Speciation of Ruthenium(III) Chloro Complexes in Hydrochloric Acid Solutions and Their Extraction Characteristics with an Amide-Containing Amine Compound
Abstract
:1. Introduction
2. Materials and Methods
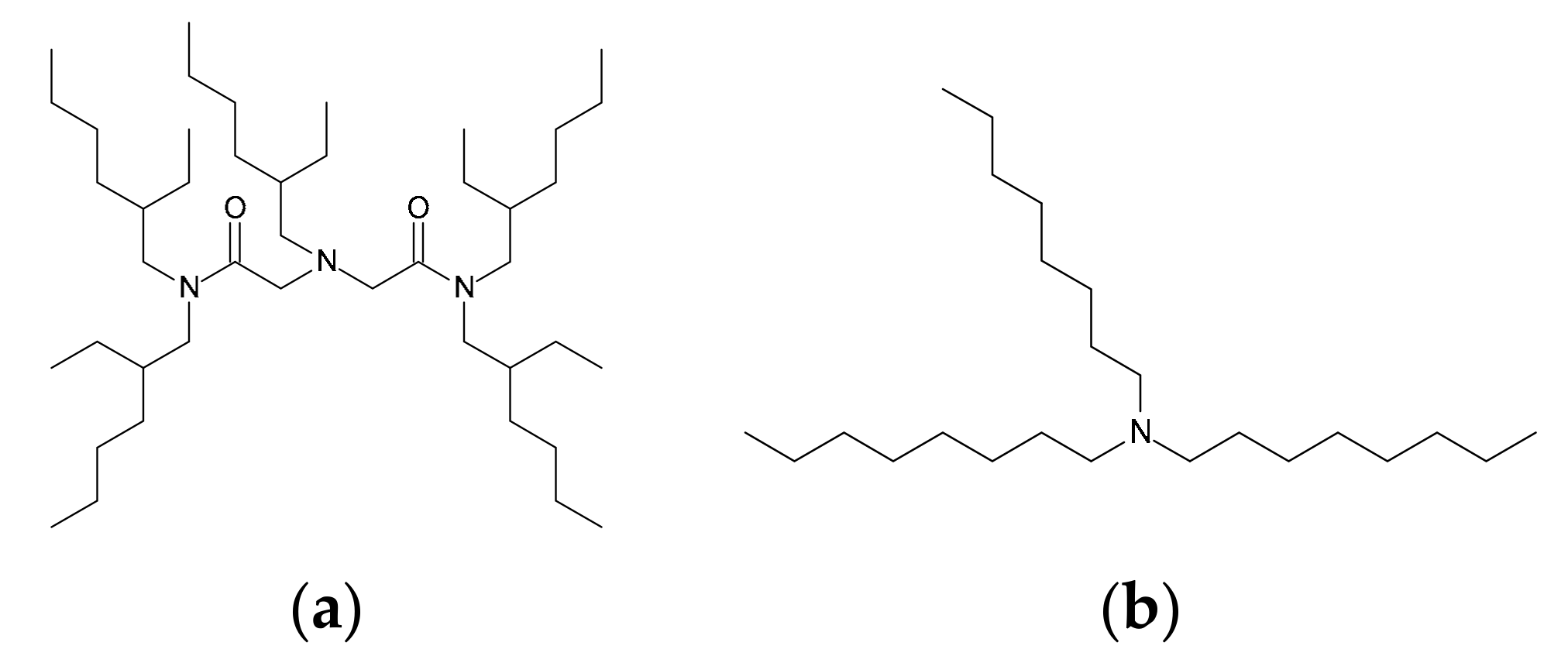
2.1. Reagents
2.2. Measurement of UV-Vis Absorption Spectra
2.3. Extraction Procedure
2.4. XAFS Measurements
2.5. Fourier-Transform Infrared (FT-IR) Spectroscopy Measurements
3. Results and Discussion
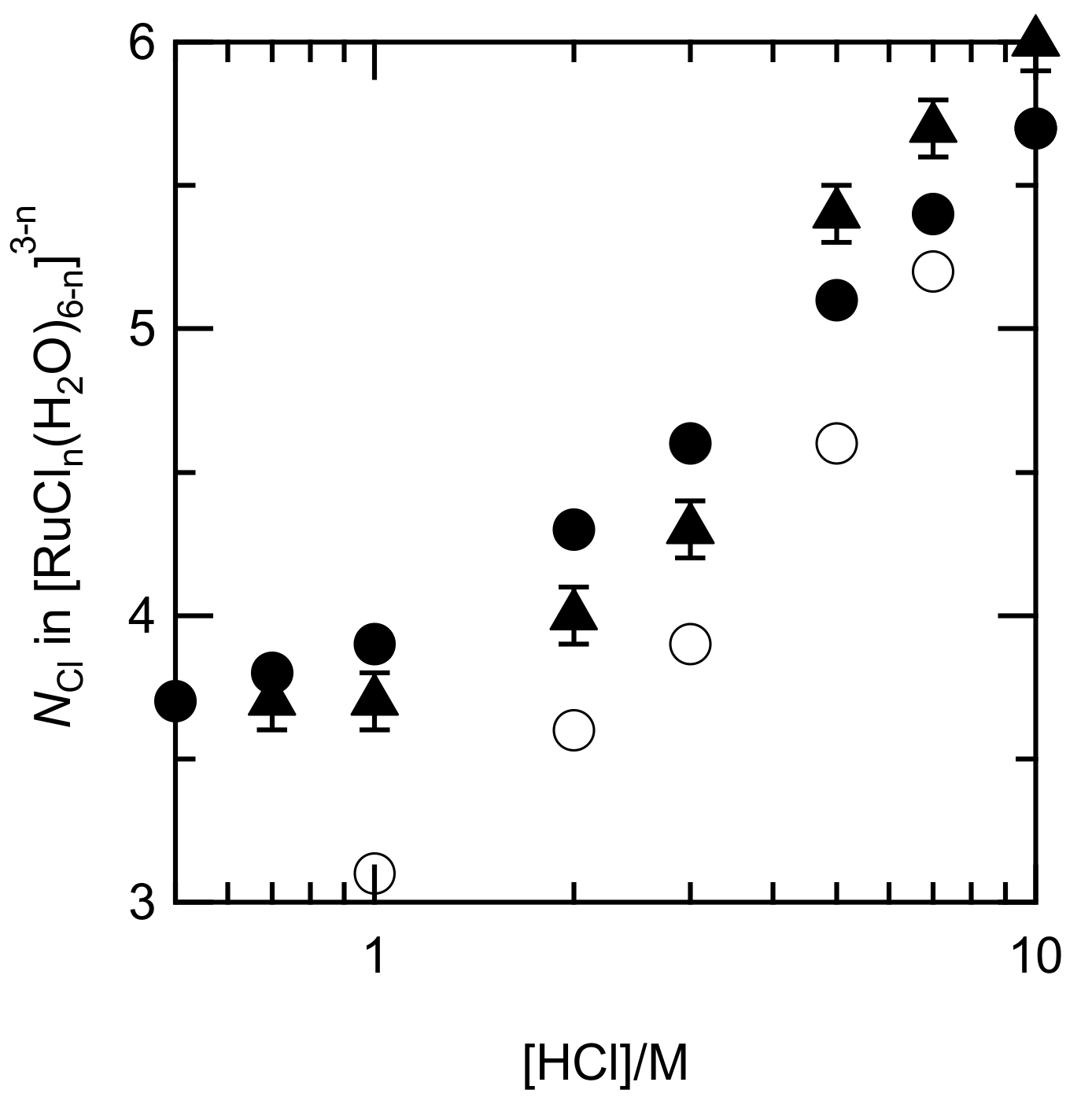
3.1. Analysis of Ru Species in Aqueous HCl
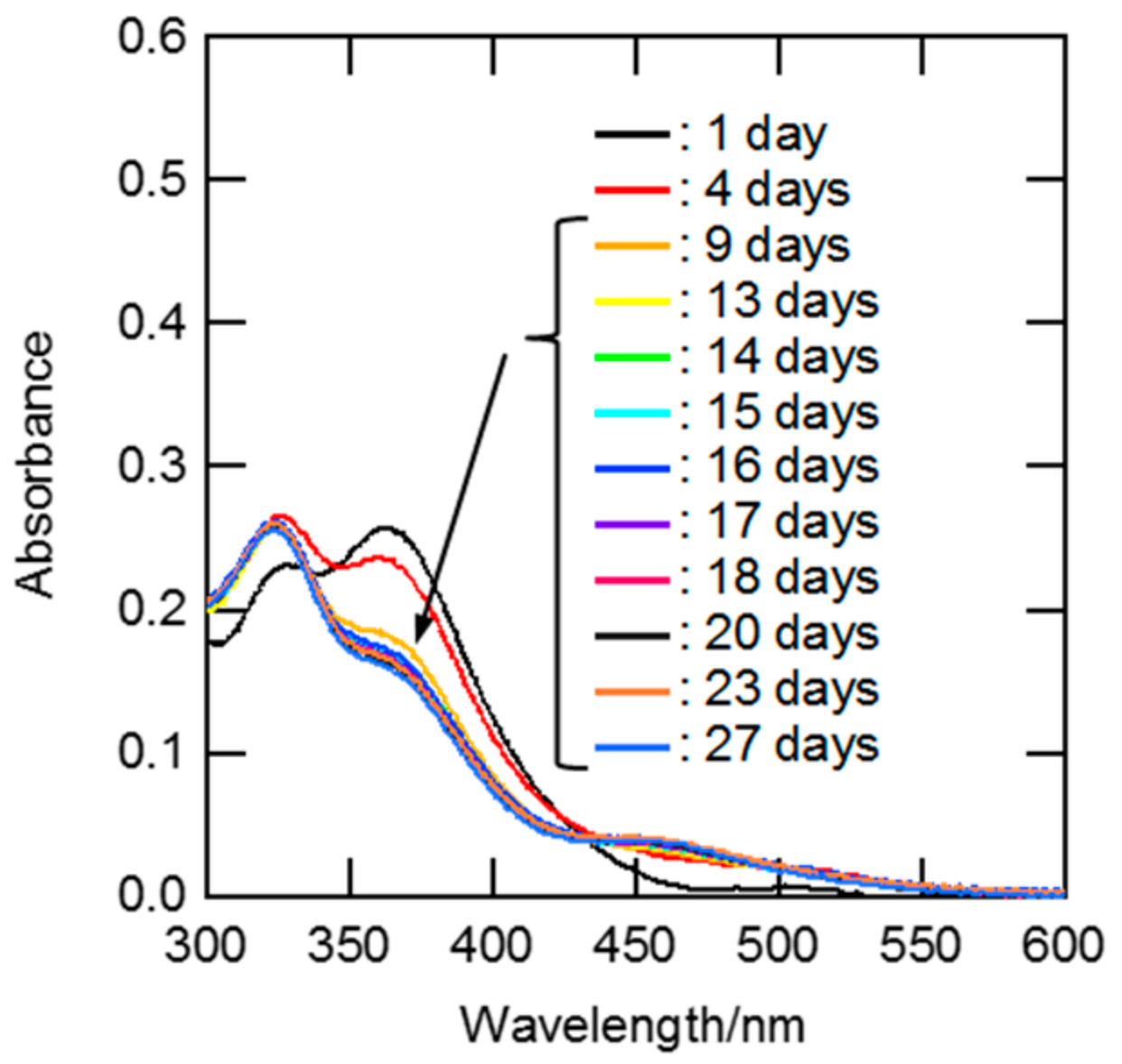
3.1.1. Estimation of Equilibration Time for Aquation/Chloridation of Ru
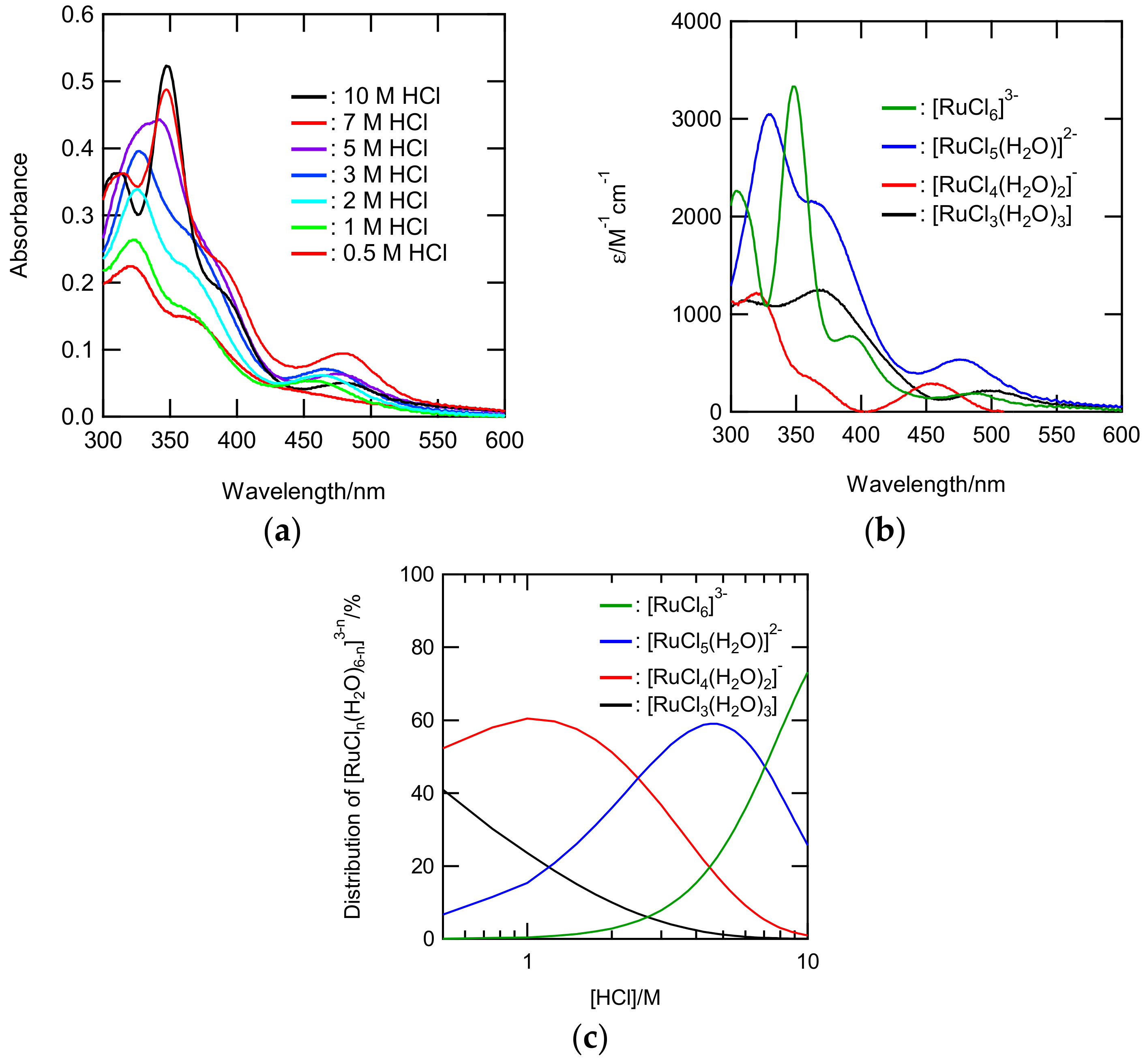
3.1.2. Speciation of Ru Species
3.2. Ru(III) Extraction Studies
3.2.1. Extraction Ability of EHBAA
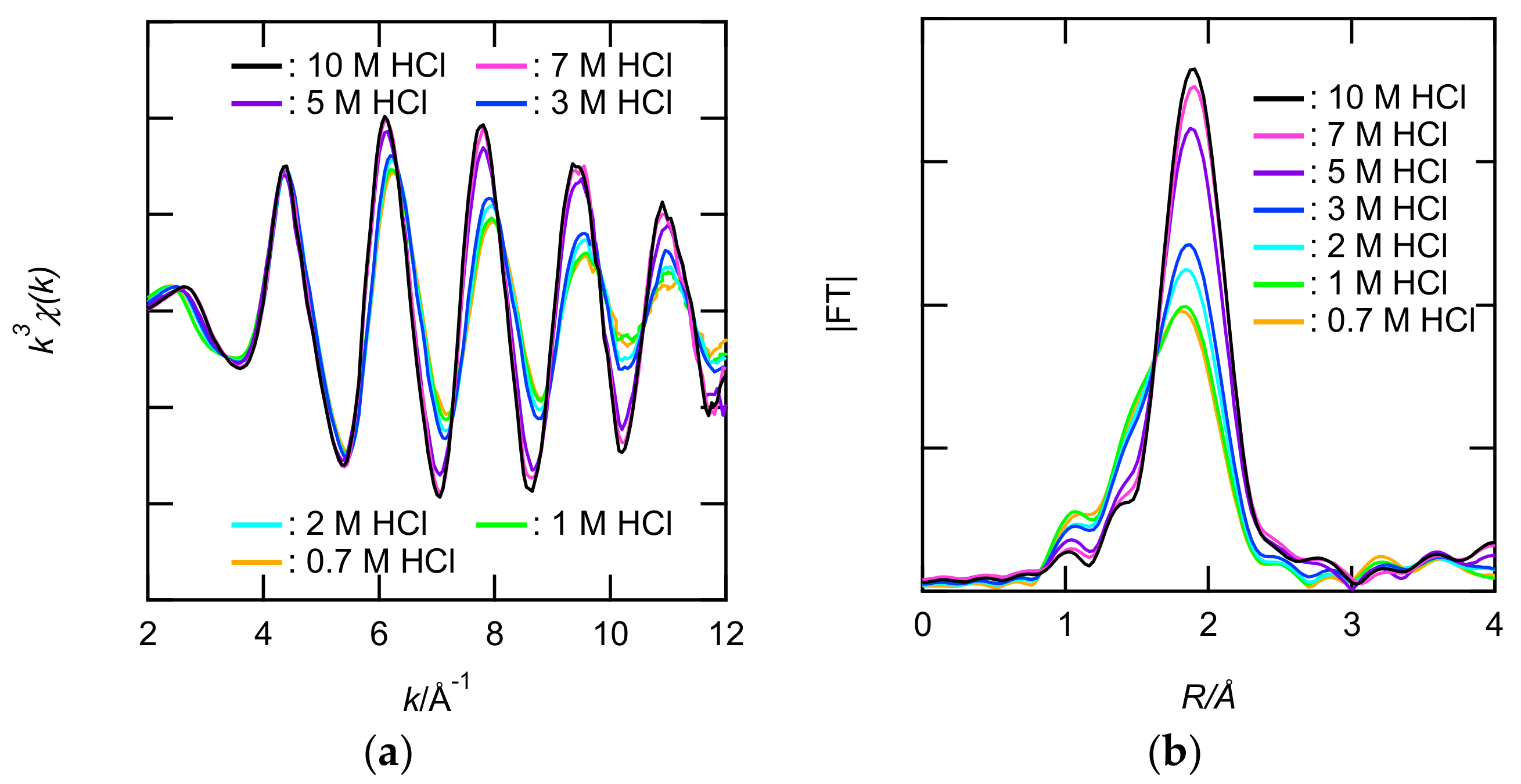
3.2.2. Structural Study of Ru Extracted by EHBAA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cox, M. Solvent Extraction in Hydrometallurgy. In Solvent Extraction Principles and Practice, 2nd ed.; Rydberg, J., Cox, M., Musikas, C., Choppin, G.R., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 455–506. ISBN 0-8247-5063-2. [Google Scholar]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A review of methods of separation of the platinum-group metals through their chloro-complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Rechnitz, G.A.; Goodkin, S.C. Some properties of ruthenium(III) and (IV) in acid solution. Platin. Met. Rev. 1963, 7, 25–29. [Google Scholar]
- Pantani, F. The behaviour of ruthenium trichloride in aqueous solutions. J. Less-Common Met. 1962, 4, 116–123. [Google Scholar] [CrossRef]
- Cady, H.H.; Connick, R.E. The determination of the formulas of aqueous ruthenium(III) species by means of ion-exchange resin: Ru+3, RuCl+2 and RuCl2+. J. Am. Chem. Soc. 1958, 80, 2646–2652. [Google Scholar] [CrossRef]
- Connick, R.E.; Fine, D.A. The identification of neutral ruthenium(III) chloride complexes: Equilibria involving neutral and cationic species. J. Am. Chem. Soc. 1960, 83, 3414–3416. [Google Scholar] [CrossRef]
- Taqui Khan, M.M.; Ramachandraiah, G.; Prakash, R. Ruthenium(III) chloride in aqueous solution: Electrochemical and spectral studies. Inorg. Chem. 1986, 25, 665–670. [Google Scholar] [CrossRef]
- Jorgensen, C.K. Complexes of the 4d- and 5d-groups II. Crystal field and electron transfer spectra of ruthenium(II) and (III), iridium(IV) and platinum(IV). Acta Chem. Scand. 1956, 10, 518–534. [Google Scholar] [CrossRef]
- Viljoen, K. Ruthenium(III) Aqua-Chloro Complex Chemistry: The Interconversion of the Hexachlororuthenate(III) and Aquapentachlororuthenate(III) Species. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, December 2003. [Google Scholar]
- Rard, J.A. Chemistry and thermodynamics of ruthenium and some of its inorganic compounds and aqueous species. Chem. Rev. 1985, 85, 1–39. [Google Scholar] [CrossRef]
- Narita, H.; Morisaku, K.; Tanaka, M. The first effective extractant for trivalent rhodium in hydrochloric acid solution. Chem. Commun. 2008, 5921–5923. [Google Scholar] [CrossRef] [PubMed]
- Narita, H.; Morisaku, K.; Tanaka, M. Highly efficient extraction of rhodium(III) from hydrochloric acid solution with amide-containing amine compounds. Solvent Extr. Ion Exch. 2015, 33, 407–417. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Lobo, V.M.M. Handbook of Electrolyte Solutions Part A; Elsevier: New York, NY, USA, 1989; pp. 515–572. ISBN 0-444-98847-5. [Google Scholar]
- Partanen, J.I.; Juusola, P.M.; Vahteristo, K.P.; de Mendonca, A.J.G. Re-evaluation of activity coefficients of aqueous hydrochloric acid solutions up to a molality of 16.0 mol·kg−1 using the Hückel and Pitzer equations at temperatures from 0 to 50 °C. J. Solut. Chem. 2007, 36, 39–59. [Google Scholar] [CrossRef]
- Uchikoshi, M. Determination of the distribution of cupric chloro-complexes in hydrochloric acid solutions at 298 K. J. Solut. Chem. 2017, 46, 704–719. [Google Scholar] [CrossRef]
- Kielland, J. Individual activity coefficients of ions in aqueous solutions. J. Am. Chem. Soc. 1937, 59, 1675–1678. [Google Scholar] [CrossRef]
- Mo, Y.; Antonio, M.R.; Scherson, D.A. In situ Ru K-edge X-ray absorption fine structure studies of electroprecipitated ruthenium dioxide films with relevance to supercapacitor applications. J. Phys. Chem. B 2000, 104, 9777–9779. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Rehr, J.J.; de Leon, J.M.; Zabinsky, S.I.; Albers, R.C. Theoretical X-ray absorption fine structure standards. J. Am. Chem. Soc. 1991, 113, 5135–5140. [Google Scholar] [CrossRef]
- Cotton, S.A. Chemistry of Precious Metals; Blackie Academic and Professional: London, UK, 1997; pp. 1–77. ISBN 0-7514-0413-6. [Google Scholar]
- Kahrovic, E.; Orioli, P.; Bruni, B.; Vaira, M.D.; Messori, L. Crystallographic evidence for decomposition of dimethylformamide in the presence of ruthenium(III) chloride. Inorg. Chim. Acta 2003, 355, 420–423. [Google Scholar] [CrossRef]
- Klementev, K.V. Statistical evaluations in fitting problems. J. Synchrotron Radiat. 2001, 8, 270–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taqui Khan, M.M.; Ramachandraiah, G.; Shukla, R.S. Ruthenium(III) chloride in aqueous solution: Kinetics of the aquation and anation reactions of the chloro complexes. Inorg. Chem. 1988, 27, 3274–3278. [Google Scholar] [CrossRef]
- Maeda, M.; Narita, H.; Tokoro, C.; Tanaka, M.; Motokawa, R.; Shiwaku, H.; Yaita, T. Selective extraction of Pt(IV) over Fe(III) from HCl with an amide-containing tertiary amine compound. Sep. Purif. Technol. 2017, 177, 176–181. [Google Scholar] [CrossRef]
- Bell, K.J.; Westra, A.N.; Warr, R.J.; Chartres, J.; Ellis, R.; Tong, C.C.; Blake, A.J.; Tasker, P.A.; Schröder, M. Outer-sphere coordination chemistry: Selective extraction and transport of the [PtCl6]2− Anion. Angew. Chem. Int. Ed. 2008, 47, 1745–1748. [Google Scholar] [CrossRef] [PubMed]

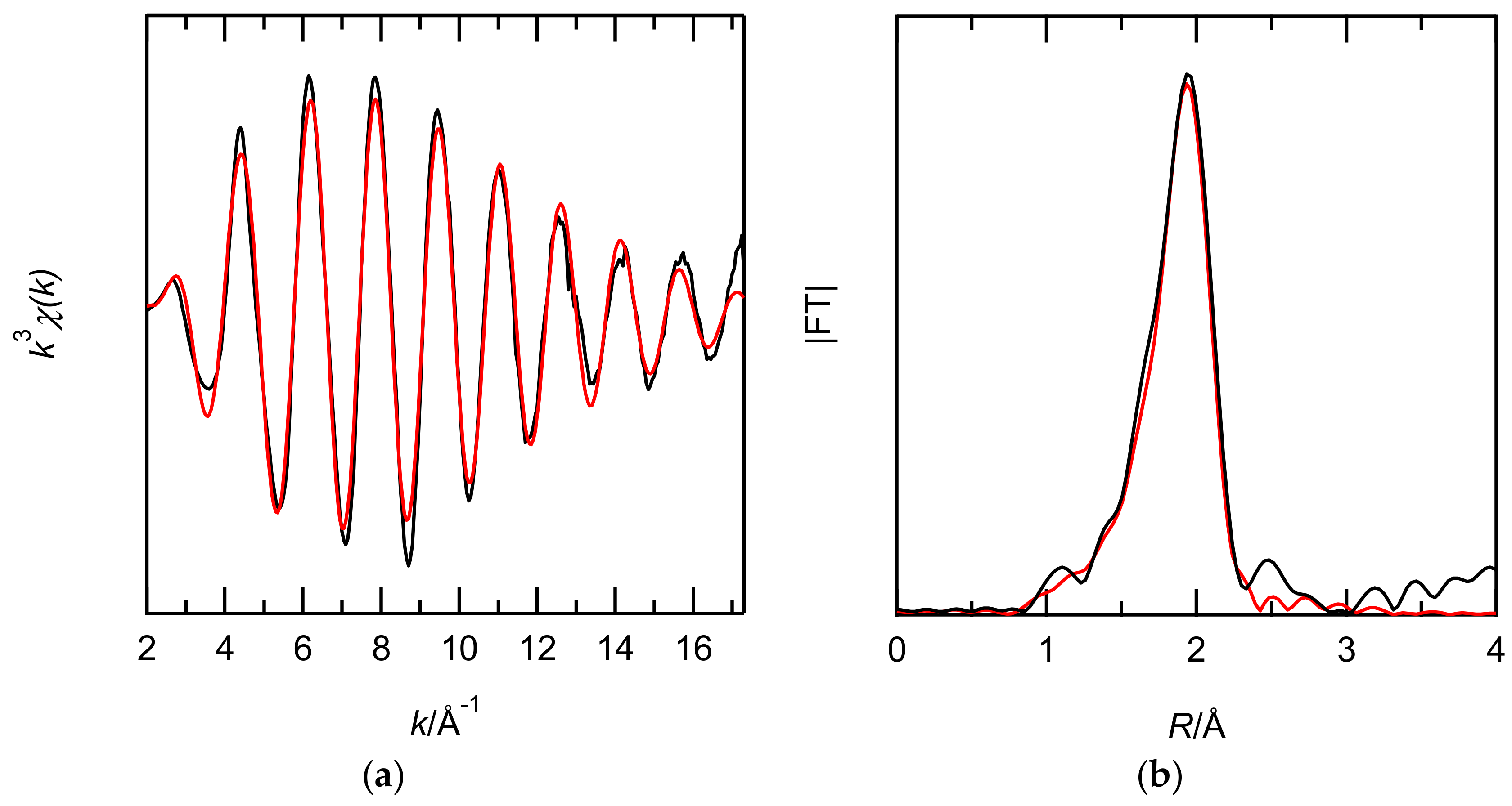
| HCl Conc. | Path | N a | R (Å) | σ2 (Å2)b | ΔE (eV)c | R-Factor |
|---|---|---|---|---|---|---|
| 0.7 M | Ru-Cl | 3.7(1) | 2.343(3) | 0.0039 d | –1.5 | 0.018 |
| Ru-O | 2.3(1) | 2.055(6) | 0.0014 d | |||
| 1.0 M | Ru-Cl | 3.7(1) | 2.342(3) | 0.0039 d | –1.8 | 0.018 |
| Ru-O | 2.3(1) | 2.056(6) | 0.0014(8) | |||
| 2.0 M | Ru-Cl | 4.0(1) | 2.350(3) | 0.0039 d | 0.1 | 0.011 |
| Ru-O | 2.0(1) | 2.065(7) | 0.0014 d | |||
| 3.0 M | Ru-Cl | 4.3(1) | 2.351(3) | 0.0039 d | 0.2 | 0.010 |
| Ru-O | 1.7(1) | 2.062(8) | 0.0014 d | |||
| 5.0 M | Ru-Cl | 5.4(1) | 2.367(2) | 0.0039 d | 0.7 | 0.007 |
| Ru-O | 0.6(1) | 2.10(1) | 0.0014 d | |||
| 7.0 M | Ru-Cl | 5.7(1) | 2.374(2) | 0.0039 d | 2.3 | 0.009 |
| Ru-O | 0.3(1) | 2.13(3) | 0.0014 d | |||
| 10 M | Ru-Cl | 6.0 | 2.371(2) | 0.0039(2) | 1.3 | 0.009 |
| [RuCl6]3– e | Ru-Cl | 6 | 2.378 g | |||
| [Ru(H2O)6]3+ f | Ru-O | 6 | 2.029 |
| Sample | Path | N a | R (Å) | σ2 (Å2) | ΔE (eV) | R-Factor |
|---|---|---|---|---|---|---|
| EHBAA phase | Ru-Cl | 4.7(2) | 2.370(3) | 0.0036(3) | 3.3 | 0.016 |
| Ru-O | 1.3(2) | 2.20(1) | 0.0010 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, T.; Ogata, T.; Tanaka, M.; Kobayashi, T.; Shiwaku, H.; Yaita, T.; Narita, H. Speciation of Ruthenium(III) Chloro Complexes in Hydrochloric Acid Solutions and Their Extraction Characteristics with an Amide-Containing Amine Compound. Metals 2018, 8, 558. https://doi.org/10.3390/met8070558
Suzuki T, Ogata T, Tanaka M, Kobayashi T, Shiwaku H, Yaita T, Narita H. Speciation of Ruthenium(III) Chloro Complexes in Hydrochloric Acid Solutions and Their Extraction Characteristics with an Amide-Containing Amine Compound. Metals. 2018; 8(7):558. https://doi.org/10.3390/met8070558
Chicago/Turabian StyleSuzuki, Tomoya, Takeshi Ogata, Mikiya Tanaka, Tohru Kobayashi, Hideaki Shiwaku, Tsuyoshi Yaita, and Hirokazu Narita. 2018. "Speciation of Ruthenium(III) Chloro Complexes in Hydrochloric Acid Solutions and Their Extraction Characteristics with an Amide-Containing Amine Compound" Metals 8, no. 7: 558. https://doi.org/10.3390/met8070558





