Transient Liquid Phase Bonding of Semi-Solid Metal 7075 Aluminum Alloy using ZA27 Zinc Alloy Interlayer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
2.3. Mechanical Testing and Metallurgy Analysis
3. Results and Discussion
3.1. Results of Microstructure in Bonded Joint
3.2. Results of Particles in the Bonded Joint by EDX-Ray Spectroscopy
3.3. Results of Bonding Strength of Joint
3.4. Results of Vickers Hardness
3.5. Analysis of Bonding Strength Data Using Statistical Method Analysis of Model Accuracy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nami, H.; Halvaee, A.; Adgi, H. Transient liquid phase diffusion bonding of Al/Mg2Si metal matrix composite. Mater. Des. 2011, 32, 3957–3965. [Google Scholar] [CrossRef]
- Jin, Y.J.; Khan, T.I. Effect of bonding time on microstructure and mechanical properties of transient liquid phase bonded magnesium AZ31 alloy. Mater. Des. 2012, 38, 32–37. [Google Scholar] [CrossRef]
- Park, M.S.; Gibbons, S.L.; Arroyave, R. Phase-field simulations of intermetallic compound growth in Cu/Sn/Cu sandwich structure under transient liquid phase bonding conditions. Acta Mater. 2012, 60, 6278–6287. [Google Scholar] [CrossRef]
- Cooke, K.O.; Khan, T.I.; Oliver, G.D. Transient liquid phase diffusion bonding Al-6061 using nano-dispersed Ni coatings. Mater. Des. 2012, 33, 469–475. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Jiao, Y.; Zhao, M.J.; Zhang, J. Investigation on in situ Al0.5FeSi0.5/Al composites prepared by transient liquid phase sintering. Mater. Des. 2014, 59, 70–75. [Google Scholar] [CrossRef]
- Kenevisi, M.S.; Mousavi Khoie, S.M. An investigation on microstructure and mechanical properties of Al7075 to Ti–6Al–4V Transient Liquid Phase (TLP) bonded joint. Mater. Des. 2012, 38, 19–25. [Google Scholar] [CrossRef]
- Sayyedain, S.S.; Salimijazi, H.R.; Toroghinejad, M.R.; Karimzadeh, F. Microstructure and mechanical properties of transient liquid phase bonding of Al2Op3/Al nanocomposite using copper interlayer. Mater. Des. 2014, 53, 275–282. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Pei, Y.; Li, S.; Chai, D. Joining of Al2O3p/Al composites by transient liquid phase (TLP) bonding and a novel process of active-transient liquid phase (A-TLP) bonding. Mater. Sci. Eng. A 2008, 488, 146–156. [Google Scholar] [CrossRef]
- Kenevisi, M.S.; Mousavi Khoie, S.M.; Alaei, M. Microstructural evaluation and mechanical properties of the diffusion bonded Al/Ti alloys joint. Mech. Mater. 2013, 64, 69–75. [Google Scholar] [CrossRef]
- Meengam, C.; Chainarong, S.; Muangjunburee, P. Friction welding of semi-solid metal 7075 aluminum alloy. Mater. Today Proc. 2017, 4, 1303–1311. [Google Scholar] [CrossRef]
- Dezellus, O.; Andrieux, J.; Bosselet, F.; Sacerdote-Peronnet, M.; Baffie, T.; Hodaj, F.; Eustathopoulos, N.; Viala, J.C. Transient liquid phase bonding of titanium to aluminium nitride. Mater. Sci. Eng. A 2008, 495, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Maity, J.; Pal, T.K.; Maiti, R. Transient liquid phase diffusion bonding of 6061-15 wt % SiCp in argon environment. J. Mater. Process. Technol. 2009, 209, 3568–3580. [Google Scholar] [CrossRef]
- Mohamed, I.S.; Tahir, I.K.; Hans, J.R. Transient liquid phase bonding of AA-6063 to UNS S32304 using Cu interlayer. Proc. Chem. 2016, 19, 517–524. [Google Scholar]
- He, Z.; Su, X.; Peng, H.; Liu, Y.; Wu, C.; Wang, J. 600 °C isothermal section of the Al-Cr-Zn ternary phase diagram. J. Alloys Compd. 2015, 649, 1239–1245. [Google Scholar] [CrossRef]
- Liang, S.-M.; Schmid-Fetzer, R. Thermodynamic assessment of the Al–Cu–Zn system, Part III: Al–Cu–Zn ternary system. Calphad 2016, 52, 21–37. [Google Scholar] [CrossRef]
- Mazar Atabaki, M.; Idris, J. Low-temperature partial transient liquid phase diffusion bonding of Al/Mg2Si metal matrix composite to AZ91D using Al-based interlayer. Mater. Des. 2012, 34, 832–841. [Google Scholar] [CrossRef]
- Zhu, Y.h. General rule of phase decomposition in Zn-Al based alloys (II) on effects of external stresses on phase transformation. Mater. Trans. 2004, 45, 3083–3097. [Google Scholar] [CrossRef]
- Seyyed Afghahi, S.S.; Ekrami, A.; Farahany, S.; Jahangiri, A. Fatigue properties of temperature gradient transient liquid phase diffusion bonded Al7075-T6 alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 1073–1079. [Google Scholar] [CrossRef]
- Dunyakul, Y.; Meengam, C.; Maunkhaw, D.; Chainarong, S. Evaluation of microstructure and mechanical properties in dissimilar joint of SSM7075 with SSM356 aluminum alloy using diffusion bonding. Eng. J. 2016, 20, 135–144. [Google Scholar] [CrossRef]
- AlHazaa, A.; Khan, T.I.; Haq, I. Transient liquid phase (TLP) bonding of Al7075 to Ti–6Al–4V alloy. Mater. Charact. 2010, 61, 312–317. [Google Scholar] [CrossRef]
- Bosco, N.S.; Zok, F.W. Strength of joints produced by transient liquid phase bonding in the Cu–Sn system. Acta Mater. 2005, 53, 2019–2027. [Google Scholar] [CrossRef]
- Jalilvand, V.; Omidvar, H.; Rahimipour, M.R.; Shakeri, H.R. Influence of bonding variables on transient liquid phase bonding behavior of nickel based superalloy IN-738LC. Mater. Des. 2013, 52, 36–46. [Google Scholar] [CrossRef]
- Ryan, T.P. Modern Engineering Statistics, 1st ed.; John Wiley & Sons Inc.: New York, NY, USA, 2007; pp. 140–189. [Google Scholar]
- Montgomery, D.C. Designing and Analysis of Experiments, 8th ed.; John Wiley & Sons Inc.: New York, NY, USA, 2000; pp. 24–146. [Google Scholar]
- Box, G.E.P.; Draper, N.R. Empirical Model-Building and Response Surface, 1st ed.; John Wiley & Sons Inc.: New York, NY, USA, 1987; pp. 52–189. [Google Scholar]

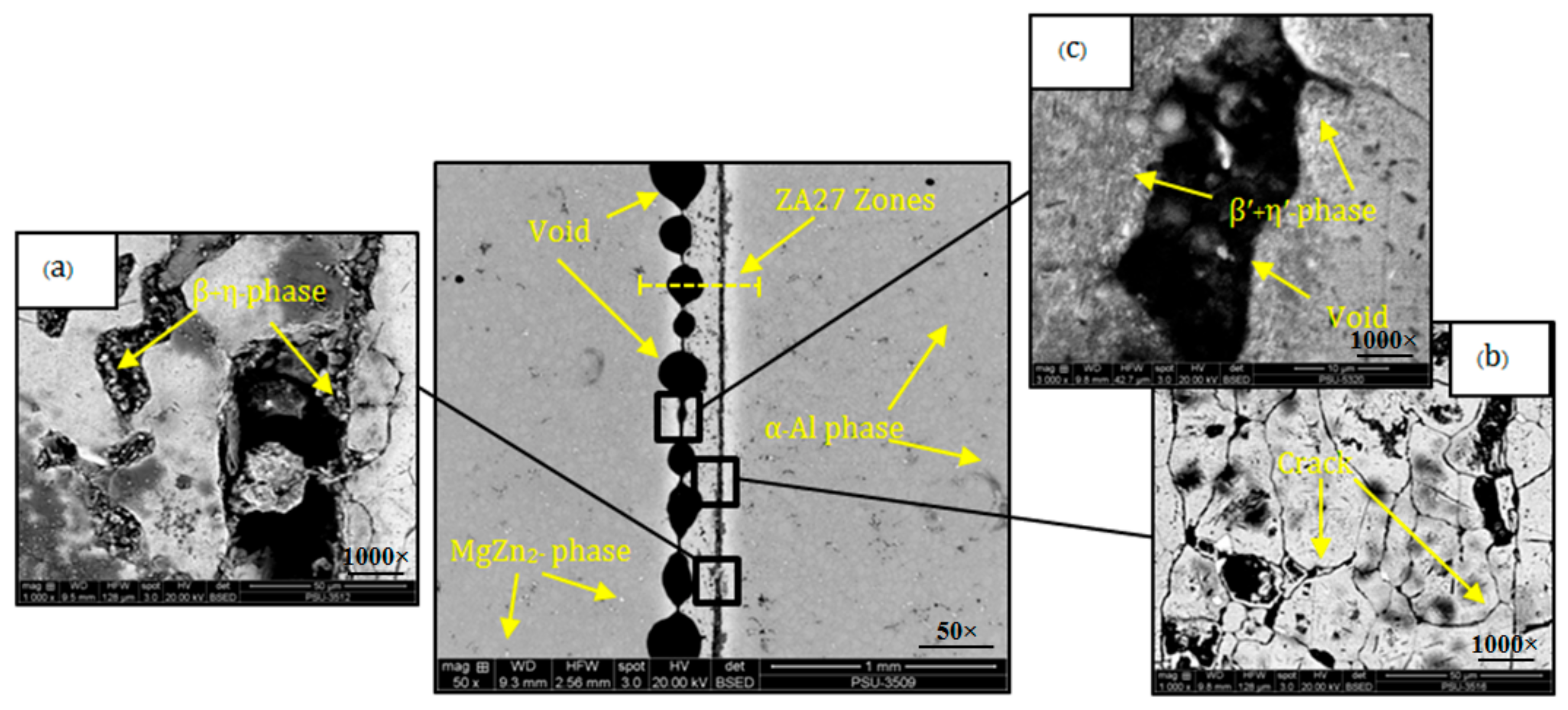



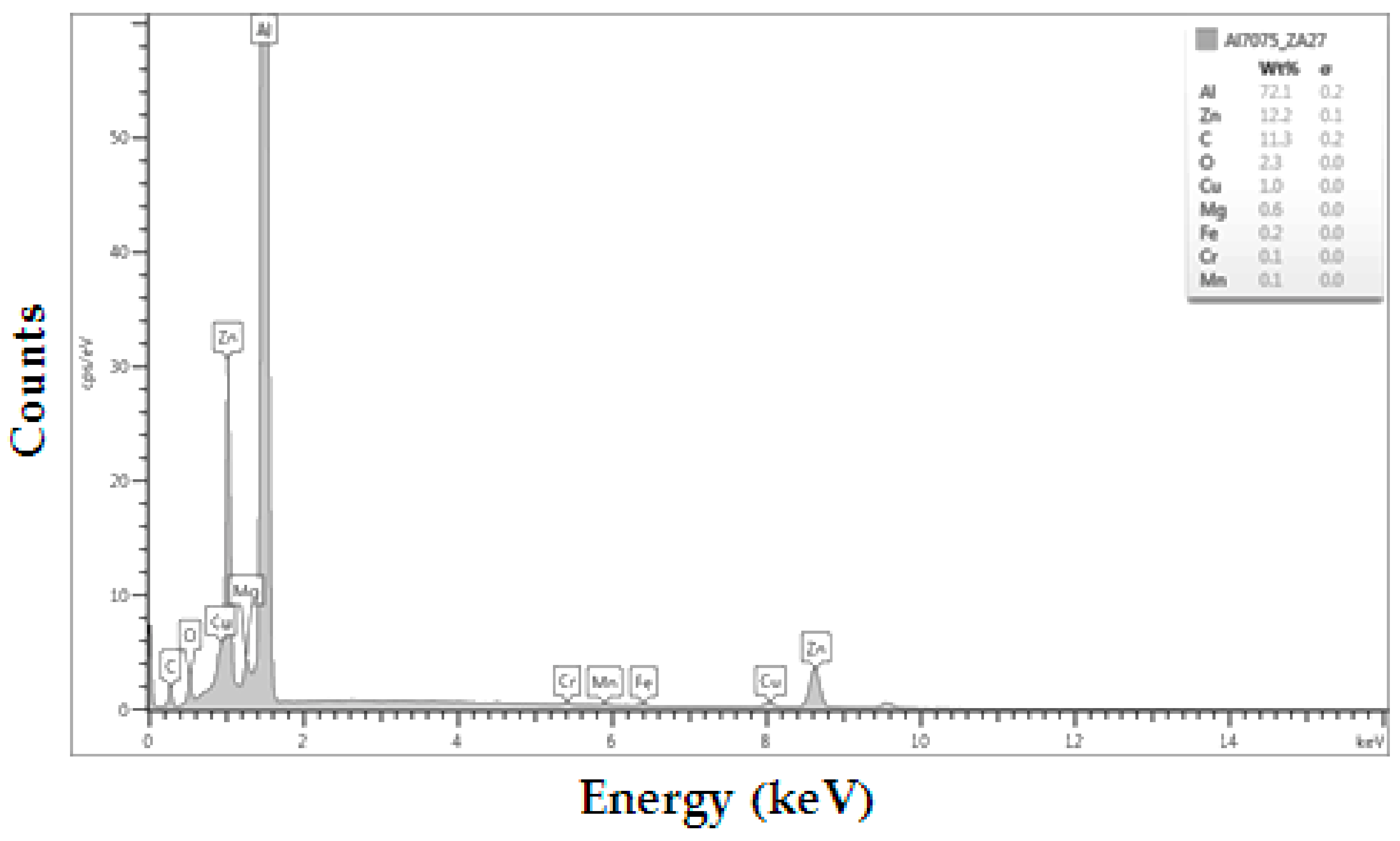
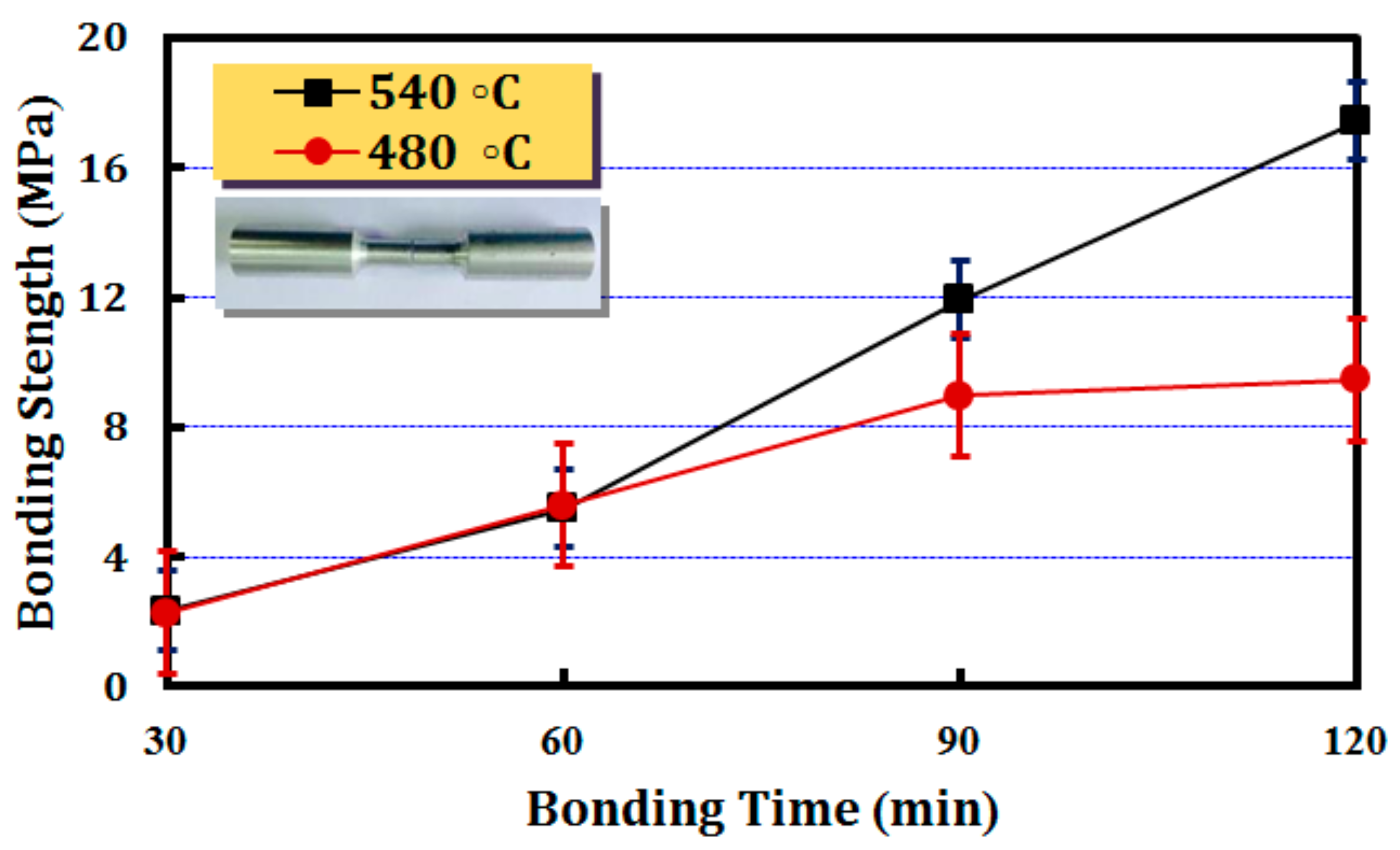




| Element | Zn | Mg | Cu | Fe | Cr | Mn | Si | Ni | Others | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| Al 7075 | 6.08 | 2.5 | 1.93 | 0.46 | 0.19 | - | - | - | 0.45 | Bal. |
| ZA27 | 89.3 | 0.82 | 3.22 | 0.01 | - | 0.91 | 0.81 | 0.05 | - | 4.20 |
| Factors | Levels | |||
|---|---|---|---|---|
| Bonding time (min) | 30 | 60 | 90 | 120 |
| Bonding temperature (degree celsius) | 480 | 540 | - | - |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Bonding Time (Btim) | 3 | 438.976 | 146.325 | 424.36 | 0.000 |
| Bonding Temperature (Btem) | 1 | 47.489 | 47.489 | 137.72 | 0.000 |
| Btim × Btem | 3 | 65.853 | 21.951 | 63.66 | 0.000 |
| Error | 16 | 5.517 | 0.345 | - | - |
| Total | 23 | 557.835 | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meengam, C.; Dunyakul, Y.; Maunkhaw, D.; Chainarong, S. Transient Liquid Phase Bonding of Semi-Solid Metal 7075 Aluminum Alloy using ZA27 Zinc Alloy Interlayer. Metals 2018, 8, 637. https://doi.org/10.3390/met8080637
Meengam C, Dunyakul Y, Maunkhaw D, Chainarong S. Transient Liquid Phase Bonding of Semi-Solid Metal 7075 Aluminum Alloy using ZA27 Zinc Alloy Interlayer. Metals. 2018; 8(8):637. https://doi.org/10.3390/met8080637
Chicago/Turabian StyleMeengam, Chaiyoot, Yongyuth Dunyakul, Dech Maunkhaw, and Suppachai Chainarong. 2018. "Transient Liquid Phase Bonding of Semi-Solid Metal 7075 Aluminum Alloy using ZA27 Zinc Alloy Interlayer" Metals 8, no. 8: 637. https://doi.org/10.3390/met8080637





