Evolutionary Medicine and Future of Humanity: Will Evolution Have the Final Word?
Abstract
:Introduction
Bio-Cultural Evolution
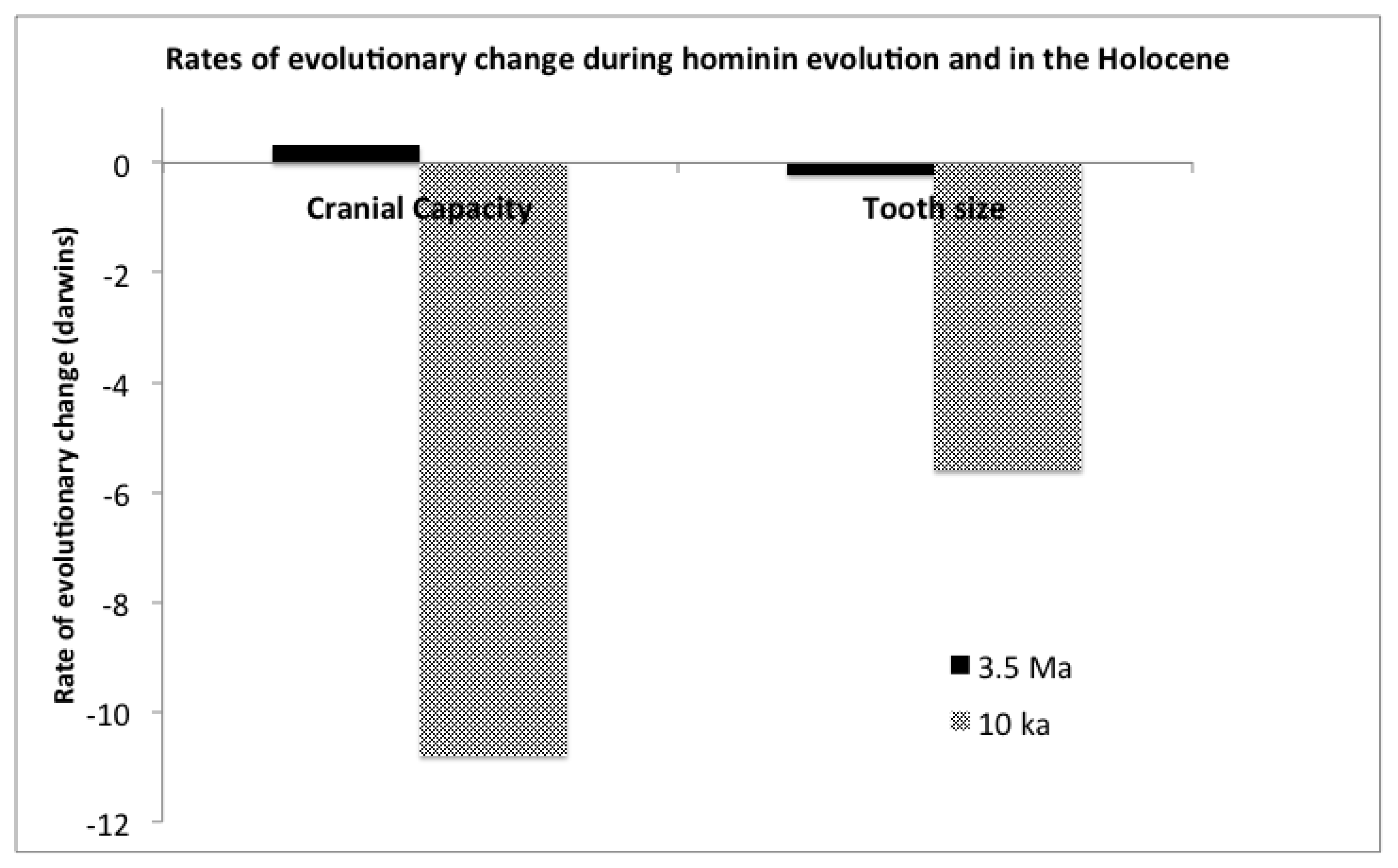
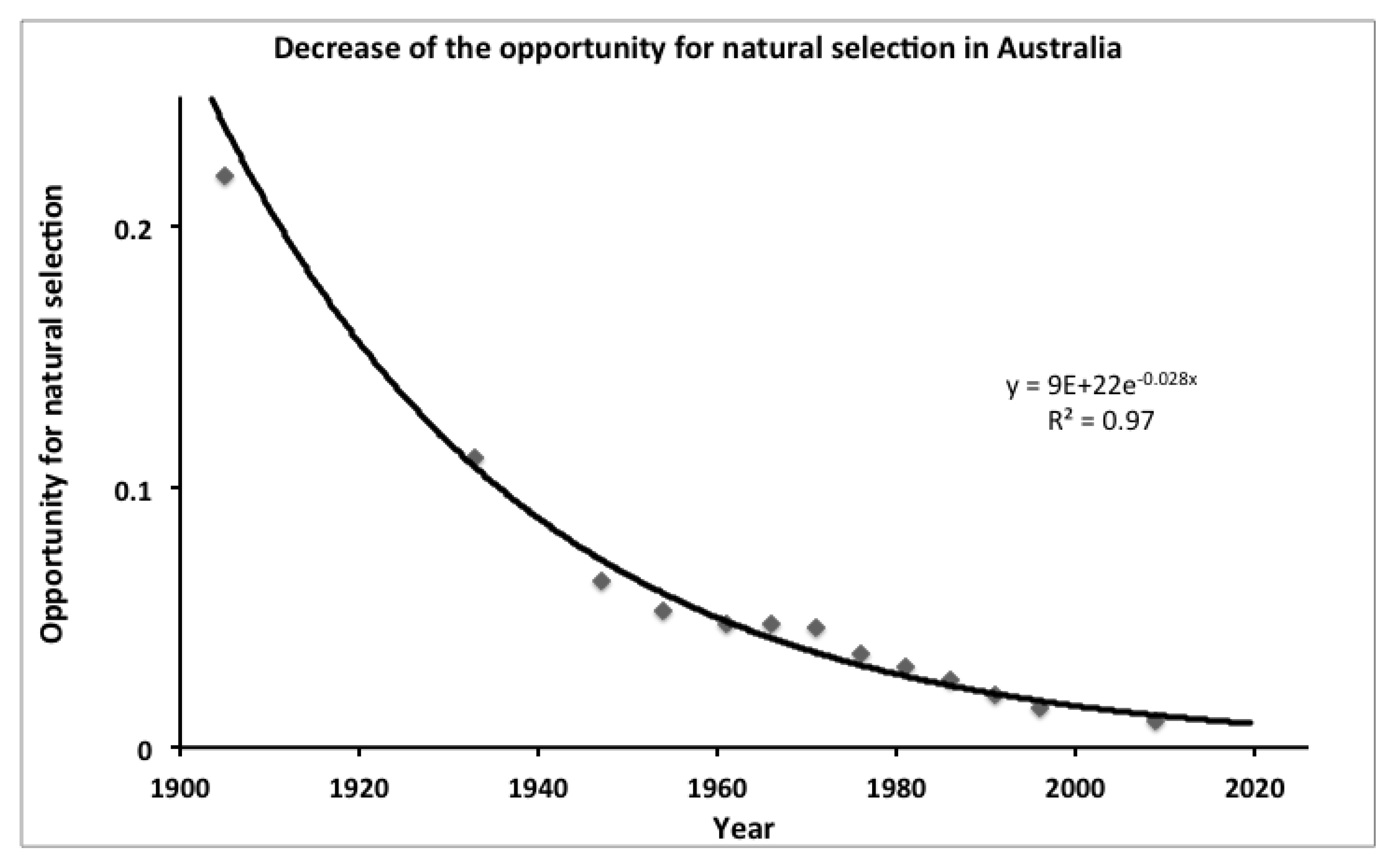
Present day Mismatches and Lamarckian Selection
Diet and Sedentism: Will Evolution Leave Humans to Their Own Fate?
Conclusions
Conflict of Interest
References
- S. Boyd Eaton, Melvin Konner, and Marjorie Shostak. “Stone agers in the fast lane: chronic degenerative diseases in evolutionary perspective.” American Journal of Medicine 84 (1998): 739–49. [Google Scholar] [CrossRef]
- George Williams, and Randolph Nesse. Why We Get Sick: The New Science of Darwinian Medicine. New York: Vintage Books, 1996. [Google Scholar]
- S. Boyd Eaton, Beverly I. Strassman, Randolph M. Nesse, James V. Neel, and et al. “Evolutionary health promotion.” Preventive Medicine 34 (2002): 109–18. [Google Scholar] [CrossRef] [PubMed]
- Wenda Travathan. “Evolutionary medicine.” Annual Review of Anthropology 36 (2007): 139–54. [Google Scholar] [CrossRef]
- Stephen S. Stearns, Randolph M. Nesse, Diddahally R. Govindaraju, and Peter T. Ellison. “Evolutionary perspectives on health and medicine.” Proceedings of the National Academy of Sciences 107, no. 1 (2010): 1691–95. [Google Scholar] [CrossRef] [PubMed]
- Sean G. Byars, Douglas Ewbank, Diddahally R. Govindaraju, and Stephen C. Stearns. “Natural selection in a contemporary human population.” Proceedings of the National Academy of Sciences 107, no. 1 (2010): 1787–92. [Google Scholar] [CrossRef] [PubMed]
- Maciej Henneberg. “The rate of human morphological microevolution and taxonomic diversity of hominids.” Studies in Historical Anthropology 4:2004 (2006): 49–59, Essays in memory of Andrzej Wiercinski. [Google Scholar]
- Yuval Itan, Adam Powell, Mark A. Beaumont, Joachim Burger, and Mark G. Thomas. “The Origins of lactase persistence in Europe.” PLoS Computational Biology 5, no. 8 (2009): e1000491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catherine J. Ingram, Charlotte A. Mulcare, Yuval Itan, and et al. “Lactose digestion and the evolutionary genetics of lactase persistence.” Human Genetics 124, no. 6 (2009): 579–91. [Google Scholar]
- Robert Bednarik. “An aetiology of hominin behaviour.” HOMO - Journal of Comparative Human Biology 63 (2012): 319–35. [Google Scholar] [CrossRef] [PubMed]
- Nicolas Claidière, and Jean-Baptiste André. “The transmission of genes and culture: A questionable analogy.” Evolutionary Biology 39, no. 1 (2012): 12–24. [Google Scholar] [CrossRef]
- Dominique Guillo. “Does culture evolve by means of Darwinian selection? The lessons of Candide’s travels.” Social Science Information 51 (2012): 364–88. [Google Scholar] [CrossRef]
- Jan Strzalko, and Maciej Henneberg. “Hominization as a necessary effect of evolution of a non-genetic mode of hereditary transmission.” In Evolution and Environment. Edited by VJA Novak and J Milkovsky. Prague: CSAV Press, 1982, pp. 367–76. [Google Scholar]
- Maciej Henneberg. “Decrease of human skull size in the Holocene.” Human Biology 63 (1988): 395–405. [Google Scholar]
- Ronald A. Fisher. The Genetical Theory of Natural Selection. Cambridge, UK: University Press, 1930. [Google Scholar]
- Maciej Henneberg. “Reproductive possibilities and estimations of the biological dynamics of earlier human populations.” Journal of Human Evolution 5 (1976): 41–48. [Google Scholar] [CrossRef]
- Arthur Saniotis, and Maciej Henneberg. “Medicine could be constructing human bodies in the future.” Medical Hypotheses 77 (2011): 560–64. [Google Scholar] [CrossRef] [PubMed]
- James F. Crow. “Some possibilities for measuring selection intensities in man.” Human Biology 30 (1958): 1–13. [Google Scholar] [PubMed]
- Gyula Acsadi, and Janos Nemeskeri. History of Human Life Span and Mortality. Budapest: Akademiai Kiado, 1970. [Google Scholar]
- Janusz Piontek, and Maciej Henneberg. “Mortality changes in a Polish rural community (1350–1972) and an estimation of their evolutionary significance.” American Journal of Physiology 54 (1981): 129–38. [Google Scholar] [CrossRef] [PubMed]
- Maciej Henneberg, and Maryna Steyn. “A preliminary report on the palaeodemography of K2 and Mapungubwe population (South Africa).” Human Biology 65 (1994): 105–20. [Google Scholar]
- Alicja Budnik, Grazyna Liczbinska, and Izabela Gumna. “Demographic trends and biological status of historic populations from Central Poland: The Ostrów Lednicki microregion.” American Journal of Physical Anthropology 125, no. 4 (2004): 369–81. [Google Scholar] [CrossRef] [PubMed]
- Carl Stephan, and Maciej Henneberg. “Medicine may be reducing the human capacity to survive.” Medical Hypotheses 57 (2001): 633–37. [Google Scholar] [CrossRef] [PubMed]
- Michael C. Whitlock, and Brad H. Davis. “Genetic Load, eLS (2011).” , 2011. [Google Scholar] [CrossRef]
- C. Loring Brace. “The probable mutation effect.” The American Naturalist 98, no. 903 (1964): 453–55. [Google Scholar] [CrossRef]
- John Hawks, Eric T. Wang, Gregory M. Cochran, and et al. “Recent acceleration of human adaptive evolution.” Proceedings of the National Academy of Sciences 104, no. 52 (2007): 20753–58. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. “Global Health Observatory (GHO).” Available online: http://www.who.int/gho/mortality_burden_disease/life_tables/life_tables/en/index.html (accessed on 21 May 2013).
- Michael F. Antolin, Kristin P. Jenkins, Carl T. Bergstrom, Bernard J. Crespi, Subhajyoti De, Angela Hancock, Kathryn A. Hanley, Thomas R. Meagher, Andres Moreno-Estrada, Randolph M. Nesse, Gilbert S. Omenn, and Stephen C. Stearns. “Evolution and medicine in undergrduate education: a prescription for all biology students.” Evolution 66, no. 6 (2012): 1991–2006. [Google Scholar] [CrossRef] [PubMed]
- Pedro Carrera-Bastos, Maelan Fontes-Villalba, James H. O’Keefe, Staffan Lindeberg, and Loren Cordain. “The western diet and lifestyle and diseases of civilization.” Research Reports in Clinical Cardiology 2 (2011): 15–35. [Google Scholar] [CrossRef]
- Staffan Lindeberg. Food and Western Disease: Health and Nutrition from an Evolutionary Perspective. Chichester, UK: Wiley-Blackwell, 2010. [Google Scholar]
- Tessa M. Pollard. Western Diseases: An Evolutionary Perspective. Cambridge: Cambridge University Press, 2008. [Google Scholar]
- Janet A. DiPietro. “Maternal Stress in Pregnancy: Considerations for Fetal Development.” Journal of Adolescent Health 51, no. 2 (2012): S3–S8. [Google Scholar] [CrossRef]
- Sheau-Fang Ng, Ruby C. Y. Lin, D. Ross Laybutt, Romain Barres, Julie A. Owens, and Margaret J. Morris. “Chronic high-fat diet in fathers programs b-cell dysfunction in female rat offspring.” Nature 467, no. 7318 (2010): 963–66. [Google Scholar] [CrossRef] [PubMed]
- Sanjay S. Kasturi, Justin Tannir, and Robert E. Brannigan. “The metabolic syndrome and male infertility.” Journal of Andrology 29 (2008): 251–59. [Google Scholar] [CrossRef] [PubMed]
- James P. Curley, Rahia Mashoodh, and Frances A. Champagne. “Epigenetics and the origins of paternal effects.” Hormones and Behavior 59 (2011): 306–14. [Google Scholar] [CrossRef] [PubMed]
- Marcus E. Pembrey, Lars O. Bygren, Gunnar Kaati, Sören Edvinsson, Kate Northstone, Michael Sjostrom, and Jean Golding. “Sex-specific, male-line transgenerational responses in humans.” European Journal of Human Genetics 14, no. 2 (2006): 159–66. [Google Scholar] [CrossRef] [PubMed]
- Andrea M. Hegedus, Arthur I. Alterman, and Ralph E. Tarter. “Learning achievement in sons of alcoholics.” Alcoholism: Clinical and Experimental Research 8 (1984): 330–33. [Google Scholar] [CrossRef]
- James P. Curley, and Rahia Mashoodh. “Parent-of-Origin and Trans-Generational Germline Influences on Behavioral Development: The Interacting Roles of Mothers, Fathers, and Grandparents.” Developmental Psychobiology 52, no. 4 (2010): 312–30. [Google Scholar] [CrossRef] [PubMed]
- Eva Jablonka, and Marion J. Lamb. Epigenetic Inheritance and Evolution: The Lamarckian Dimension. Oxford: Oxford University Press, 1995. [Google Scholar]
- Eva Jablonka, and Gal Raz. “Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution.” The Quarterly Review of Biology 84 (2009): 131–76. [Google Scholar] [CrossRef] [PubMed]
- Lars O. Bygren, Gunnar Kaati, and Soren Edvinsson. “Longevity determined by ancestors’ overnutrition during their slow growth period.” Acta Biotheoretica 49 (2001): 53–59. [Google Scholar] [CrossRef] [PubMed]
- Gunnar Kaati, Lars O. Bygren, and Soren Edvinsson. “Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period.” European Journal of Human Genetics 10 (2002): 682–88. [Google Scholar] [CrossRef] [PubMed]
- Gregory Bateson. Steps to an Ecology of Mind. St. Albans: Paladin, 1973. [Google Scholar]
- Herman Pontzer, David A. Raichlen, Brian M. Wood, Audax Z. P. Mabulla, Susan B. Racette, and Frank W. Marlowe. “Hunter-gatherer energetics and human obesity.” PLoS One 7, no. 7 (2012): e40503. [Google Scholar] [CrossRef] [PubMed]
- S. Boyd Eaton, and Melvin Konner. “Paleolithic nutrition. A consideration of its nature and current implications.” New England Journal of Medicine 312, no. 5 (1985): 283–89. [Google Scholar] [CrossRef] [PubMed]
- Peter Gluckman, and Mark Hanson. Mismatch: Why Our World No Longer Fits Our Bodies. Oxford: Oxford University Press, 2006. [Google Scholar]
- Bethany L. Turner, Kenneth Maes, Jennifer Sweeney, and George J. Armelagos. “Human evolution, diet, and nutrition.” In Evolutionary Medicine and Health: New Perspectives. Edited by Wenda R. Trevathan, E.O. Smith and James J. McKenna. Oxford: Oxford University Press, 2008, pp. 55–71. [Google Scholar]
- Arthur Saniotis. “Evolutionary medicine and bioethics: an Asian perspective.” Calicut Medical Journal 8, no. 4 (2010): e4.1–5. [Google Scholar]
- Henry Blackburn, and Ronald Prineas. “Diet and hypertension: anthropology, epidemiology, and public health implications.” Progress in Biochemical Pharmacology 19 (1983): 31–79. [Google Scholar] [PubMed]
- Loren Cordain, S. Boyd Eaton, Janette Brand-Miller, Neil Mann, and Kim Hill. “The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic.” European Journal of Clinical Nutrition 56 (2002): S42–S52. [Google Scholar] [CrossRef] [PubMed]
- S. Boyd Eaton, and Loren Cordain. “Evolutionary health promotion: a consideration of common counter-arguments.” Preventive Medicine 34 (2002): 119–23. [Google Scholar] [CrossRef] [PubMed]
- Loren Cordain, Michael R. Eades, and Mary D. Eades. “Hyperinsulinemic diseases of civilization: more than just syndrome X.” Comp Biochem Physiol Part A 136 (2003): 95–112. [Google Scholar] [CrossRef]
- James H. O’Keefe, Robert Vogel, Carl J. Lavie, and Loren Cordain. “Achieving hunter-gatherer fitness in the 21st century: back to the future.” The American Journal of Medicine 123 (2010): 1082–86. [Google Scholar] [CrossRef] [PubMed]
- Graeme D. Ruxton, and David M. Wilkinson. “Avoidance of overheating and selection for both hair loss and bipedality in hominins.” Proceedings of the National Academy of Sciences 108, no. 52 (2011): 20965–69. [Google Scholar] [CrossRef] [PubMed]
- Mark P. Mattson. “Evolutionary aspects of human exercise—Born to run purposefully.” Ageing Research Reviews 11, no. 3 (2012): 347–52. [Google Scholar] [CrossRef] [PubMed]
- Catherine Panter-Brick. “Sexual division of labor: energetic and evolutionary scenarios.” American Journal of Human Biology 14, no. 5 (2002): 627–40. [Google Scholar] [CrossRef] [PubMed]
- David P. Strachan. “Hay fever, hygiene, and household size.” British Medical Journal 299 (1989): 1259–60. [Google Scholar] [CrossRef] [PubMed]
- David P. Strachan. “Family size, infection and atopy: the first decade of the “ygiene hypothesis”.” Thorax 55 (2000): S2–S10. [Google Scholar]
- Graham A.W. Rook, Victoria Adams, John Hunt, Rebecca Palmer, Roberta Martinelli, and Laura Rosa Brunet. “Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders.” Springer Semin Immunopathol 25 (2004): 237–55. [Google Scholar] [CrossRef] [PubMed]
- George J. Armelagos. “The paleolithic disease-scape, the hygiene hypothesis, and the second epidemiological transition.” In The Hygiene Hypothesis and Darwinian Medicine. Edited by Graham AW Rook. Basel: Birkhäuser, 2009, pp. 29–43. [Google Scholar]
- Rick M. Maizels, and Ursula Wiedermann. “Immunoregulation by microbes and parasites in the control of allergy and autoimmunity.” In The Hygiene Hypothesis and Darwinian Medicine. Edited by Graham AW Rook. Basel: Birkhäuser, 2009, pp. 45–75. [Google Scholar]
- Lucile Capuron, and Andrew H. Miller. “Cytokines and psychopathology: lessons from interferonalpha.” Biological Psychiatry 56 (2004): 819–24. [Google Scholar] [CrossRef] [PubMed]
- Sinead M. O’Brien, Lucinda V. Scott, and Timothy G. Dinan. “Cytokines: abnormalities in major depression and implications for pharmacological treatment.” Human Psychopharmacology 19 (2004): 397–403. [Google Scholar] [CrossRef] [PubMed]
- Olga J. Schiepers, Marieke C. Wichers, and Michael Maes. “Cytokines and major depression.” Progress in Neuro-Psychopharmacology and Biological Psychiatry 29 (2005): 201–17. [Google Scholar] [CrossRef] [PubMed]
- Graham A.W. Rook, and Christopher A. Lowry. “The hygiene hypothesis and affective and anxiety disorders.” In The Hygiene Hypothesis and Darwinian Medicine. Edited by Graham AW Rook. Basel: Birkhäuser, 2009, pp. 189–220. [Google Scholar]
- Timothy Noakes, and Michael Spedding. “Run for your life.” Nature 487 (2012): 295–96. [Google Scholar] [CrossRef] [PubMed]
- Bente K. Pedersen, Maria Pedersen, Karen S. Krabbe, Helle Bruunsgaard, Vance B. Matthews, and Mark A. Febbraio. “Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals.” Experimental Physiology 94 (2009): 1153–60. [Google Scholar] [CrossRef] [PubMed]
- Mark P. Mattson, and Ruiqian Wan. “Neurotrophic factors in autonomic nervous system plasticity and dysfunction.” NeuroMolecular Medicine 10 (2008): 157–68. [Google Scholar] [CrossRef] [PubMed]
- David A. Raichlen, and Adam D. Gordon. “Relationship between exercise capacity and brain size in mammals.” PLoS One 6, no. 6 (2011): e20601. [Google Scholar] [CrossRef] [PubMed]
- Robert M. Sapolsky. “Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders.” Archives of General Psychiatry 57 (2000): 925–35. [Google Scholar] [CrossRef] [PubMed]
- David A. Padgett, and Ronald Glaser. “How stress influences the immune Response.” Trends in Immunology 24, no. 8 (2003): 444–48. [Google Scholar] [CrossRef]
- Fernando Gómez-Pinilla, V. So, and J Patrick Kesslak. “Spatial learning and physical activity contribute to the induction of fibroblast growth factor: neural substrates for increased cognition associated with exercise.” Neuroscience 85 (1998): 53–61. [Google Scholar] [CrossRef]
- Klaus Fabel, Konstanze Fabel, Betty Tam, Daniela Kaufer, Armin Baiker, Natalie Simmons, Calvin J Kuo, and Theo D. Palmer. “VEGF is necessary for exercise-induced adult hippocampal neurogenesis.” European Journal of Neuroscience 18 (2003): 2803–12. [Google Scholar] [CrossRef] [PubMed]
- Laurence H. Tecott, Sheree F. Logue, Jeanne M. Wehner, and Julie A. Kauer. “Perturbed dentate gyrus function in serotonin 5-HT2C receptor mutant mice.” Proceedings of the National Academy of Sciences 95, no. 25 (1998): 15026–31. [Google Scholar] [CrossRef]
- Gregory Cochran, and Henry Harpending. The 10,000 Year Explosion: How Civilization Accelerated Human Evolution. New York: Basic Books, 2009. [Google Scholar]
- Clark S. Larsen. “Biological changes in human populations with agriculture.” Annual Review of Anthropology 24 (1995): 185–213. [Google Scholar] [CrossRef]
- Hussam Abuissa, James H. O’Keefe, and Loren Cordain. “Realigning our 21st century diet and lifestyle with our hunter-gatherer genetic identity.” Directions in Psychiatry 25 (2005): SR1–SR10. [Google Scholar]
- Russell Powell. “The future of human evolution.” British Journal for the Philosophy of Science 63, no. 1 (2012): 145–75. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saniotis, A.; Henneberg, M. Evolutionary Medicine and Future of Humanity: Will Evolution Have the Final Word? Humanities 2013, 2, 278-291. https://doi.org/10.3390/h2020278
Saniotis A, Henneberg M. Evolutionary Medicine and Future of Humanity: Will Evolution Have the Final Word? Humanities. 2013; 2(2):278-291. https://doi.org/10.3390/h2020278
Chicago/Turabian StyleSaniotis, Arthur, and Maciej Henneberg. 2013. "Evolutionary Medicine and Future of Humanity: Will Evolution Have the Final Word?" Humanities 2, no. 2: 278-291. https://doi.org/10.3390/h2020278



