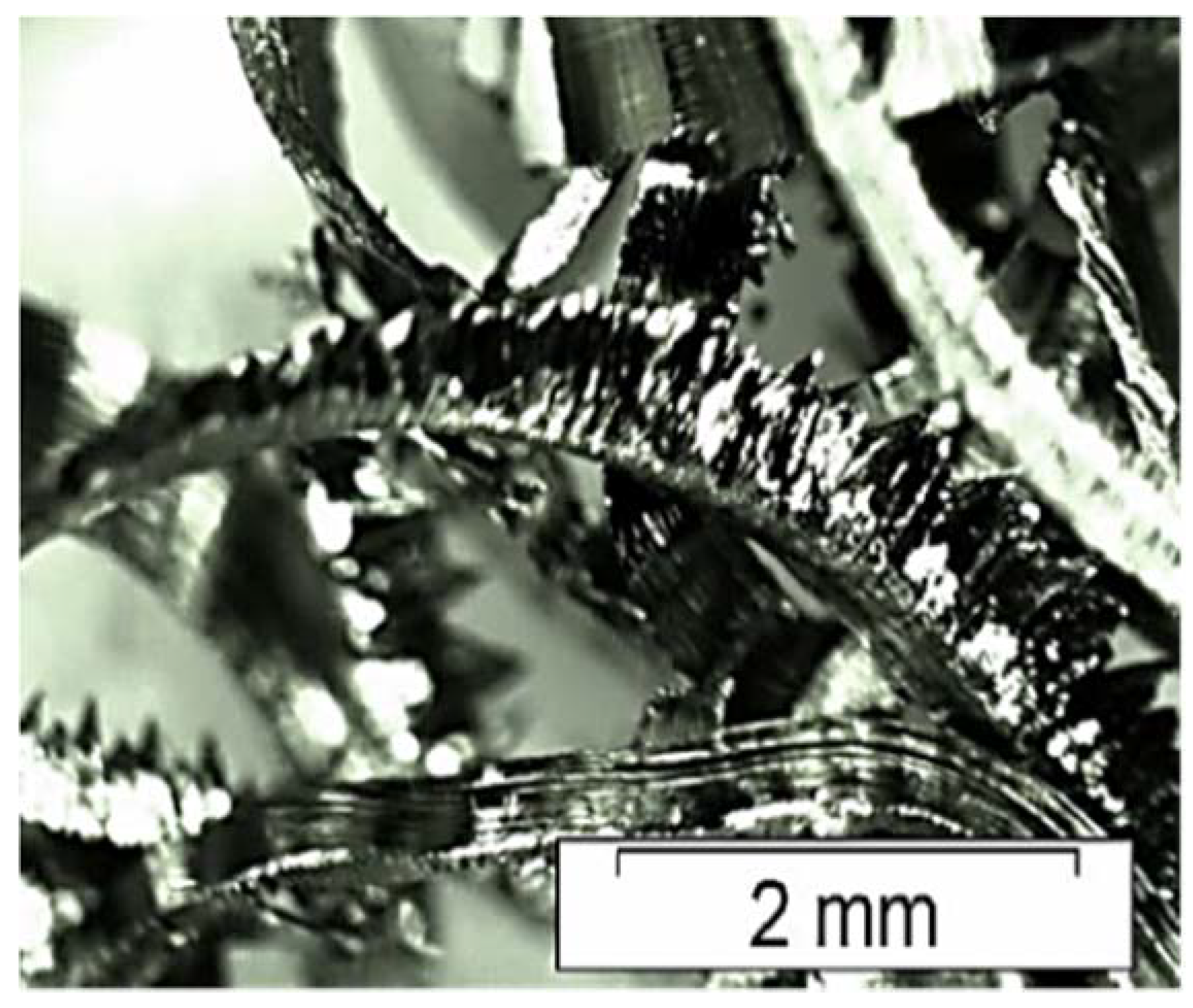

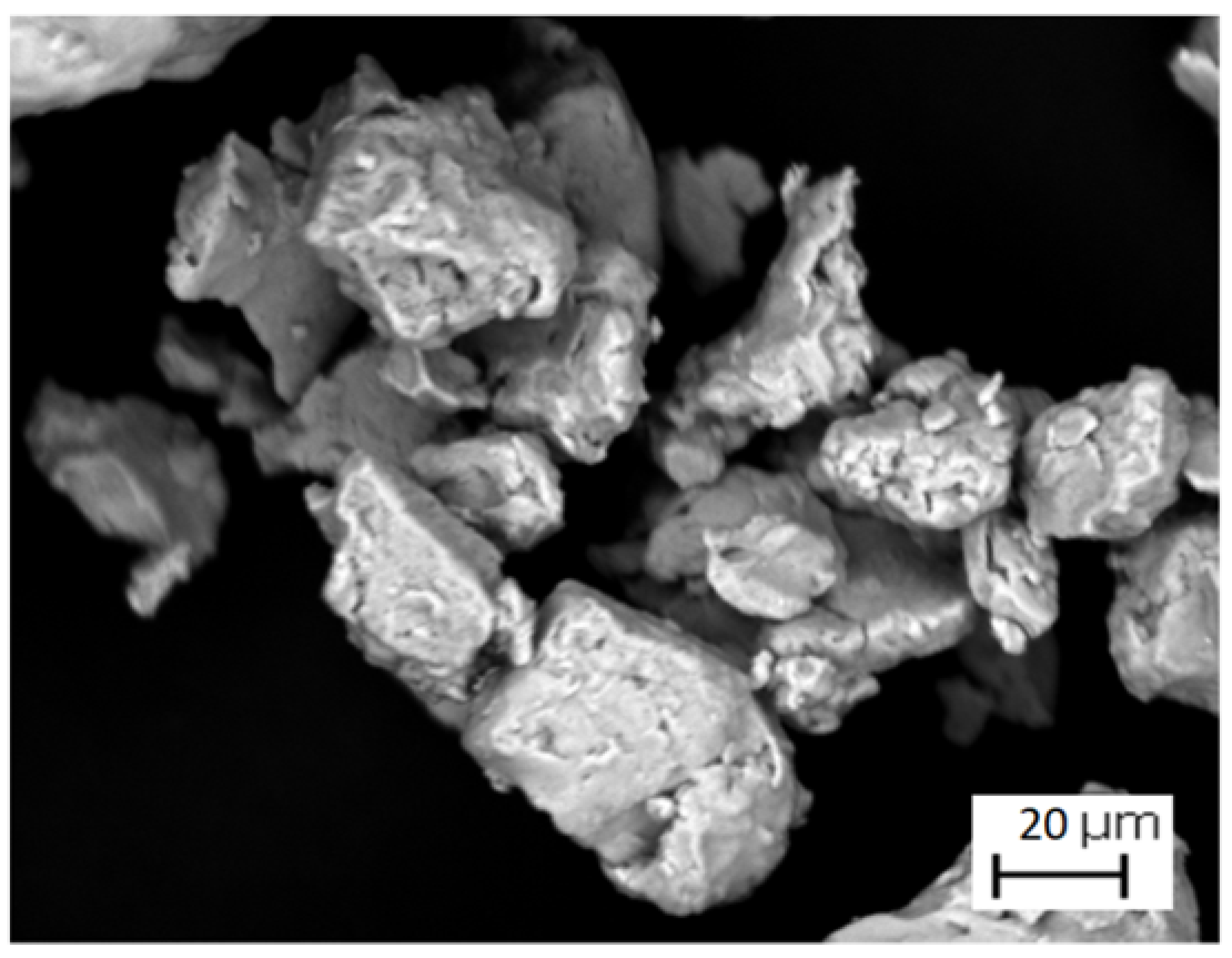

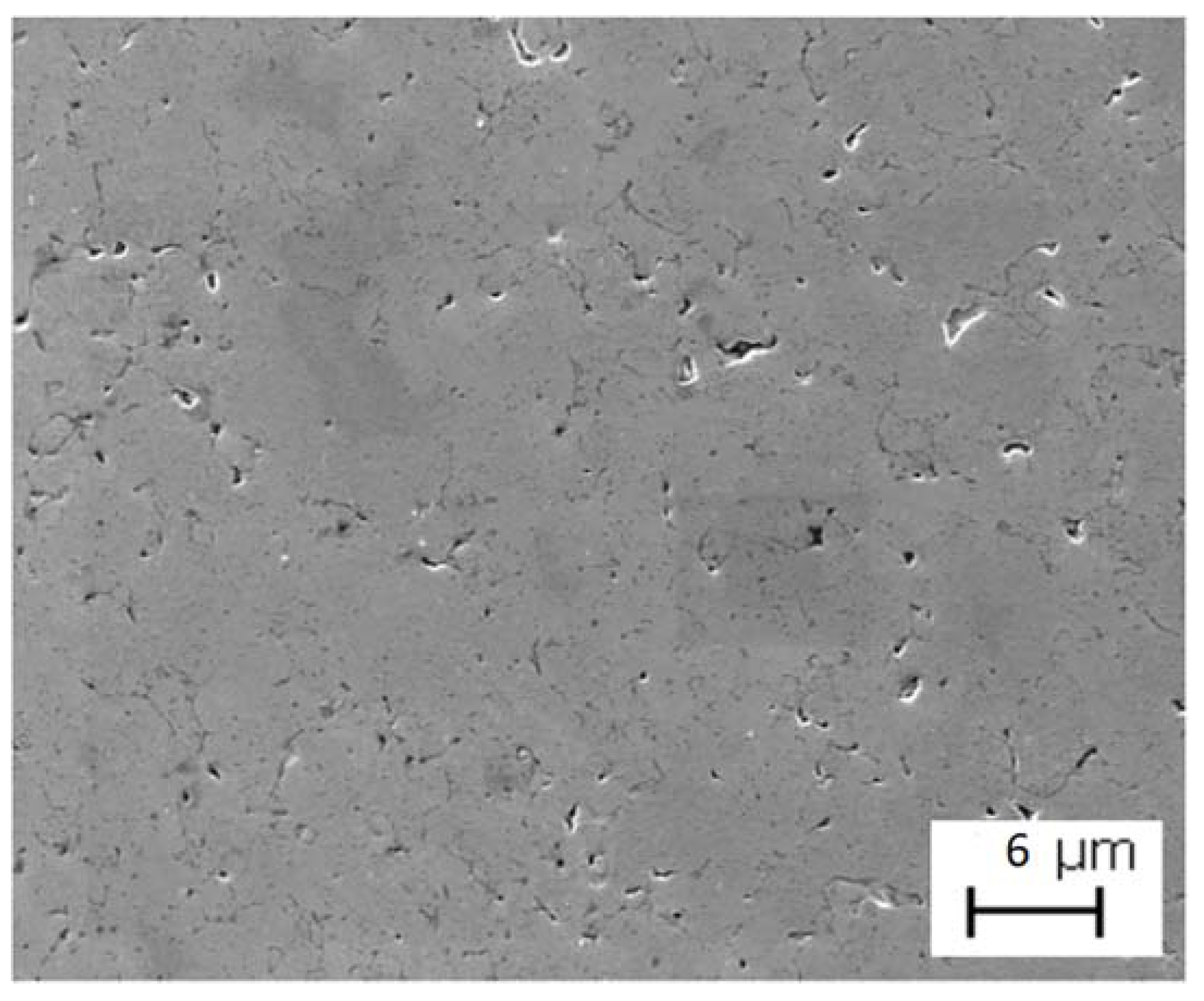
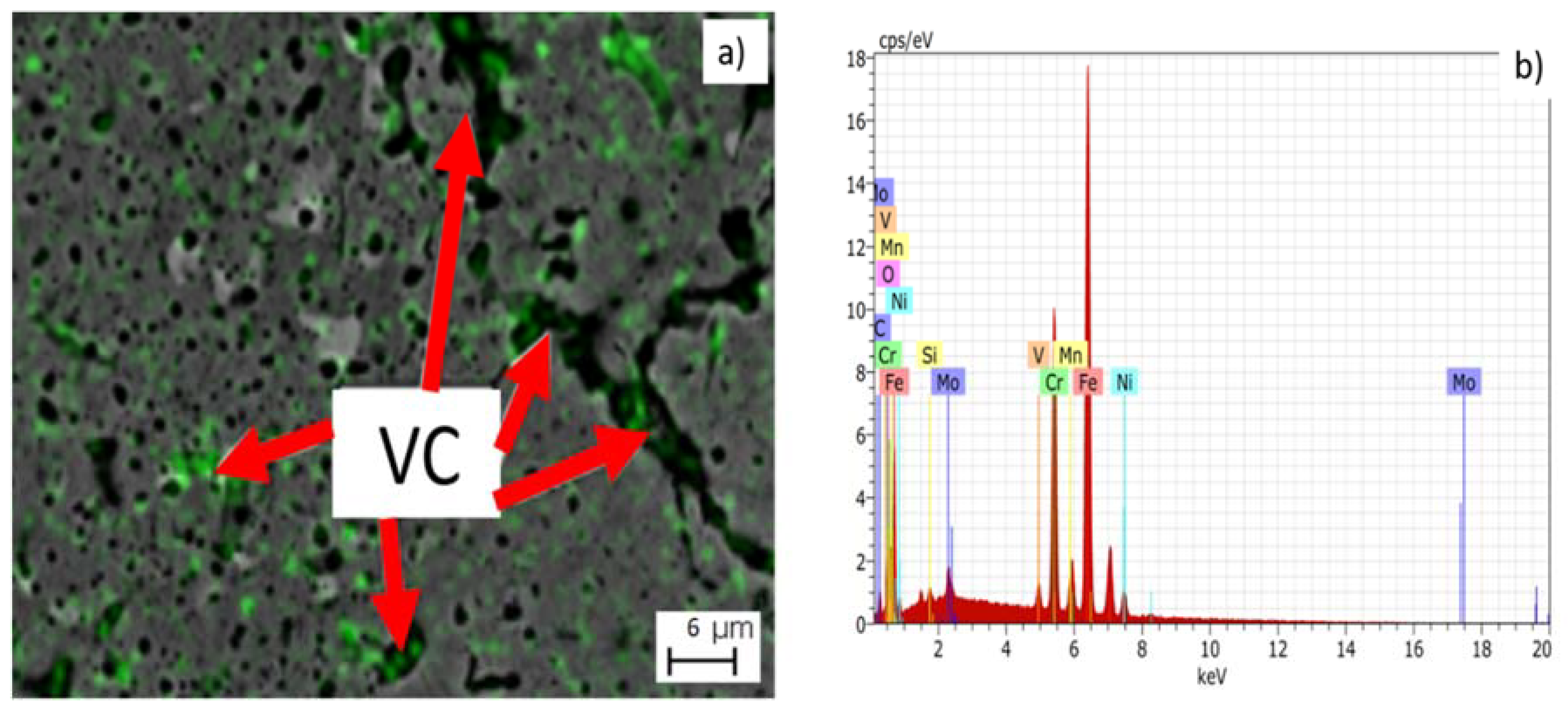



| Sample | Density in Green (g/cm3) | Density of Arquimedes (g/cm3) |
|---|---|---|
| Duplex stainless–steel/3 wt % VC | 5.08 ± 0.06 | 6.57 ± 0.08 |
| Condition | Microhardness (HV) |
|---|---|
| Sample sintering—1250 °C—1 h | 232 ± 8 |
| Material in condition as received | 265 ± 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claudiney, M.; Adhimar, O.; Daniela, S.; Patricia, C.; Vander, R.; Mateus, J.; Mirian, M.; Gilbert, S. A New Method to Recycle Stainless–Steel Duplex UNS S31803 Chips. Metals 2018, 8, 546. https://doi.org/10.3390/met8070546
Claudiney M, Adhimar O, Daniela S, Patricia C, Vander R, Mateus J, Mirian M, Gilbert S. A New Method to Recycle Stainless–Steel Duplex UNS S31803 Chips. Metals. 2018; 8(7):546. https://doi.org/10.3390/met8070546
Chicago/Turabian StyleClaudiney, Mendonça, Oliveira Adhimar, Sachs Daniela, Capellato Patricia, Ribeiro Vander, Junqueira Mateus, Melo Mirian, and Silva Gilbert. 2018. "A New Method to Recycle Stainless–Steel Duplex UNS S31803 Chips" Metals 8, no. 7: 546. https://doi.org/10.3390/met8070546






