Recovery of Gold and Iron from Cyanide Tailings with a Combined Direct Reduction Roasting and Leaching Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Direct Reduction Roasting of Cyanide Tailings
2.3. Sulfuric Acid Leaching and Preparation of Ferrous Sulfate Crystals
2.4. Cyanidation Extraction of Gold from Acid-Leaching Residue
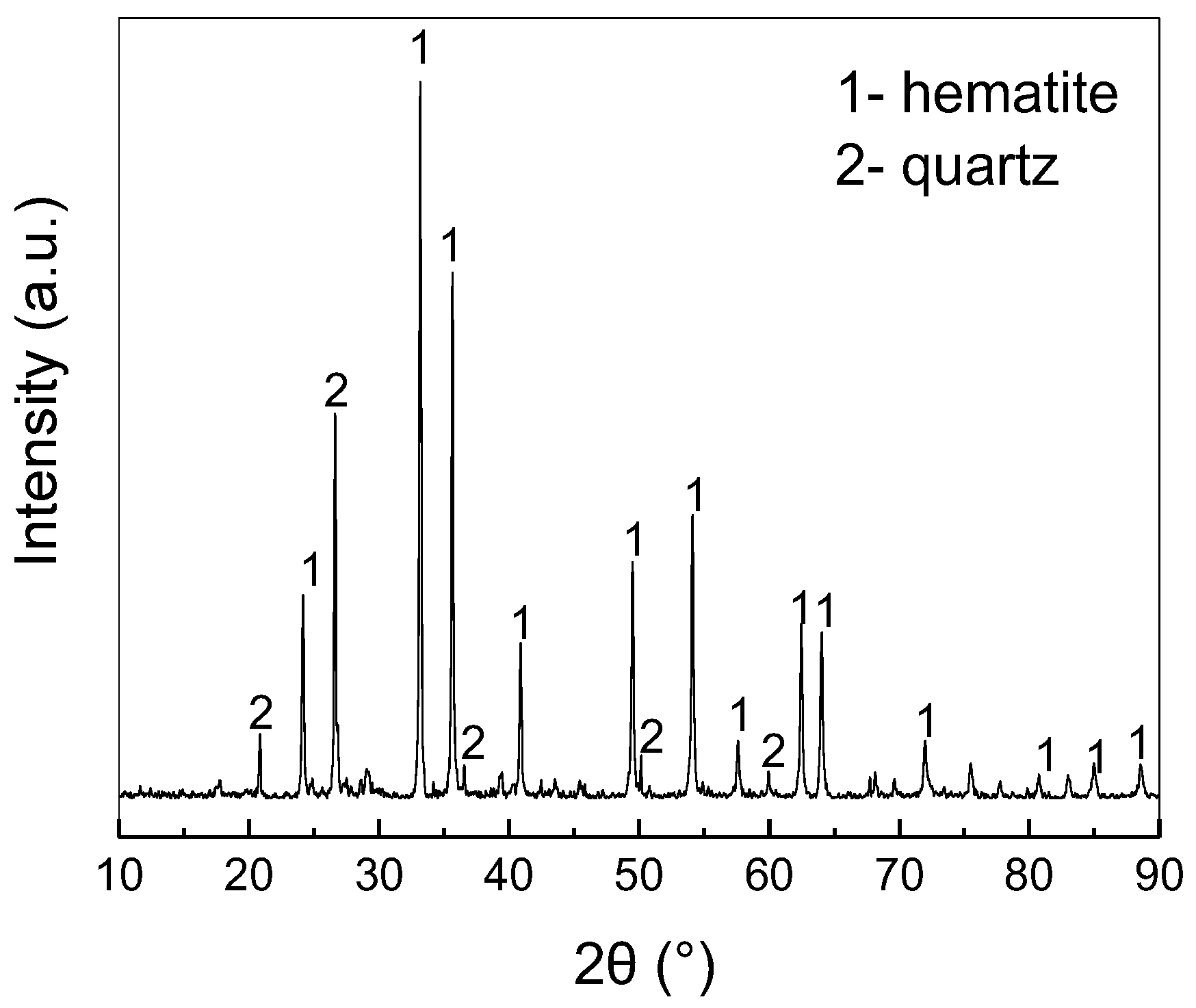
2.5. Analysis and Characterization
3. Results and Discussion
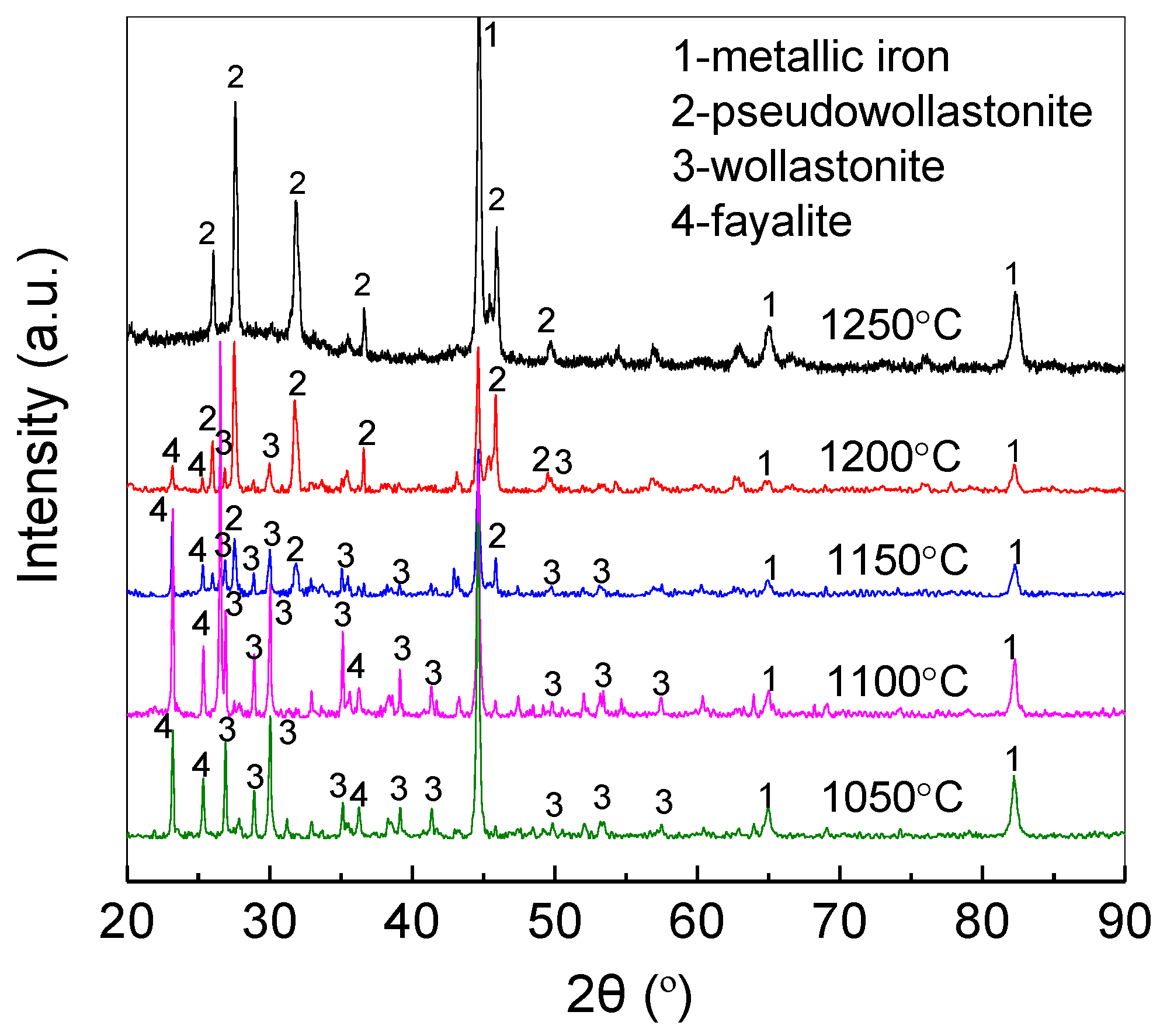
3.1. Recovery of Gold and Iron with Direct Reduction Roasting
3.1.1. Effect of Reduction Temperature
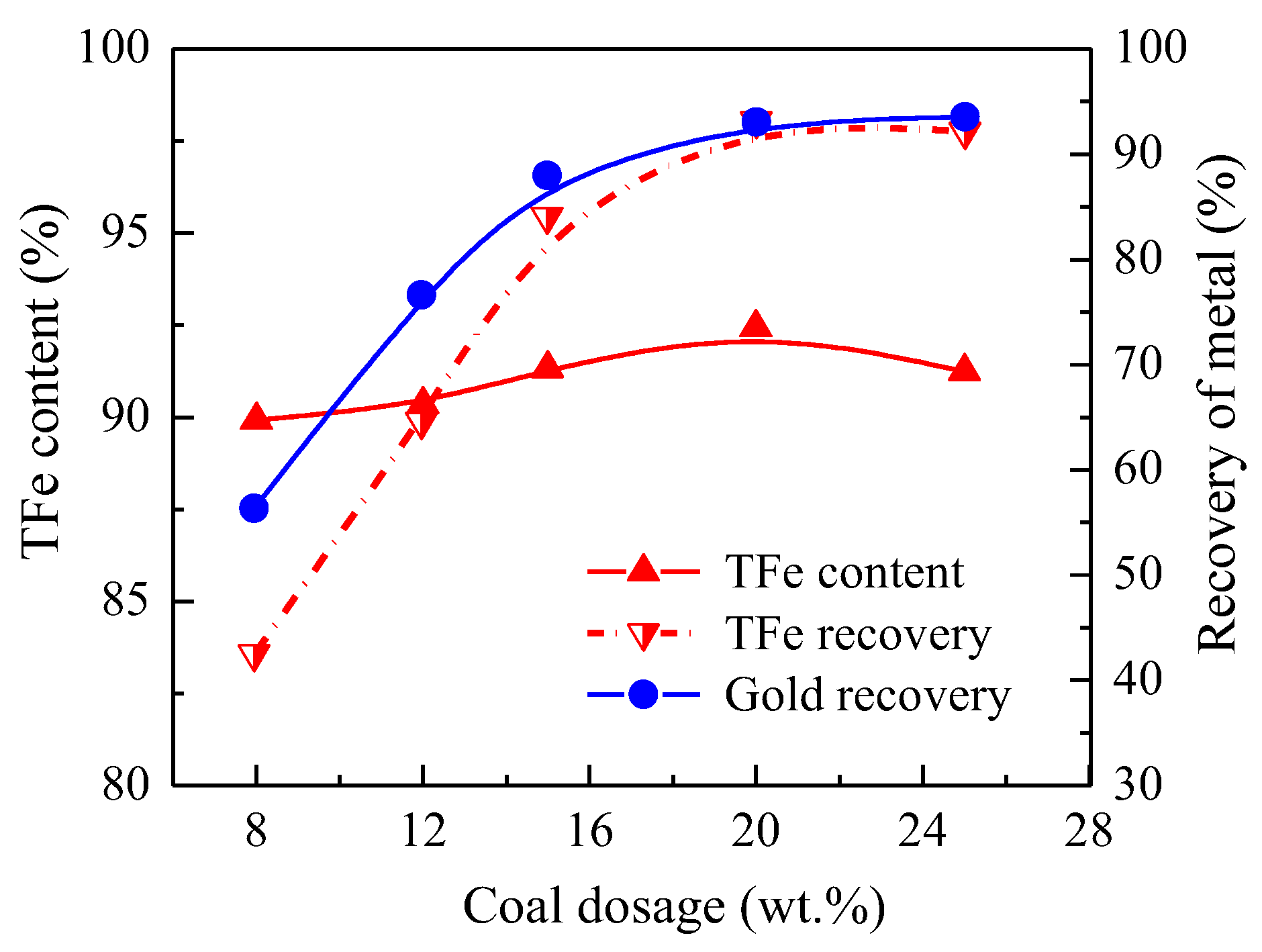
3.1.2. Effect of Coal Dosage
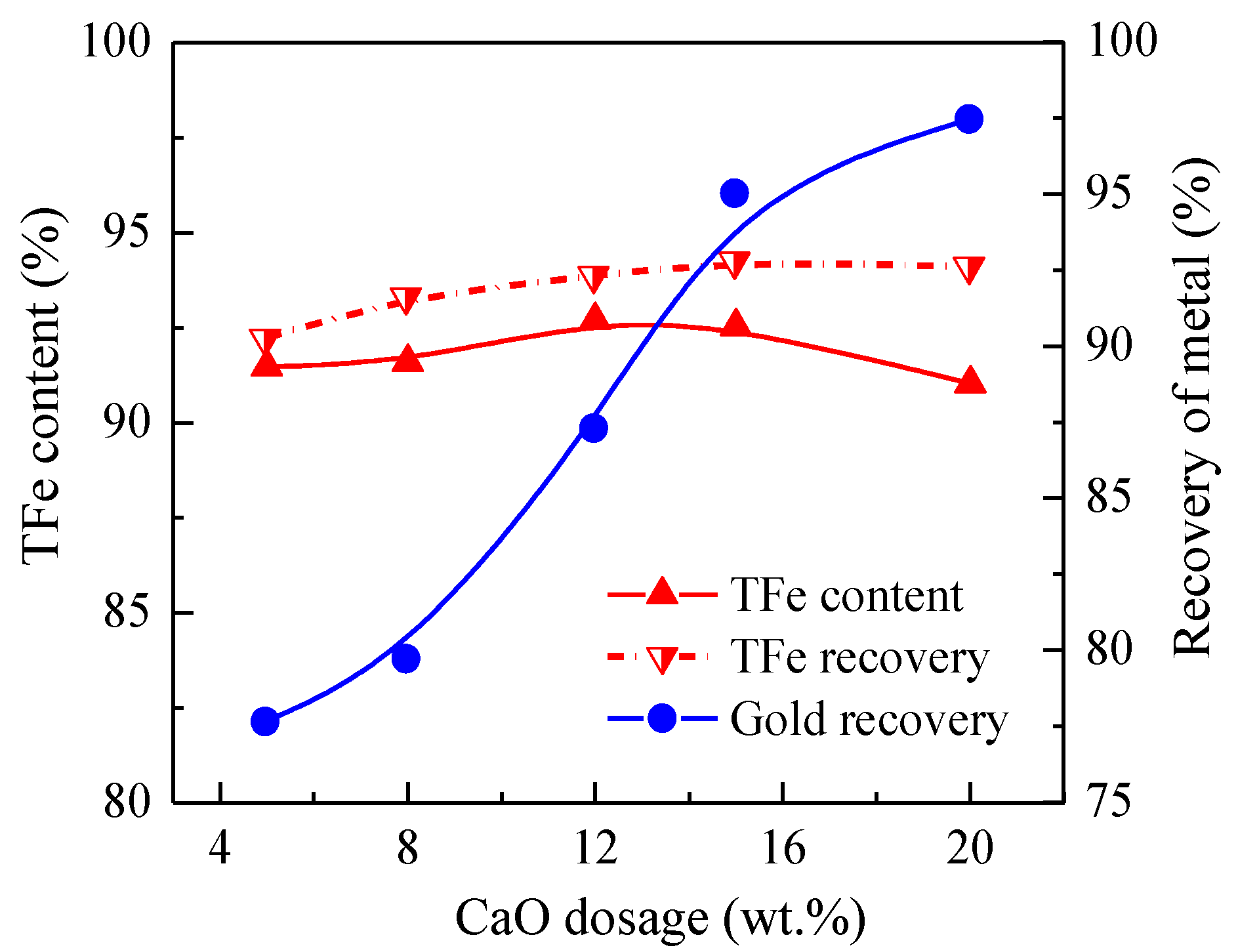
3.1.3. Effect of CaO Dosage
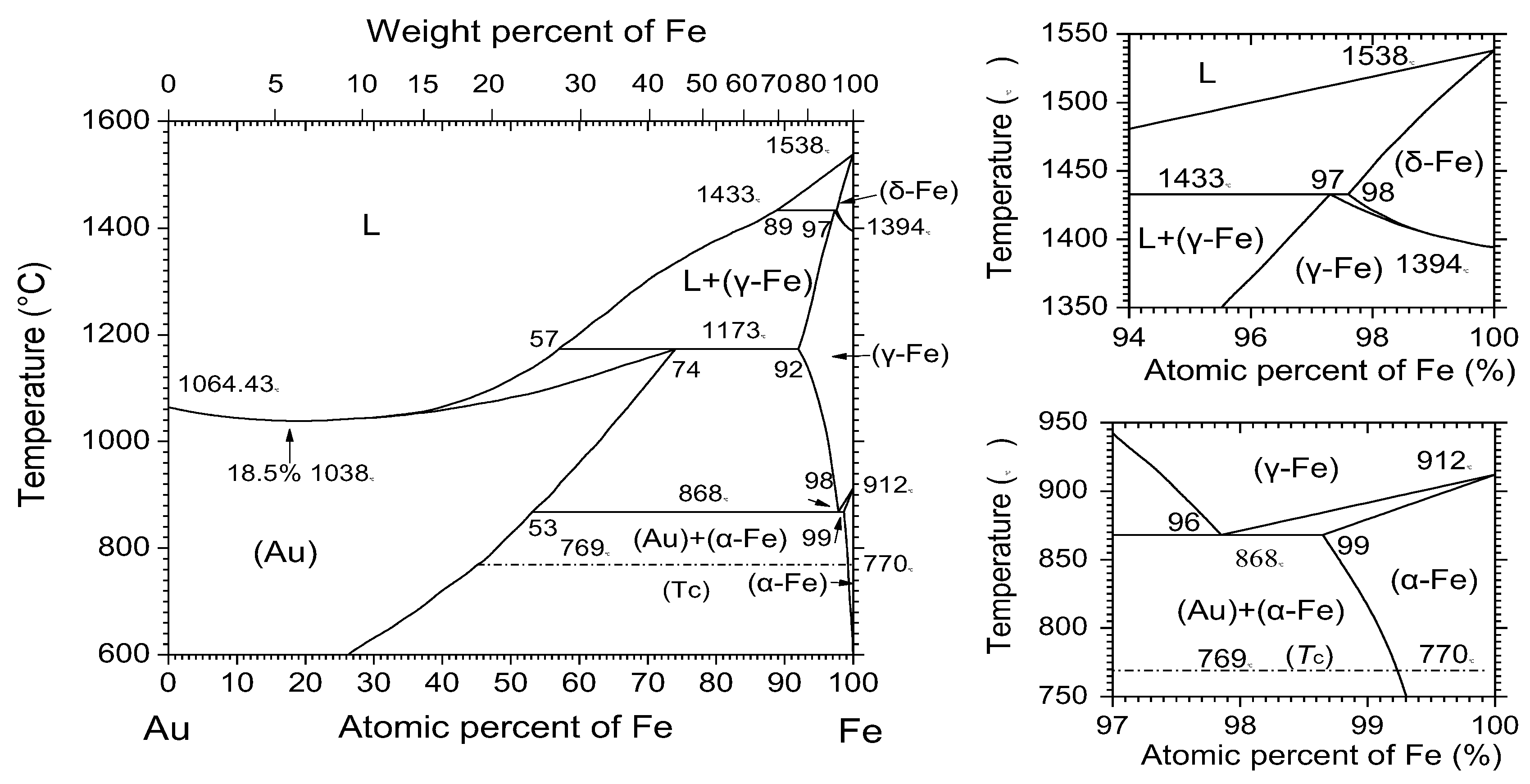
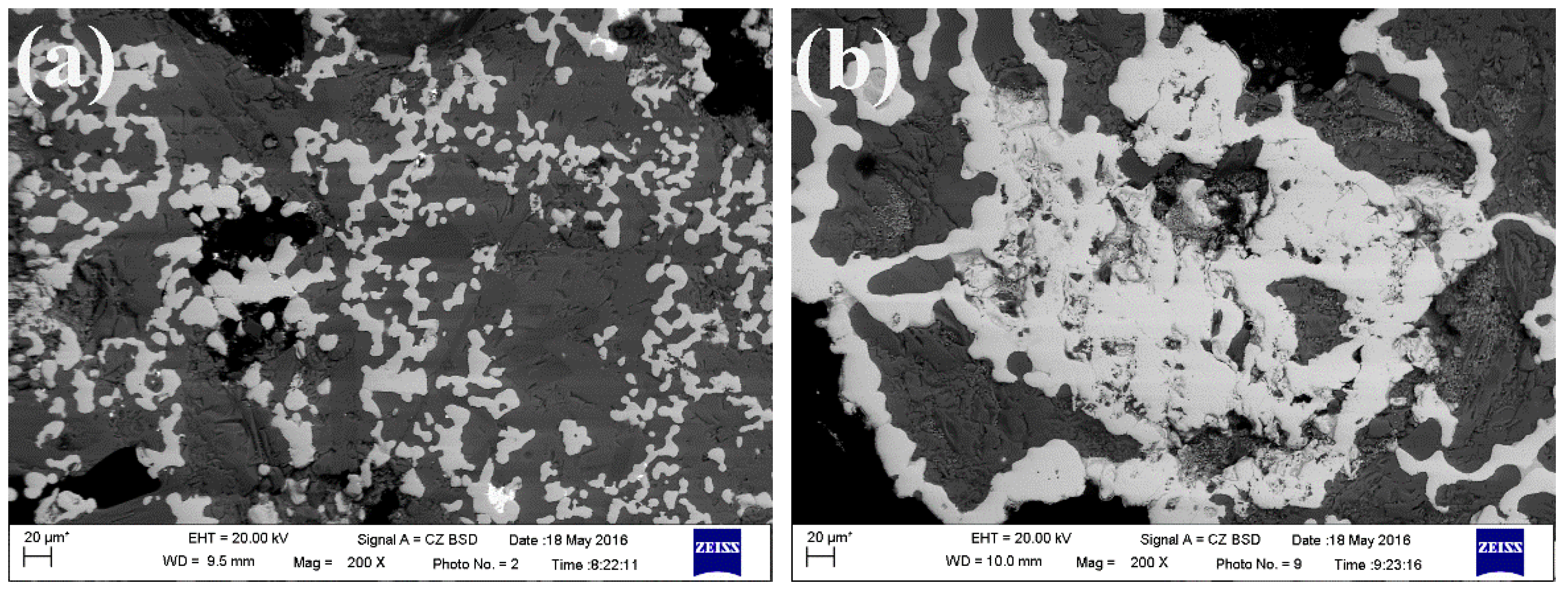
3.2. Proposed Mechanism for Enrichment of Gold from Cyanide Tailings
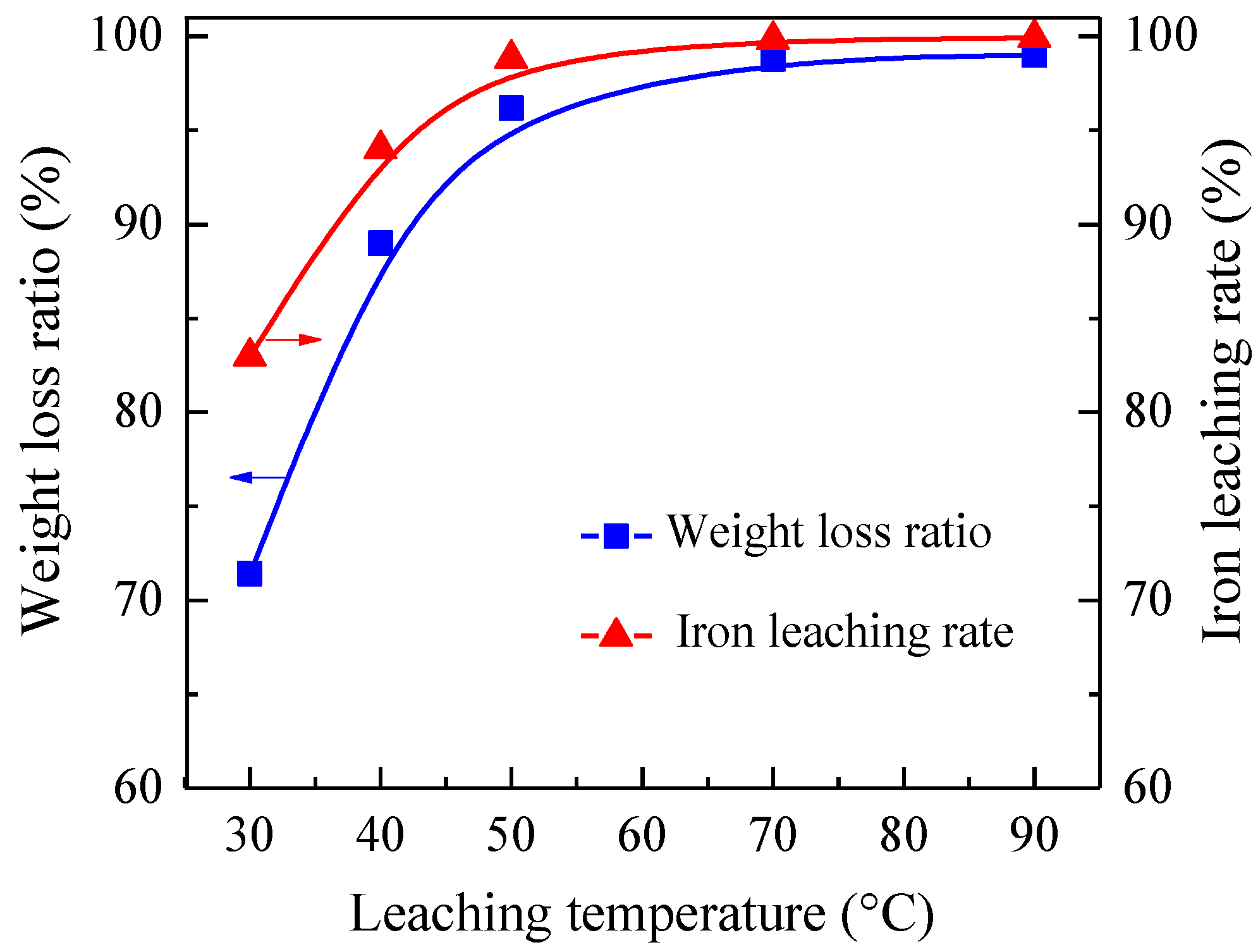
3.3. Sulfuric Acid Leaching of Gold Containing Iron Powders
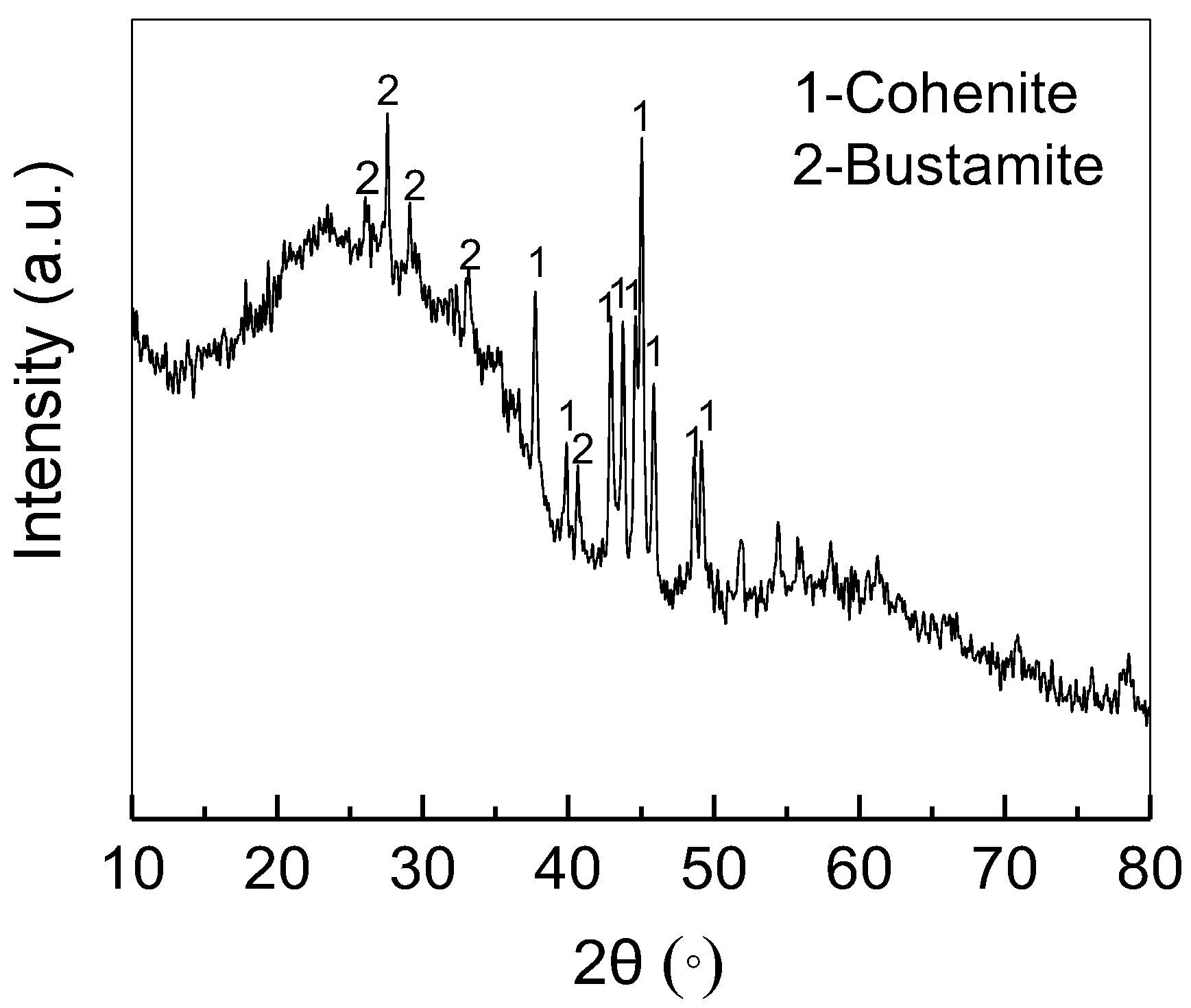
3.4. Recovery of Gold from Acid-Leaching Residue
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sayiner, B. Influence of lead nitrate on cyanide leaching of gold and silver from Turkish gold ores. Physicochem. Probl. Miner. Process. 2014, 50, 507–513. [Google Scholar] [CrossRef]
- Yazici, E.Y.; Ahlatci, F.; Koc, E.; Celep, O.; Deveci, H. Pre-treatment of a copper-rich gold ore for elimination of copper interference. In Proceedings of the European Metallurgical Conference, Weimar, Germany, 23–26 June 2016. [Google Scholar]
- Donato, D.B.; Nichols, O.; Possingham, H.; Moore, M.; Ricci, P.F.; Noller, B.N. A critical review of the effects of gold cyanide-bearing tailings solutions on wildlife. Environ. Int. 2007, 33, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Yang, Y.B.; Chen, L.J.; Li, Q.; Xu, B.; Jiang, T. Intensification behavior of mercury ions gold cyanide leaching. Metals 2018, 8, 80. [Google Scholar] [CrossRef]
- Clement, A.J.H.; Novakova, T.; Hudson-Edwards, K.A.; Fuller, I.C.; Macklin, M.G.; Fox, E.G.; Zapico, I. The environmental and geomorphological impacts of historical gold mining in the Ohinemuri and Waihou river catchments, Coromandel, New Zealand. Geomorphology 2017, 295, 159–175. [Google Scholar] [CrossRef]
- Koohestani, B.; Darban, A.K.; Darezereshki, E.; Mokhtari, P.; Yilmaz, E.; Yilmaz, E. The influence of sodium and sulfate ions on total solidification and encapsulation potential of iron-rich acid mine drainage in silica gel. J. Environ. Chem. Eng. 2018, 6, 3520–3527. [Google Scholar] [CrossRef]
- Yilmaz, E.; Ahlatci, F.; Yazici, E.Y.; Celep, O.; Deveci, H. Passivation of pyritic tailings and mitigation of its acid mine drainage (AMD) potential. In Proceedings of the International Symposium on Mining and Environment, Mugla, Turkey, 27–29 September 2017. [Google Scholar]
- Zhang, Y.L.; Li, H.M.; Yu, X.J. Recovery of iron from cyanide tailings with reduction roasting-water leaching followed by magnetic separation. J. Hazard. Mater. 2012, 213-214, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Zhang, Z.H.; Li, L.B.; Wang, Y.J. Recovery of gold and iron from the cyanide tailings by magnetic roasting. Rare. Metal. Mater. Eng. 2013, 42, 1805–1809. [Google Scholar]
- Pilśniak-Rabiega, M.; Trochimczuk, A.W. Selective recovery of gold on functionalized resins. Hydrometallurgy 2014, 146, 111–118. [Google Scholar] [CrossRef]
- De Andrade Lima, L.R.P.; Bernardez, L.A.; Barbosa, L.A.D. Characterization and treatment of artisanal gold mine tailings. J. Hazard. Mater. 2008, 150, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Syed, S. Recovery of gold from secondary sources—A review. Hydrometallurgy 2012, 115–116, 30–51. [Google Scholar] [CrossRef]
- Koc, E.; Ahlatci, F.; Kuzu, M.; Yazici, E.Y.; Celep, O.; Deveci, H. Recovery of silver from cyanide leach solutions of a pyritic gold concentrate by sodium sulphide precipitation. In Proceedings of the 15th International Mineral Processing Symposium and Exhibition, Istanbul, Turkey, 11–13 June 2016. [Google Scholar]
- Yazici, E.Y.; Yilmaz, E.; Ahlatci, F.; Celep, O.; Deveci, H. Recovery of silver from cyanide leach solutions by precipitation using Trimercapto-s-triazine (TMT). Hydrometallurgy 2016, 174, 175–183. [Google Scholar] [CrossRef]
- Ilankoon, I.M.S.K.; Tang, Y.; Ghorbani, Y.; Northey, S.; Yellishetty, M.; Deng, X.Y.; McBride, D. The current state and future directions of percolation leaching in the Chinese mining industry: Challenges and opportunities. Miner. Eng. 2018, 125, 206–222. [Google Scholar] [CrossRef]
- Dehghani, A.; Ostad-rahimi, M.; Mojtahedzadeh, S.H.; Gharibi, K.K. Recovery of gold from the mouteh gold mine tailings dam. J. South African Inst. Min. Metall. 2009, 109, 417–421. [Google Scholar]
- Lv, C.C.; Ding, J.; Qian, P.; Li, Q.C.; Ye, S.F.; Chen, Y.F. Comprehensive recovery of metals from cyanidation tailing. Miner. Eng. 2015, 70, 141–147. [Google Scholar] [CrossRef]
- Yang, X.L.; Huang, X.; Qiu, T.S. Recovery of zinc from cyanide tailings by flotation. Miner. Eng. 2015, 84, 100–105. [Google Scholar] [CrossRef]
- Li, D.X.; Gao, G.L.; Meng, F.L.; Ji, C. Preparation of nano-iron oxide red pigment powders by use of cyanided tailings. J. Hazard. Mater. 2008, 155, 369–377. [Google Scholar] [CrossRef]
- Bas, A.D.; Safizadeh, F.; Ghali, E.; Choi, Y. Leaching and electrochemical dissolution of gold in the presence of iron oxide minerals associated with roasted gold ore. Hydrometallurgy 2016, 166, 143–153. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, W.W.; Yue, K.; Chen, M.X. High-temperature chlorination of gold with transformation of iron phase. Rare Met. 2016, 35, 881–886. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhang, L.B.; Koppala, S.; Ma, A.Y.; Peng, J.H.; Li, S.W.; Yin, S.H. Extraction of gold and silver in the selective chlorination roasting process of cyanidation tailing. Sep. Sci. Technol. 2018, 53, 458–466. [Google Scholar] [CrossRef]
- Okamoto, H.; Massalski, T.B.; Swartzendruber, L.J.; Beck, P.A. The Au-Fe (gold-iron) system. Bull. Alloy Phase Diagrams 1984, 5, 592–601. [Google Scholar] [CrossRef]
- Bansal, C.; Sarkar, S.; Mishra, A.K.; Abraham, T.; Lemier, C.; Hahn, H. Electronically tunable conductivity of a nanoporous Au–Fe alloy. Scr. Mater. 2007, 56, 705–708. [Google Scholar] [CrossRef]
- Lee, Y.P.; Kudryavtsev, Y.V.; Nemoshkalenko, V.V.; Gontarz, R.; Rhee, J.Y. Magneto-optical and optical properties of Fe-rich Au-Fe alloy films near the fcc-bcc structural transformation region. Phys. Rev. B 2003, 67, 104424. [Google Scholar] [CrossRef]
- Konieczny, R.; Idczak, R.; Chojcan, J. A study of thermodynamic properties of dilute Fe-Au alloys by the 57Fe Mössbauer Spectroscopy. Acta Phys. Pol. A 2017, 131, 255–258. [Google Scholar] [CrossRef]
- Kumar, P.S.M.; Sivakumar, T.; Fujita, T.; Jayavel, R.; Abe, H. Synthesis of metastable Au-Fe alloy using ordered nanoporous silica as a hard template. Metals 2018, 8, 17. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Huang, Q.Y.; Lv, X.W.; Bai, C.G. Multistage utilization process for the gradient-recovery of V, Fe, and Ti from vanadium-bearing converter slag. J. Hazard. Mater. 2017, 336, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.F.; Jing, L.L.; Zhang, B.G. Recovery of iron from vanadium tailings with coal-based direct reduction followed by magnetic separation. J. Hazard. Mater. 2011, 185, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.C.; Sun, T.C.; Xue, X.; Liu, Z.H. Iron recovery from discarded copper slag in a RHF direct reduction and subsequent grinding/magnetic separation process. Minerals 2016, 6, 119. [Google Scholar] [CrossRef]
- Zhang, B.J.; Niu, L.P.; Zhang, T.G.; Li, Z.Q.; Zhang, D.L.; Zheng, C. Alternative reduction of copper matte in reduction process of copper slag. ISIJ Int. 2017, 57, 775–781. [Google Scholar] [CrossRef]
- Li, W.F.; Zhan, J.; Fan, Y.Q.; Wei, C.; Zhang, C.F.; Hwang, J.Y. Research and industrial application of a process for direct reduction of molten high-lead smelting slag. JOM 2017, 69, 784–789. [Google Scholar] [CrossRef]
- Xu, B.; Kong, W.H.; Li, Q.; Yang, Y.B.; Jiang, T.; Liu, X.L. A review of thiosulfate leaching of gold: Focus on thiosulfate consumption and gold recovery from pregnant solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Ahtiainen, R.; Lundstrom, M. Preg-robbing of gold in chloride-bromide solution. Physicochem. Probl. Miner. Process. 2016, 52, 244–251. [Google Scholar] [CrossRef]
- Liu, G.S.; Strezov, V.; Lucas, J.A.; Wibberley, L.J. Thermal investigations of direct iron ore reduction with coal. Thermochim. Acta 2004, 410, 133–140. [Google Scholar] [CrossRef]
- Li, Y.L.; Sun, T.C.; Kou, J.; Guo, Q.; Xu, C.Y. Study on phosphorus removal of high-phosphorus oolitic hematite by coal-based direct reduction and magnetic separation. Min. Proc. Ext. Met. Rev. 2014, 35, 66–73. [Google Scholar] [CrossRef]
- Han, H.; Duan, D.; Yuan, P.; Chen, S. Recovery of metallic iron from high phosphorous oolitic hematite by carbothermic reduction and magnetic separation. Ironmak. Steelmak. 2015, 42, 542–547. [Google Scholar] [CrossRef]
- Yu, W.; Tang, Q.Y.; Chen, J.A.; Sun, T.C. Thermodynamic analysis of the carbothermic reduction of a high-phosphorus oolitic iron ore by FactSage. Int. J. Min., Met. Mater. 2016, 23, 1126–1132. [Google Scholar] [CrossRef]
- Yu, W.; Sun, T.C.; Cui, Q.; Xu, C.Y.; Kou, J. Effect of coal type on the reduction and magnetic separation of a high-phosphorus oolitic hematite ore. ISIJ Int. 2015, 55, 536–543. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhu, D.Q.; Pan, J.; Wu, T.J. Utilization of waste copper slag to produce directly reduced iron for weathering resistant steel. ISIJ Int. 2015, 55, 1347–1352. [Google Scholar] [CrossRef]
- Cheng, X.L.; Zhao, K.; Qi, Y.H.; Shi, X.F.; Zheng, C.L. Direct reduction experiment on iron-bearing waste slag. J. Iron Steel Res. Int. 2013, 20, 24–29. [Google Scholar] [CrossRef]
- Mousa, E.A. Effect of basicity on wustite sinter reducibility under simulated blast furnace conditions. Ironmak. Steelmak. 2014, 41, 418–429. [Google Scholar] [CrossRef]
- Favez, D.; Wagnière, J.D.; Rappaz, M. Au-Fe alloy solidification and solid-state transformations. Acta Mater. 2010, 58, 1016–1025. [Google Scholar] [CrossRef]
- Liu, Z.R.; Zeng, K.; Zhao, W.; Li, Y. Effect of temperature on iron leaching from bauxite residue by sulfuric acid. Bull. Environ. Contam. Toxicol. 2009, 82, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.J.; Liu, Z.C. Preparation of monodispersed micaceous iron oxide pigment from pyrite cinders. Powder Technol. 2011, 207, 335–342. [Google Scholar] [CrossRef]
- Bas, A.D.; Koc, E.; Yazici, E.Y.; Deveci, H. Treatment of copper-rich gold ore by cyanide leaching, ammonia pretreatment and ammoniacal cyanide leaching. Trans. Nonferrous Met. Soc. China 2015, 25, 597–607. [Google Scholar] [CrossRef]


| Components | TFe | Au * | SiO2 | Al2O3 | CaO | MgO | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|
| Content (%) | 44.96 | 3.83 | 24.35 | 2.47 | 0.58 | 0.67 | 1.26 | 1.15 |
| Components | FeSO4·7H2O | Mn | Pb | As | Si | Al | Cu |
|---|---|---|---|---|---|---|---|
| Content (%) | 96.73 | 0.011 | 0.002 | 0.0001 | 0.03 | 0.01 | 0.003 |
| Cyanide Dosage (kg/t) | 5 | 10 | 20 | 40 | 80 |
|---|---|---|---|---|---|
| Gold extraction rate (%) | 56.78 | 87.46 | 94.48 | 96.72 | 96.93 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, P.; Li, Z.; Feng, J.; Bian, Z. Recovery of Gold and Iron from Cyanide Tailings with a Combined Direct Reduction Roasting and Leaching Process. Metals 2018, 8, 561. https://doi.org/10.3390/met8070561
Fu P, Li Z, Feng J, Bian Z. Recovery of Gold and Iron from Cyanide Tailings with a Combined Direct Reduction Roasting and Leaching Process. Metals. 2018; 8(7):561. https://doi.org/10.3390/met8070561
Chicago/Turabian StyleFu, Pingfeng, Zhenyu Li, Jie Feng, and Zhenzhong Bian. 2018. "Recovery of Gold and Iron from Cyanide Tailings with a Combined Direct Reduction Roasting and Leaching Process" Metals 8, no. 7: 561. https://doi.org/10.3390/met8070561





