Flow-Accelerated Corrosion of Type 316L Stainless Steel Caused by Turbulent Lead–Bismuth Eutectic Flow
Abstract
:1. Introduction
2. Physical Models
2.1. Turbulent Flow Model
2.2. Corrosion Model
- (1)
- If the oxygen concentration in LBE is sufficiently low, as in the present experimental study, there will be no effective oxide protective layer formed on the steel surface. In this situation, the steel contacts with the LBE directly, and the main constituents of the steel are thus dissolved into the LBE directly.
- (2)
- If the oxygen concentration is within an appropriate range, oxidation of steel will occur, and an active oxide film (-based) will eventually be formed on the steel surface. Direct dissolution of steel into LBE will be prevented due to separation of the oxide film. In this case, the iron diffuses from the base metal, and the oxygen transfers from the bulk flow to the oxide/LBE interface to engage in the oxidation–reduction chemical reaction ().
3. Experiments
3.1. JLBL-1
3.2. Specimen Testing Tube
3.3. Operation Conditions
3.4. Post-Testing Material Characterization
4. Numerical Simulation Conditions
4.1. Hydrodynamic Study
4.2. Mass Transfer Study
5. Results and Discussion
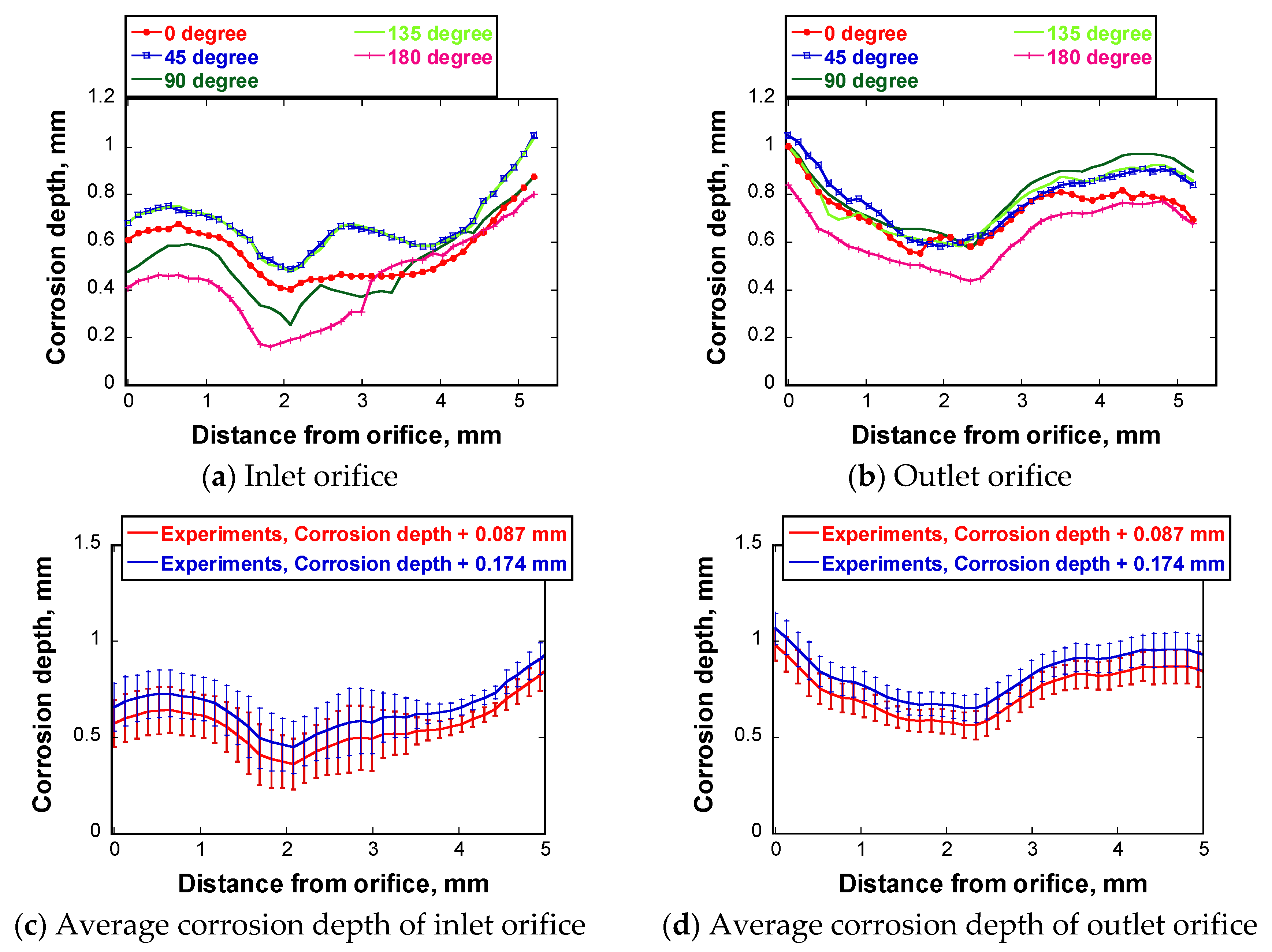
5.1. Corrosion Depth Profile of the Test Tube
5.2. Effects of the TKE, Shear Stress, and Pressure
5.3. Other Effects
5.3.1. Cavitation
5.3.2. Gravity
5.3.3. Corroded Morphology
5.3.4. Orifice Angle
5.4. Mass Transfer
5.4.1. Effective Viscosity and Effective Diffusivity
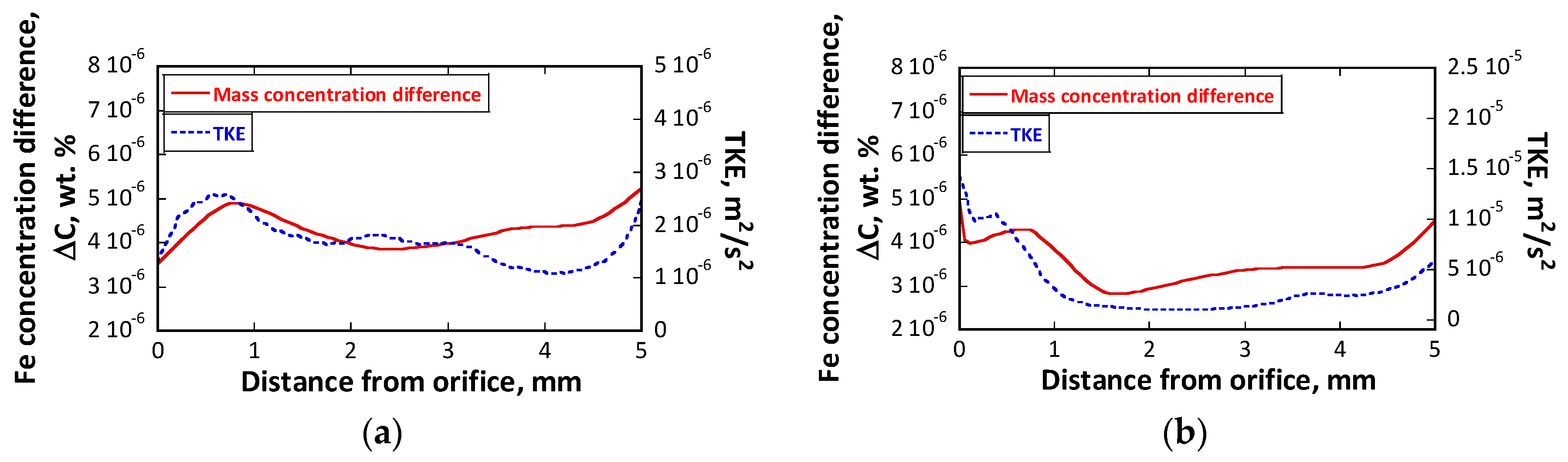
5.4.2. Mass Concentration Difference Close to the Wall
5.4.3. Comparison of Predicted Corrosion Depth and Measured Corrosion Depth
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| constants in empirical equations linking to and | |
| cavitation number | |
| , , | species concentration in the bulk fluid, at the wall, and in the first node, kmol/m3 |
| oxygen concentration in a liquid, kmol/m3 | |
| hydraulic diameter, m | |
| molecular diffusion coefficient, m2/s | |
| eddy or turbulent diffusivity, , m2/s | |
| effective diffusion coefficient, , m2/s | |
| , | damping factor in the LRN turbulence model |
| turbulence level in the near-wall region | |
| mass flux of iron, | |
| turbulent kinetic energy, m2/s2 | |
| mass transfer coefficient, m/s | |
| chemical component of a material | |
| molar mass of iron, kg/kmol | |
| generation of turbulence, | |
| additional term of turbulence generation in the LRN turbulence model, | |
| local pressure in a liquid, Pa | |
| vapor pressure of a liquid, Pa | |
| wall roughness, m | |
| Reynolds number, | |
| , | turbulence Reynolds number, , |
| Schmidt number, | |
| turbulent Schmidt number, | |
| Sherwood number, | |
| temperature, K | |
| temperature difference, K | |
| dimensionless velocity | |
| characteristic flow speed of a liquid, m/s | |
| distance from the wall, m | |
| dimensionless distance from the wall | |
| distance of the first node from the wall, m | |
| Greek symbols | |
| thickness of diffusion boundary layer, m | |
| thickness of viscous sublayer, m | |
| dissipation of turbulent kinetic energy, m2/s3 | |
| molecular dynamic viscosity, | |
| eddy or turbulent viscosity, | |
| effective viscosity, , | |
| density, | |
| wall shear stress, Pa |
References
- Bergman, T.L.; Incropera, F.P.; Devitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 378–418. [Google Scholar]
- Mahato, B.K.; Voora, S.K.; Schemilt, L.W. Steel pipe corrosion under flow conditions—I. an isothermal correlation for a mass transfer model. Corros. Sci. 1968, 8, 173–193. [Google Scholar] [CrossRef]
- Corpson, H.R. Effects of velocity on corrosion. Corrosion 1960, 16, 130–136. [Google Scholar]
- Silverman, D.C. Rotating cylinder electrode for velocity sensitivity testing. Corrosion 1984, 40, 220–226. [Google Scholar] [CrossRef]
- Silverman, D.C. Rotating cylinder electrode—Geometry relationships for prediction of velocity-sensitive corrosion. Corrosion 1987, 44, 42–49. [Google Scholar] [CrossRef]
- Nesic, S.; Postlethwaite, J. Relationship between the structure of disturbed flow and erosion-corrosion. Corrosion 1990, 46, 874–880. [Google Scholar] [CrossRef]
- Nesic, S.; Postlethwaite, J. Hydrodynamics of disturbed flow and erosion-corrosion. Part I—Single-phase flow study. Can. J. Chem. Eng. 1991, 69, 698–703. [Google Scholar] [CrossRef]
- Mahato, B.K.; Cha, C.Y.; Shemilt, L.W. Unsteady state mass transfer coefficients controlling steel pipe corrosion under isothermal flow conditions. Corros. Sci. 1980, 20, 421–441. [Google Scholar] [CrossRef]
- Chang, Y.S.; Kim, S.H.; Chang, H.S.; Lee, S.M.; Choi, J.B.; Kim, Y.J.; Choi, Y.H. Fluid effects on structural integrity of pipes with an orifice and elbows with a wall-thinned part. J. Loss Prev. Proc. 2009, 22, 854–859. [Google Scholar] [CrossRef]
- Utanohara, Y.; Nagaya, Y.; Nakamura, A.; Murase, M. Influence of local flow field on flow accelerated corrosion downstream from an orifice. J. Power Energy Syst. 2012, 6, 18–33. [Google Scholar] [CrossRef]
- Utanohara, Y.; Nagaya, Y.; Nakamura, A.; Murase, M.; Kamahori, K. Correlation between flow accelerated corrosion and wall shear stress downstream from an orifice. J. Power Energy Syst. 2013, 7, 138–146. [Google Scholar] [CrossRef]
- Crawford, N.M.; Cunningham, G.; Spence, S.W.T. An experimental investigation into the pressure drop for turbulent flow in 90° elbow bends. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2007, 221, 77–88. [Google Scholar] [CrossRef]
- Nesic, S. Using computational fluid dynamics in combating erosion-corrosion. Chem. Eng. Sci. 2006, 61, 4086–4097. [Google Scholar] [CrossRef]
- Poulson, B. Complexities in predicting erosion corrosion. Wear 1999, 233–235, 497–504. [Google Scholar] [CrossRef]
- El-Gammal, M.; Mazhar, H.; Cotton, J.S.; Shefski, C.; Pietralik, J.; Ching, C.Y. The hydrodynamic effects of single-phase flow on flow accelerated corrosion in a 90-degree elbow. Nucl. Eng. Des. 2010, 240, 1589–1598. [Google Scholar] [CrossRef]
- El-Gammal, M.; Ahmed, W.H.; Ching, C.Y. Investigation of wall mass transfer characteristics downstream of an orifice. Nucl. Eng. Des. 2012, 242, 353–360. [Google Scholar] [CrossRef]
- Takano, T.; Ikarashi, Y.; Uchiyama, K.; Yamagata, T.; Fujisawa, N. Influence of swirling flow on mass and momentum transfer downstream of a pipe with elbow and orifice. Int. J. Heat Mass Transf. 2016, 92, 394–402. [Google Scholar] [CrossRef]
- Ikarashi, Y.; Taguchi, S.; Yamagata, T.; Fujisawa, N. Mass and momentum transfer characteristics in and downstream of 90° elbow. Int. J. Heat Mass Transf. 2017, 107, 1085–1093. [Google Scholar] [CrossRef]
- Gromov, B.F.; Belomitcev, Y.S.; Yefimov, E.I.; Leonchuk, M.P.; Martinov, P.N.; Orlov, Y.I.; Pankratov, D.V.; Pashkin, Y.G.; Toshinsky, G.I.; Chekunov, V.V.; et al. Use of lead-bismuth coolant in nuclear reactors and accelerator-driven systems. Nucl. Eng. Des. 1997, 173, 207–217. [Google Scholar] [CrossRef]
- Zhang, J. Lead-Bismuth Eutectic (LBE): A coolant candidate for Gen. IV advanced nuclear reactor concepts. Adv. Eng. Mater. 2014, 16, 349–356. [Google Scholar] [CrossRef]
- Zhang, J. A review of steel corrosion by liquid lead and lead-bismuth. Corros. Sci. 2009, 51, 1207–1227. [Google Scholar] [CrossRef]
- Zhang, J.; Hoseman, P.; Maloy, S. Models of liquid metal corrosion. J. Nucl. Mater. 2010, 404, 82–96. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N. Review of the studies on fundamental issues in LBE corrosion. J. Nucl. Mater. 2008, 373, 351–377. [Google Scholar] [CrossRef]
- Schroer, C.; Wedemeyer, O.; Novotny, J.; Skrypnik, A.; Konys, J. Long-term service of austenitic steel 1.4571 as a container material for flowing lead–bismuth eutectic. J. Nucl. Mater. 2011, 418, 8–15. [Google Scholar] [CrossRef]
- STAR-CD methodology, Ver. 4.26; Siemens Product Lifecycle Management Inc.: Plano, TX, USA, 2016.
- Launder, B.E.; Spalding, D.B. The numerical computation of turbulent flows. Comp. Meth. Appl. Mech. Eng. 1974, 3, 269–289. [Google Scholar] [CrossRef]
- Nesic, S.; Postlethwaite, J. Calculation of wall-mass transfer rates in separated aqueous flow using a low Reynolds number k − ε model. Int. J. Heat Mass Transf. 1992, 35, 1977–1985. [Google Scholar] [CrossRef]
- Lien, F.S.; Chen, W.L.; Leschziner, M.A. Low-Reynolds-Number Eddy-Viscosity Modelling Based on Non-Linear Stress-Strain/Vorticity Relations. In Proceedings of the 3rd Symposium on Engineering Turbulence Modelling and Measurements, Crete, Greece, 27–29 May 1996. [Google Scholar]
- He, X.; Li, N.; Mineev, M. A kinetic model for corrosion and precipitation in non-isothermal LBE flow loop. J. Nucl. Mater. 2001, 297, 214–219. [Google Scholar] [CrossRef]
- Simon, N.; Terlain, A.; Flament, T. The compatibility of austenitic materials with liquid Pb-17Li. Corros. Sci. 2001, 43, 1041–1052. [Google Scholar] [CrossRef]
- Yamaki, E.; Ginestar, K.; Martinelli, L. Dissolution mechanism of 316L in lead-bismuth eutectic at 500 °C. Corros. Sci. 2011, 53, 3075–3085. [Google Scholar] [CrossRef]
- Schad, M. To the corrosion of austenitic steel in sodium loops. Nucl. Tech. 1980, 50, 267–288. [Google Scholar] [CrossRef]
- Distefano, J.R.; Hoffman, E.E. Corrosion Mechanisms in Refractory Metal-Alkali Metal Systems. At. Energy Rev. 1964, 2, 3–33. [Google Scholar]
- Keating, A. A Model for the Investigation of Two-Phase Erosion-Corrosion in Complex Geometries. Maters’s Thesis, University of Queensland, Brisbane, QLD, Australia, 1999. [Google Scholar]
- Davis, C.; Frawley, P. Modelling of erosion-corrosion in practical geometries. Corros. Sci. 2009, 51, 769–775. [Google Scholar] [CrossRef]
- Kikuchi, K.; Karata, Y.; Saito, S.; Fukazawa, M.; Salsa, T.; Okinawa, H.; Wake, E.; Miura, K. Corrosion–erosion test of SS316 in flowing Pb–Bi. J. Nucl. Mater. 2003, 318, 348–354. [Google Scholar] [CrossRef]
- Kikuchi, K.; Saito, S.; Kurata, Y.; Futakawa, M.; Sasa, T.; Oigawa, H.; Wakai, E.; Umeno, M.; Mizubayashi, H.; Miura, K. Lead-bismuth eutectic compatibility with materials in the concept of spallation target for ADS. JSME Int. J. B-Fluid Therm. 2004, 47, 332–339. [Google Scholar] [CrossRef]
- OECD NEA. Handbook on Lead-Bismuth Eutectic Alloy and Lead Properties, Materials Compatibility, Thermal-Hydraulics and Technologies; 2015 Edition; OECD NEA: Boulogne-Billancourt, France, 2015. [Google Scholar]
- Li, N. Active control of oxygen in molten lead–bismuth eutectic systems to prevent steel corrosion and coolant contamination. J. Nucl. Mater. 2002, 300, 73–81. [Google Scholar] [CrossRef]
- Balbaud-Celerier, F.; Barbier, F. Influence of the Pb-Bi hydrodynamics on the corrosion of T91 martensitic steel and pure iron. J. Nucl. Mater. 2004, 335, 204–209. [Google Scholar] [CrossRef]
- Abella, J.; Verdaguer, A.; Colominas, S.; Ginestar, K.; Martinelli, L. Fundamental data: Solubility of nickel and oxygen and diffusivity of iron and oxygen in molten LBE. J. Nucl. Mater. 2011, 415, 329–337. [Google Scholar] [CrossRef]
- Syrett, B.C. Erosion-corrosion of copper-nickel alloys in sea water and other aqueous environments—A literature review. Corrosion 1976, 32, 242–252. [Google Scholar] [CrossRef]
- Heitz, E. Chemo-mechanical effects of flow on corrosion. Corrosion 1991, 47, 135–145. [Google Scholar] [CrossRef]
- Brennen, C.E. Cavitation and Bubble Dynamics; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Yada, H.; Kanagawa, A.; Hattori, S. Cavitation inception and erosion in flowing system of water and liquid metal. Trans. Jpn. Soc. Mech. Eng. Ser. B 2011, 78, 811–820. (In Japanese) [Google Scholar] [CrossRef]
- Fujisawa, N.; Uchiyama, K.; Yamagata, T. Mass transfer measurements on periodic roughness in a circular pipe and downstream of orifice. Int. J. Heat Mass Transf. 2017, 105, 316–325. [Google Scholar] [CrossRef]
- Shan, F.; Liu, Z.; Liu, W.; Tsuji, Y. Effects of the orifice to pipe diameter ratio on orifice flows. Chem. Eng. Sci. 2016, 152, 497–506. [Google Scholar] [CrossRef]
- Postlethwaite, J.; Lotz, U. Mass transfer at erosion-corrosion roughened surfaces. Can. J. Chem. Eng. 1988, 66, 75–78. [Google Scholar] [CrossRef]
- Fazio, C.; Ricapito, I.; Scaddozzo, G.; Benamati, G. Corrosion behaviour of steels and refractory metals and tensile features of steels exposed to flowing PbBi in the LECOR loop. J. Nucl. Mater. 2003, 318, 325–332. [Google Scholar] [CrossRef]
- Poulson, B. Mass transfer from rough surfaces. Corros. Sci. 1990, 30, 743–746. [Google Scholar] [CrossRef]
- Balbaud-Celerier, F.; Barbier, F. Investigation of models to predict the corrosion of steels in flowing liquid lead alloys. J. Nucl. Mater. 2001, 289, 227–242. [Google Scholar] [CrossRef]
- Malang, S.; Smith, D.L. Modeling of Liquid Metal Corrosion/Deposition in a Fusion Reactor Blanket (No. ANL/FPP/TM-192); Argonne National Lab.: Lemont, IL, USA, 1984.
- Sugawara, T.; Nishihara, K.; Obayashi, H.; Kurata, Y.; Oigawa, H. Conceptual design study of beam window for accelerator-driven system. J. Nucl. Sci. Technol. 2010, 47, 953–962. [Google Scholar] [CrossRef]
- Wan, T.; Naoe, T.; Wakui, T.; Futakawa, M.; Obayashi, H.; Sasa, T. Study on the evaluation of erosion damage by using laser ultrasonic integrated with a wavelet analysis technique. J. Phys. Conf. Ser. 2017, 842, 012010. [Google Scholar] [CrossRef] [Green Version]
- Yoon, M.; Hwang, J.; Lee, J.; Sung, H.J.; Kim, J. Large-scale motions on a turbulent channel flow with the slip boundary condition. Int. J. Heat Mass Transf. 2016, 61, 96–107. [Google Scholar] [CrossRef]
- Rivai, A.K.; Saito, S.; Tezuka, M.; Kato, C.; Kikuchi, K. Effect of cold working on the corrosion resistance of JPCA stainless steel in flowing Pb–Bi at 450 °C. J. Nucl. Mater. 2012, 431, 97–104. [Google Scholar] [CrossRef]
- Lambrinou, K.; Charalampopoulou, E.; Donck, T.V.; Delville, R.; Schryvers, D. Dissolution corrosion of 316L austenitic stainless steels in contact with static liquid lead-bismuth eutectic (LBE) at 500 °C. J. Nucl. Mater. 2017, 490, 9–27. [Google Scholar] [CrossRef]
- Kurata, Y.; Masatoshi, F.; Saito, S. Corrosion behavior of steels in liquid lead–bismuth with low oxygen concentrations. J. Nucl. Mater. 2008, 373, 164–178. [Google Scholar] [CrossRef]
- Kurata, Y. Corrosion experiments and materials developed for the Japanese HLM systems. J. Nucl. Mater. 2011, 415, 254–259. [Google Scholar] [CrossRef]

















© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, T.; Saito, S. Flow-Accelerated Corrosion of Type 316L Stainless Steel Caused by Turbulent Lead–Bismuth Eutectic Flow. Metals 2018, 8, 627. https://doi.org/10.3390/met8080627
Wan T, Saito S. Flow-Accelerated Corrosion of Type 316L Stainless Steel Caused by Turbulent Lead–Bismuth Eutectic Flow. Metals. 2018; 8(8):627. https://doi.org/10.3390/met8080627
Chicago/Turabian StyleWan, Tao, and Shigeru Saito. 2018. "Flow-Accelerated Corrosion of Type 316L Stainless Steel Caused by Turbulent Lead–Bismuth Eutectic Flow" Metals 8, no. 8: 627. https://doi.org/10.3390/met8080627




