Microstructure and Wear Resistance of Fe-Cr-Mo-Co-C-B Amorphous Composite Coatings Synthesized by Laser Cladding
Abstract
:1. Introduction
2. Experimental
2.1. Laser Cladding Process
2.2. Phase and Microstructure Analysis
2.3. Microhardness and Wear Tests
3. Results and Discussion
3.1. Phase and Microstructure
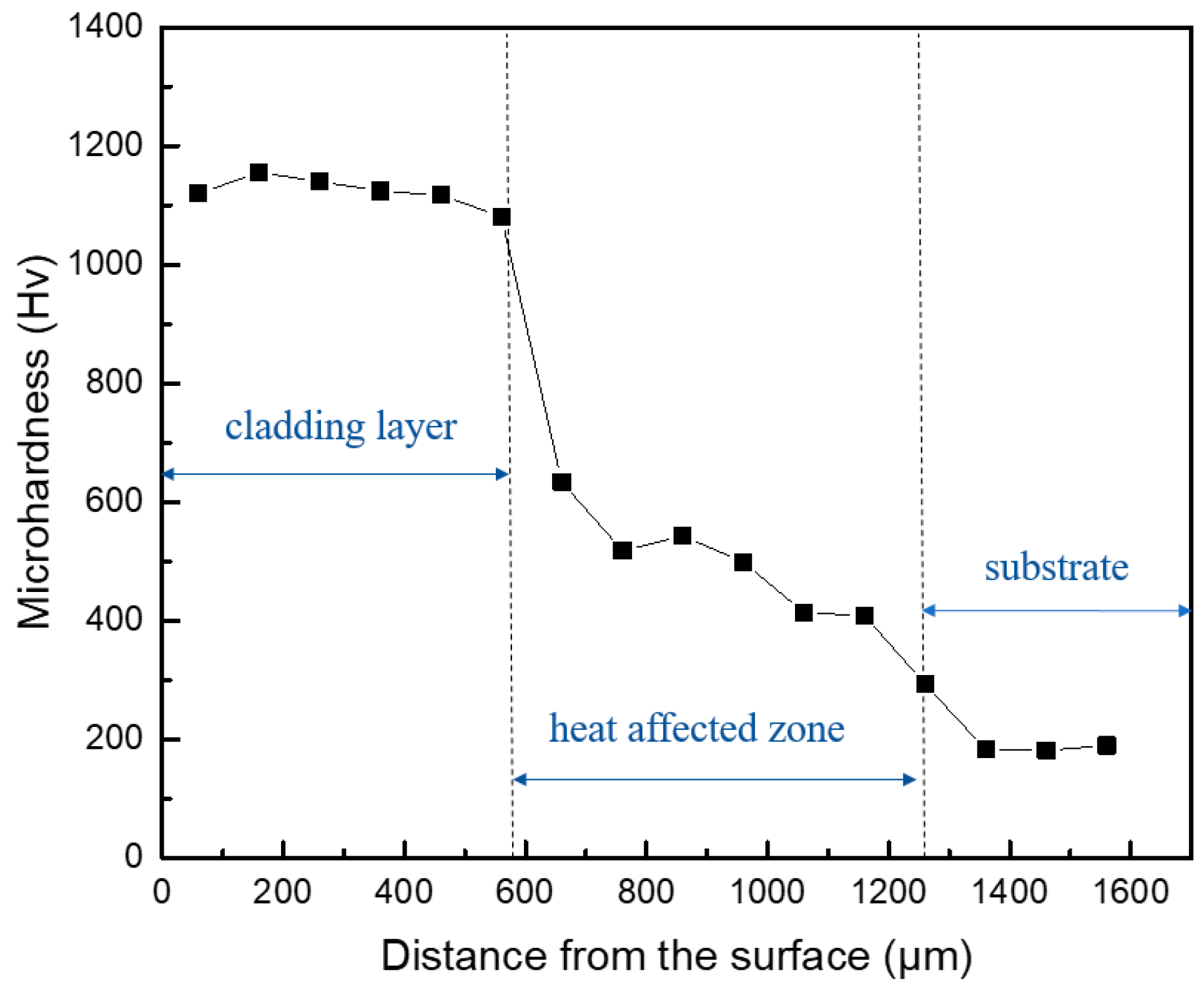
3.2. Microhardness
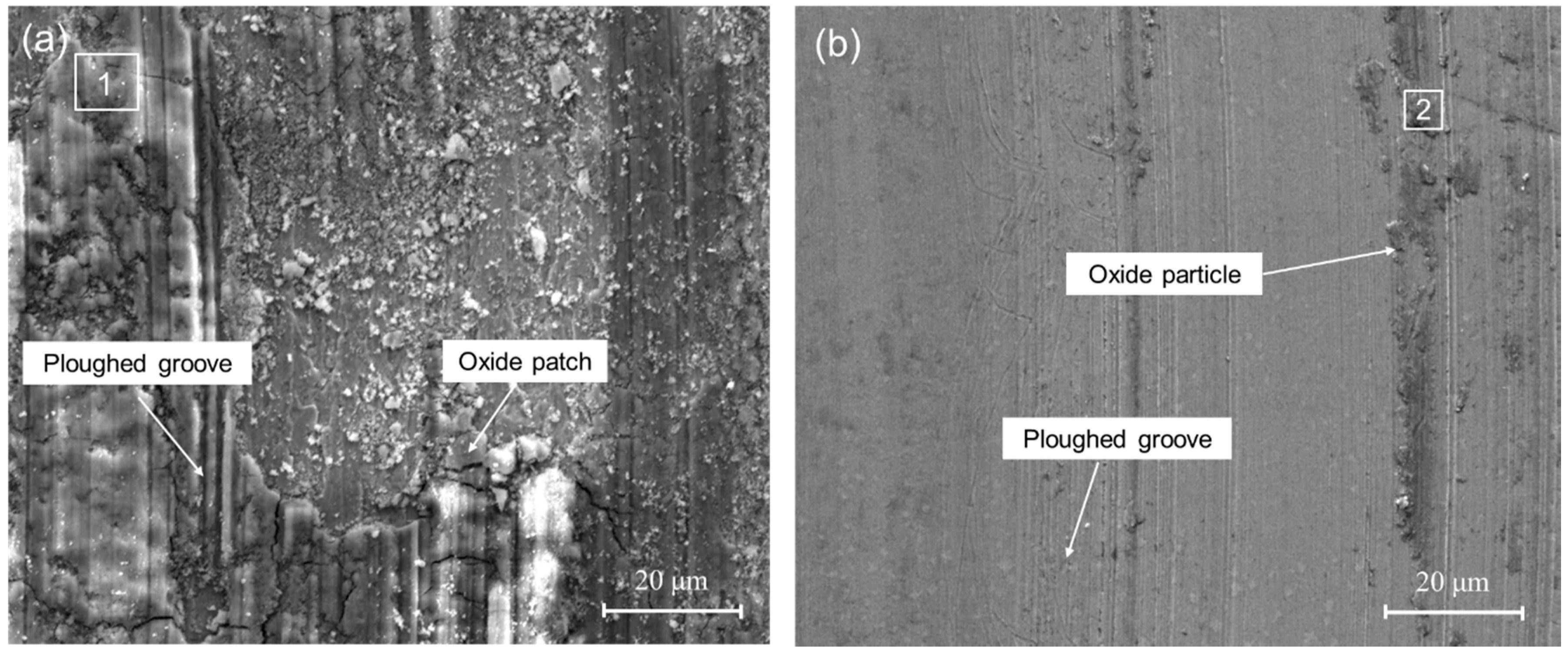
3.3. Wear Resistance
4. Conclusions
- (1)
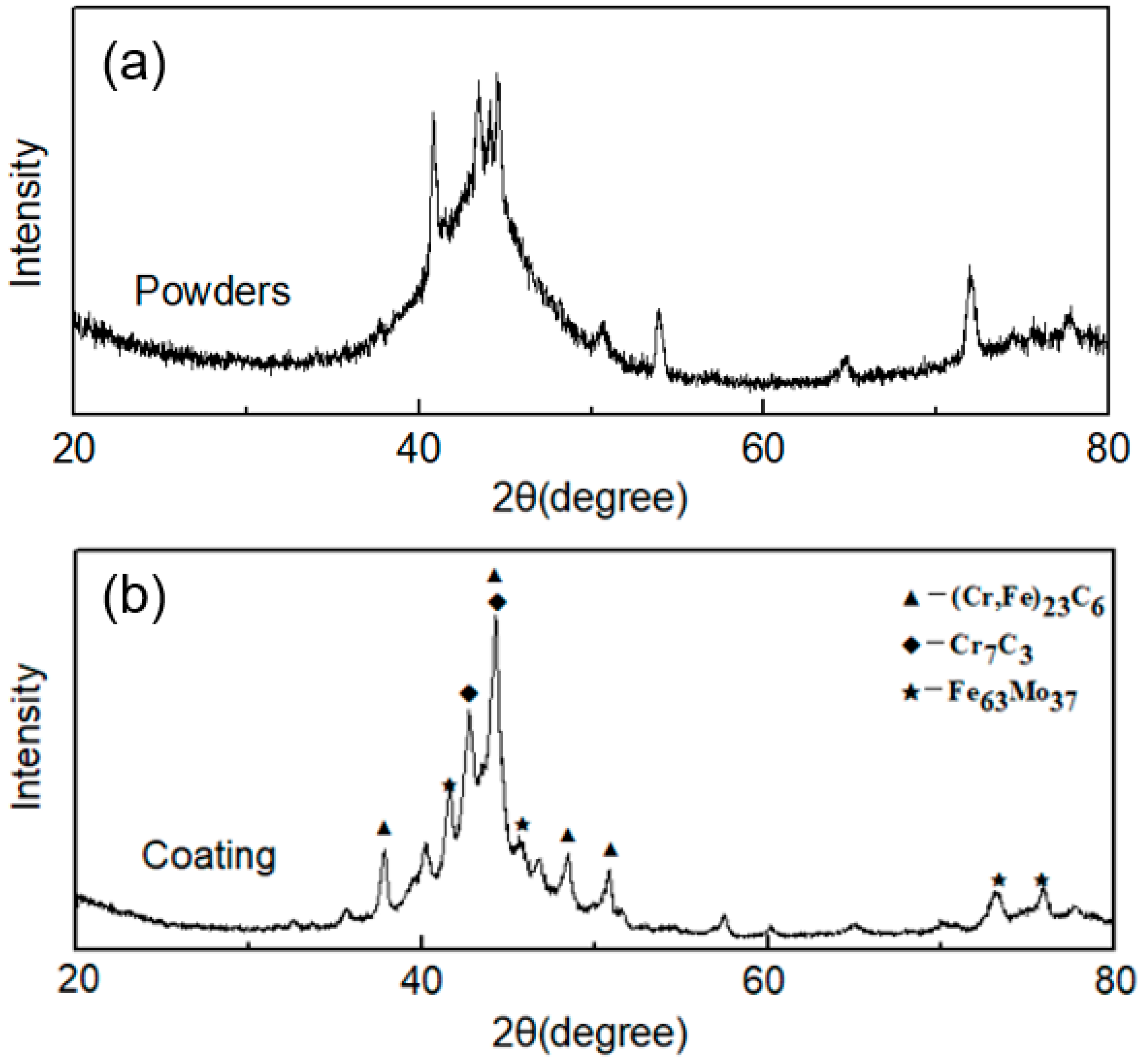
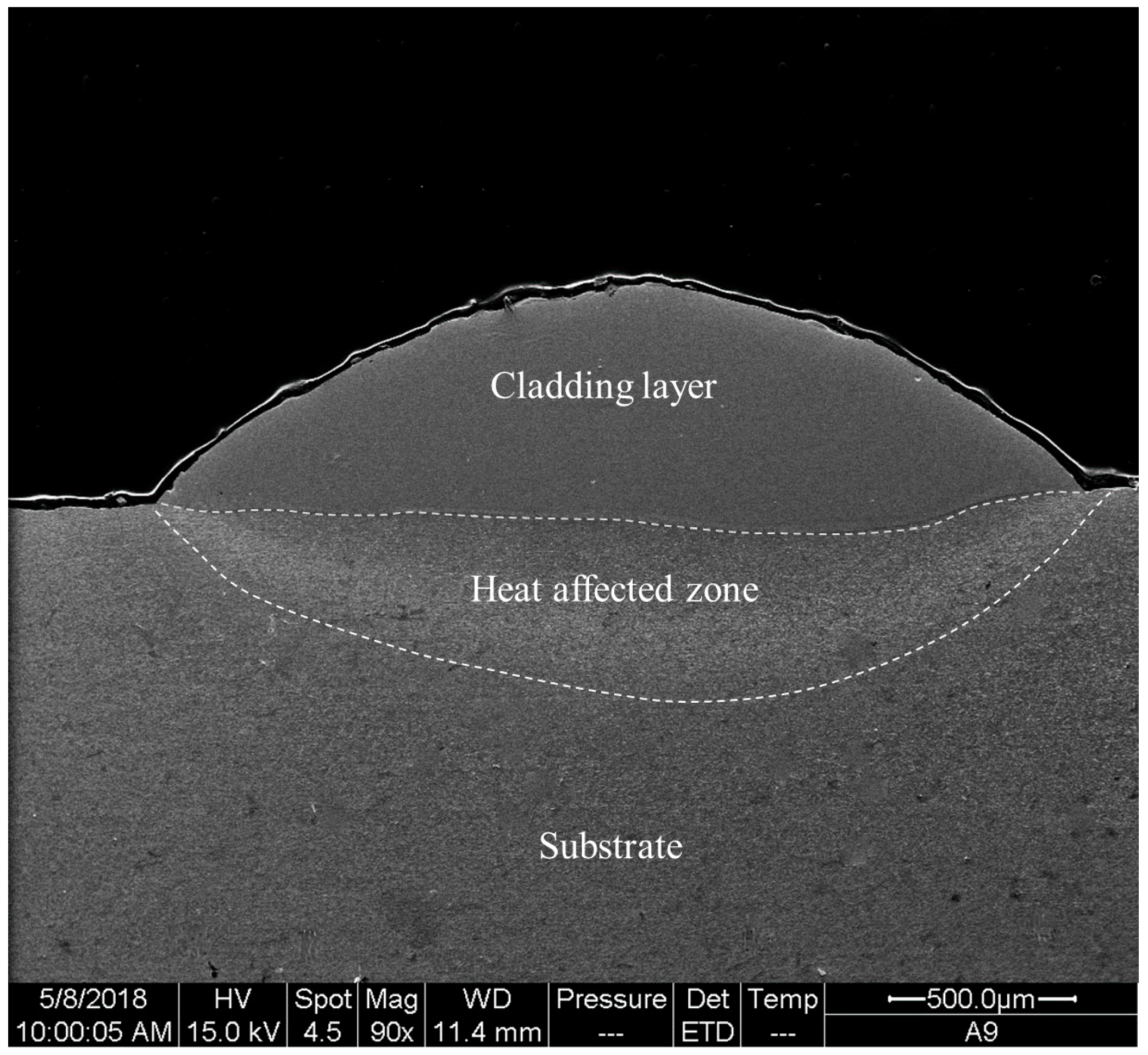
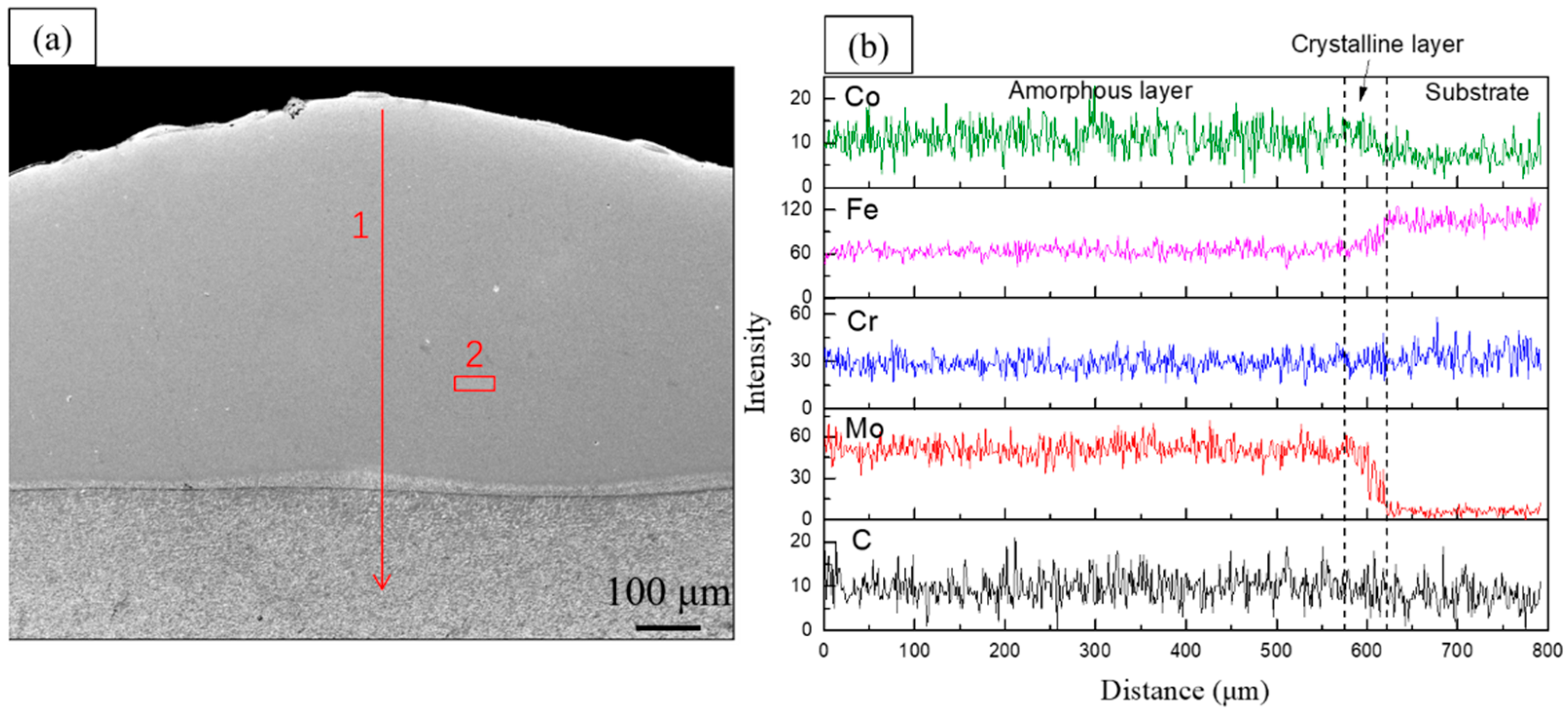
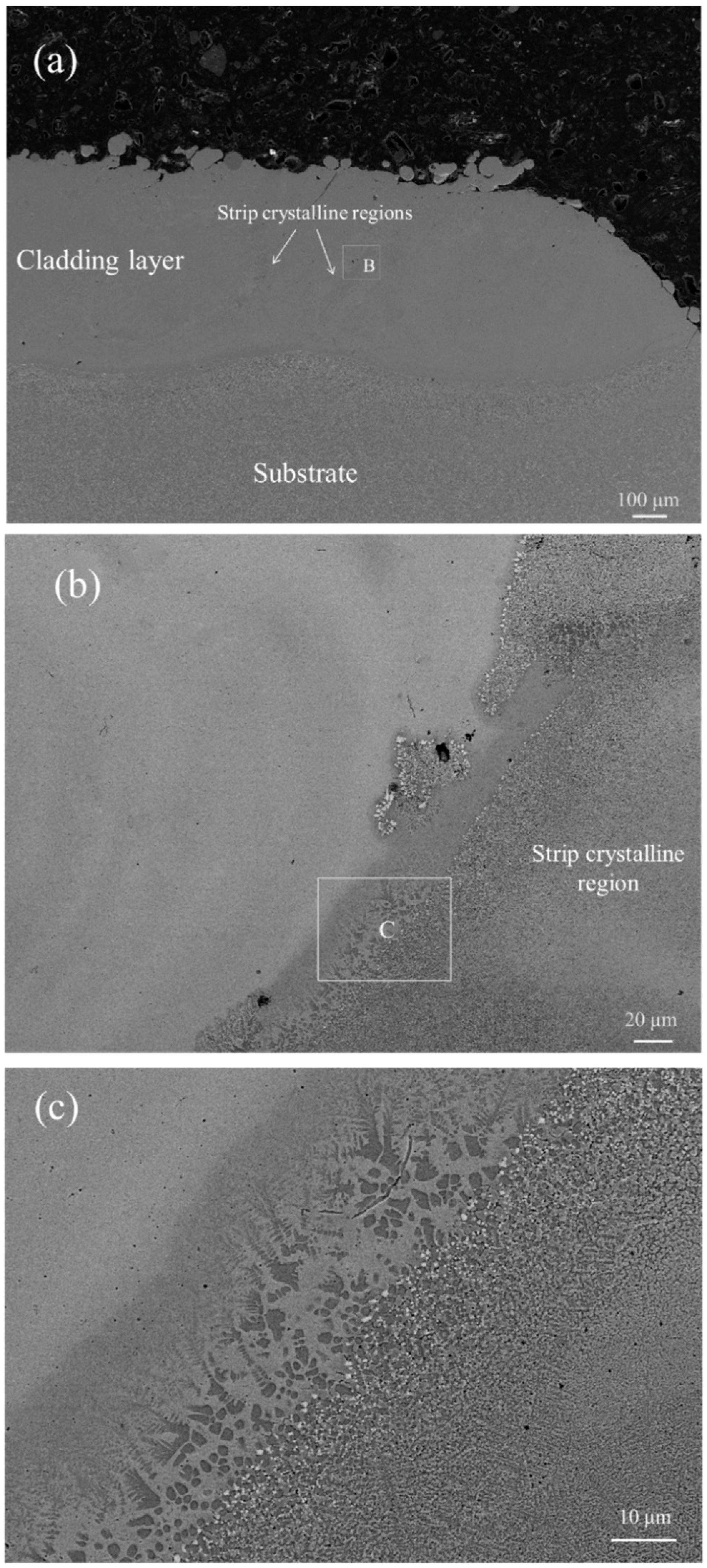
- The cladding layer is composed of a featureless amorphous phase in the upper part and a columnar crystal region at the bottom part. The crystalline phases include carbides , , and solid solution , and the volume fraction of the amorphous phase is calculated to be 52.8%. Significant elemental dilution occurs in the crystallization region, which resulted in the crystallization.
- (2)
- The highest hardness of the cladding layer is 1200 , which is about 6 times of that of the 3Cr13 stainless steel substrate (200 ).
- (3)
- The cladding layer have a much better wear resistance than the substrate: the friction coefficient of the laser cladding (0.11) is much lower than that of substrate (0.29), and the wear volume loss of the cladding layer is only about one fourth of that of the 3Cr13 substrate.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ponnambalam, V.; Poon, S.J.; Shiflet, G.J. Fe-based bulk metallic glasses with diameter thickness larger than one centimeter. J. Mater. Res. 2004, 19, 1320–1323. [Google Scholar] [CrossRef]
- Schuh, C.A.; Hufnagel, T.C.; Ramamurty, U. Mechanical behavior of amorphous alloys. Acta Mater. 2007, 55, 4067–4109. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, M.X.; Liu, L.; Zhao, Y.H.; Zhao, D.Q.; Wang, W.H. Synthesis of Fe-based bulk metallic glasses with low purity materials by multi-metalloids addition. Mater. Lett. 2003, 57, 2698–2701. [Google Scholar] [CrossRef]
- Cheney, J.; Vecchio, K. Development of quaternary Fe-based bulk metallic glasses. Mater. Sci. Eng. A 2008, 492, 230–235. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, L.; Wang, F.C.; Zhang, H.F.; Liu, Y.B.; Xu, S.H. Formation and corrosion behavior of Fe-based amorphous metallic coatings by HVOF thermal spraying. Surf. Coat. Technol. 2009, 204, 563–570. [Google Scholar] [CrossRef]
- Guo, R.Q.; Zhang, C.; Chen, Q.; Yang, Y.; Li, N.; Liu, L. Study of structure and corrosion resistance of Fe-based amorphous coatings prepared by HVAF and HVOF. Corros. Sci. 2011, 53, 2351–2356. [Google Scholar] [CrossRef]
- Kishitake, K.; Era, H.; Otsubo, F. Thermal-sprayed Fe-10CM3P-7C amorphous coatings possessing excellent corrosion resistance. J. Therm. Spray Technol. 1996, 5, 476–482. [Google Scholar] [CrossRef]
- Cadney, S.; Brochu, M. Formation of amorphous Zr41.2Ti13.8Ni10Cu12.5Be22.5 coatings via the ElectroSpark Deposition process. Intermetallics 2008, 16, 518–523. [Google Scholar] [CrossRef]
- Bergmann, H.W.; Mordike, B.L. Laser and electron-beam melted amorphous layers. J. Mater. Sci. 1981, 16, 863–869. [Google Scholar] [CrossRef]
- Yoshioka, H.; Asami, K.; Kawashima, A.; Hashimoto, K. Laser-processed corrosion-resistant amorphous Ni Cr P B surface alloys on a mild steel. Corros. Sci. 1987, 27, 981–995. [Google Scholar] [CrossRef]
- Sahasrabudhe, H.; Bandyopadhyay, A. Laser processing of Fe based bulk amorphous alloy coating on zirconium. Surf. Coat. Technol. 2010, 205, 2661–2667. [Google Scholar] [CrossRef]
- Katakam, S.; Hwang, J.Y.; Paital, S.; Banerjee, R.; Vora, H.; Dahotre, N.B. In Situ Laser Synthesis of Fe-Based Amorphous Matrix Composite Coating on Structural Steel. Metall. Mater. Trans. A 2012, 43, 4957–4966. [Google Scholar] [CrossRef]
- Zheng, B.; Zhou, Y.; Smugeresky, J.E.; Lavernia, E.J. Processing and Behavior of Fe-Based Metallic Glass Components via Laser-Engineered Net Shaping. Metall. Mater. Trans. A 2009, 40, 1235–1245. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Chang, B.; Lei, Y.; Fu, H.; Lin, Y. Effect of Cobalt on Microstructure and Wear Resistance of Ni-Based Alloy Coating Fabricated by Laser Cladding. Metals 2017, 7, 551. [Google Scholar] [CrossRef]
- Majumdar, J.D.; Manna, I. Introduction to Laser Assisted Fabrication of Materials; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Wang, Y.; Lu, Q.; Xiao, L.; Shi, Z. Laser Cladding Fe-Cr-Si-P Amorphous Coatings on 304L Stainless. Rare Met. Mater. Eng. 2014, 43, 274–277. [Google Scholar]
- Basu, A.; Samant, A.N.; Harimkar, S.P.; Majumdar, J.D.; Manna, I.; Dahotre, N.B. Laser surface coating of Fe-Cr-Mo-Y-B-C bulk metallic glass composition on AISI 4140 steel. Surf. Coat. Technol. 2008, 202, 2623–2631. [Google Scholar] [CrossRef]
- Wu, X.; Hong, Y. Fe-based thick amorphous-alloy coating by laser cladding. Surf. Coat. Technol. 2001, 141, 141–144. [Google Scholar] [CrossRef]
- Zhu, Q.; Qu, S.; Wang, X.; Zou, Z. Synthesis of Fe-based amorphous composite coatings with low purity materials by laser cladding. Appl. Surf. Sci. 2007, 253, 7060–7064. [Google Scholar] [CrossRef]
- Balla, V.K.; Bandyopadhyay, A. Laser processing of Fe-based bulk amorphous alloy. Surf. Coat. Technol. 2010, 205, 2661–2667. [Google Scholar] [CrossRef]
- Maurya, R.S.; Sahu, A.; Laha, T. Quantitative phase analysis in Al86Ni8Y6 bulk glassy alloy synthesized by consolidating mechanical alloyed amorphous powder via spark plasma sintering. Mater. Des. 2016, 93, 96–103. [Google Scholar] [CrossRef]
- Shu, F.Y.; Liu, S.; Zhao, H.Y.; He, W.X.; Sui, S.H.; Zhang, J.; He, P.; Xu, B.S. Structure and high-temperature property of amorphous composite coating synthesized by laser cladding FeCrCoNiSiB high-entropy alloy powder. J. Alloys Compd. 2018, 731, 662–666. [Google Scholar] [CrossRef]
- Rabinowicz, E.; Tanner, R.I. Friction and Wear of Materials. J. Appl. Mech. 1995, 33, 606–611. [Google Scholar] [CrossRef]
- Wen, S.; Huang, P. The Principle of Tribology; Tsinghua University Press: Beijing, China, 2008; pp. 458–460. [Google Scholar]




| Element | C | Cr | Ni | Si | Mn | P | S | Fe |
|---|---|---|---|---|---|---|---|---|
| wt. % | 0.26~0.35 | 12.00~14.00 | ≤0.60 | ≤1.00 | ≤1.00 | ≤0.035 | ≤0.03 | Bal. |
| Laser Power (W) | Scanning Speed (mm/min) | Powder Feeding Rate (g/min) | Overlapping Rate (%) |
|---|---|---|---|
| 500 | 600 | 10 | 30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, X.; Du, D.; Wang, K.; Hong, Y.; Chang, B. Microstructure and Wear Resistance of Fe-Cr-Mo-Co-C-B Amorphous Composite Coatings Synthesized by Laser Cladding. Metals 2018, 8, 622. https://doi.org/10.3390/met8080622
Hou X, Du D, Wang K, Hong Y, Chang B. Microstructure and Wear Resistance of Fe-Cr-Mo-Co-C-B Amorphous Composite Coatings Synthesized by Laser Cladding. Metals. 2018; 8(8):622. https://doi.org/10.3390/met8080622
Chicago/Turabian StyleHou, Xiangchun, Dong Du, Kaiming Wang, Yuxiang Hong, and Baohua Chang. 2018. "Microstructure and Wear Resistance of Fe-Cr-Mo-Co-C-B Amorphous Composite Coatings Synthesized by Laser Cladding" Metals 8, no. 8: 622. https://doi.org/10.3390/met8080622






