The Effect of Tellurium on the Formation of MnTe-MnS Composite Inclusions in Non-Quenched and Tempered Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results
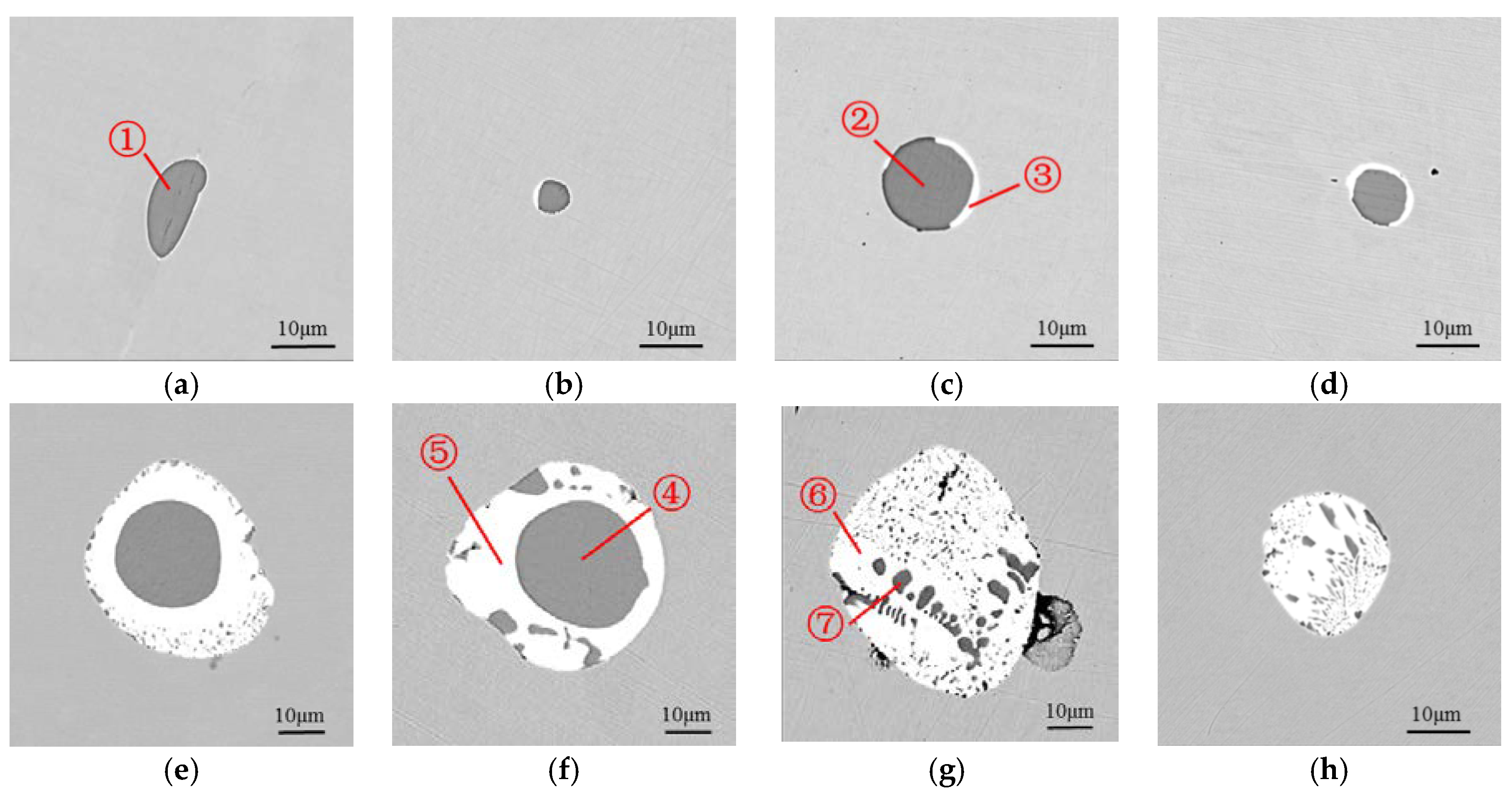
3.1. Optical and SEM-EDS Analysis
3.2. Statistics of Inclusions
3.3. Analysis of the Three-Dimensional Morphology of Inclusions
4. Discussion
4.1. The Effect of Te on the Aspect Ratio of the Inclusion
4.2. The Precipitation of the MnTe-MnS Binary Phase from Steel
4.3. The Precipitation of MnS and MnTe from the MnTe-MnS Binary Phase
5. Conclusions
- After adding a certain amount of Te in the 38MnVS6 steel, MnTe was generated and wrapped the MnS inclusion. With the increase of Te content from 130 ppm to 2400 ppm, the diameter of the inclusion increased. The aspect ratio of inclusions in all steels was nearly the same. Most inclusions were in the aspect ratio range of 1~3.
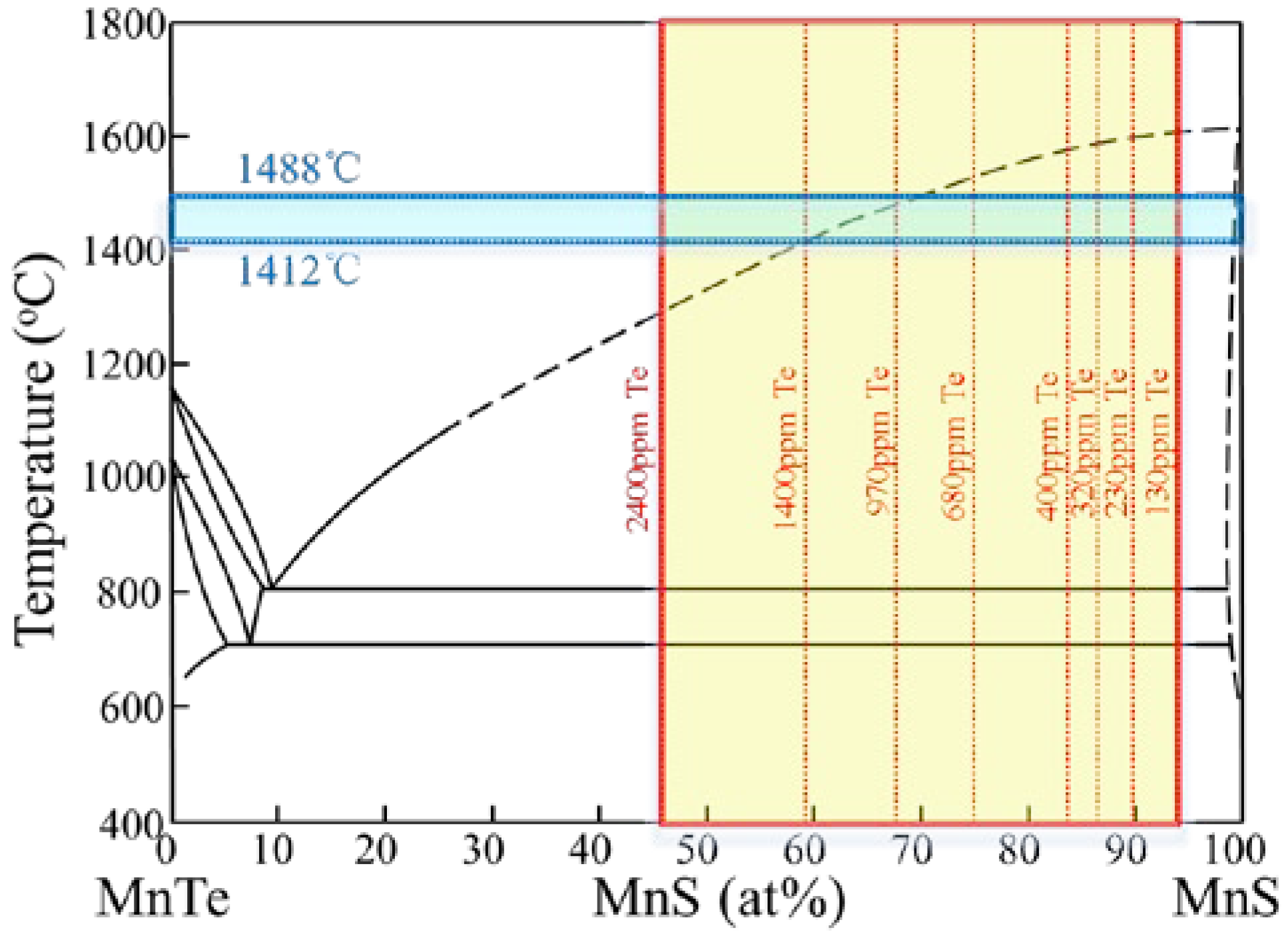
- The 38MnVS6 steel solidified in the temperature range of 1488~1412 °C, and MnTe and MnS were precipitated from the liquid steel and formed a MnTe-MnS binary liquid phase. In the steel with low Te content, a small amount of dendritic MnS inclusion was first precipitated in the interdendritic regions. With the increase of Te content, few dendritic MnS inclusions were observed. After the solidification of the steel, the MnTe-MnS binary phase kept a liquid state and maintained a spherical shape for the minimum surface free energy.
- When the temperature fell down from 1412 °C to 810 °C, MnS was precipitated from the MnTe-MnS binary liquid phase. Once the temperature reached 810 °C, both MnTe and MnS precipitated from the MnTe-MnS binary liquid phase. In the steel with low Te content, the MnTe and MnS precipitated in the form of the divorced eutectic, forming a composite inclusion with one MnS core. In the steel with high Te content, both divorced eutectic reactions and eutectic reactions occurred, leading to the inclusion containing many small MnS particles.
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, L.; Malfliet, A.; Wollants, P.; Blanpain, B.; Guo, M. Effect of surfactant te on the formation of MnS inclusions in steel. Metall. Mater. Trans. B 2017, 48, 1–12. [Google Scholar] [CrossRef]
- Yaguchi, H.; Onodera, N. The effect of tellurium on the machinability of AISI 12L14+Te steel. Trans. Inst. Iron Steel Inst. Jpn. 1988, 28, 1051–1059. [Google Scholar] [CrossRef]
- Popova, I.V.; Nasibov, A.G.; Gulei, G.G.; Sveshnikova, G.A. Nonmetallic inclusions and austenite grains of steel containing tellurium. Met. Sci. Heat Treat. 1986, 28, 52–55. [Google Scholar] [CrossRef]
- Mahmutoviü, A.; Rimac, M. Modification of non-metallic inclusions by tellurium in austenitic stainless. J. Trands Dev. Mach. Assoc. Technol. 2015, 19, 53–56. [Google Scholar]
- Costa, E.; Luiz, N.; Silva, M.D.; Machado, A.; Ezugwu, E. Influence of tellurium addition on drilling of microalloyed steel (DIN 38MnS6). Ind. Lubr. Tribol. 2011, 63, 420–426. [Google Scholar] [CrossRef]
- Luiz, N.E.; Machado, Á.R. Development trends and review of free-machining steels. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2008, 222, 347–360. [Google Scholar] [CrossRef]
- Smith, T.B.; Clayton, D.B. Distribution of tellurium in free-cutting steels. Nature 1963, 198, 380–381. [Google Scholar] [CrossRef]
- Li, D.; Gao, S.; Zhang, L.; Wang, Z.; Dong, X. Formation and behavior of telluride in free cutting steels. Iron Steel 1987, 22, 38–44. [Google Scholar] [CrossRef]
- Gupta, G.; Robertson, D.G.C.; Schlesinger, M.E. Tellurium thermodynamics in austenitic iron. Can. Metall. Q. 2013, 44, 351–356. [Google Scholar] [CrossRef]
- Abeyama, S.; Nakamura, S. An ultra-free maching stainless steel “DSR6F”. Jpn. Inst. Met. 2011, 21, 363–365. [Google Scholar] [CrossRef]
- Kimura, A.; Nishikiori, K. Nitrided super free-machining steel “SFC3FT-N”. Denki Seiko (Electr. Furn. Steel) 1989, 59, 59–64. [Google Scholar] [CrossRef]
- Speich, G.R.; Spitzig, W.A. Effect of volume fraction and shape of sulfide inclusions on through-thickness ductility and impact energy of high-strength 4340 plate steels. Metall. Trans. A 1982, 13, 2239–2258. [Google Scholar] [CrossRef]
- Luyckx, L.; Bell, J.R.; Mclean, A.; Korchynsky, M. Sulfide shape control in high strength low alloy steels. Metall. Trans. 1970, 1, 3341–3350. [Google Scholar]
- Katoh, T.; Abeyama, S.; Kimura, A.; Nakamura, S. A study on resulfurized free-machining steel containing a small amount of tellurium. Denki Seiko (Electr. Furn. Steel) 1982, 53, 195–202. [Google Scholar] [CrossRef]
- Watson, J.D. Microscopy and the development of free-machining steels. In Applied Metallography; Vander Voort, G.F., Ed.; Springer: New York, NY, USA, 1986; pp. 211–236. [Google Scholar]
- Tien, T.Y.; Van Vlack, L.H.; Martin, R.J. The System MnTe-MnS: Progress Report; The University of Michigan: New York, NY, USA, September 1967. [Google Scholar]












| Composition | C | Si | Mn | P | S | Cr | V | Al | Ti | Nb | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.383 | 0.574 | 1.399 | 0.01 | 0.051 | 0.179 | 0.099 | 0.018 | 0.017 | 0.016 | 0.123 |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Content | 130 | 230 | 320 | 400 | 680 | 970 | 1400 | 2400 |
| Element | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Mn | 56.53 | 55.87 | 23.00 | 59.35 | 26.15 | 25.45 | 56.41 |
| S | 28.64 | 29.14 | 2.33 | 30.38 | 2.52 | 2.18 | 28.51 |
| Fe | 11.35 | 9.62 | 13.00 | 5.97 | 5.51 | 6.93 | 8.23 |
| Te | 3.47 | 5.37 | 61.67 | 4.29 | 65.82 | 65.43 | 6.85 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, P.; Yang, Q.; Zhang, D.; Yang, S.; Fu, J. The Effect of Tellurium on the Formation of MnTe-MnS Composite Inclusions in Non-Quenched and Tempered Steel. Metals 2018, 8, 639. https://doi.org/10.3390/met8080639
Shen P, Yang Q, Zhang D, Yang S, Fu J. The Effect of Tellurium on the Formation of MnTe-MnS Composite Inclusions in Non-Quenched and Tempered Steel. Metals. 2018; 8(8):639. https://doi.org/10.3390/met8080639
Chicago/Turabian StyleShen, Ping, Qiankun Yang, Dong Zhang, Shufeng Yang, and Jianxun Fu. 2018. "The Effect of Tellurium on the Formation of MnTe-MnS Composite Inclusions in Non-Quenched and Tempered Steel" Metals 8, no. 8: 639. https://doi.org/10.3390/met8080639





