Order of Inoculation during Heligmosomoides bakeri and Hymenolepis microstoma Coinfection Alters Parasite Life History and Host Responses
Abstract
:1. Introduction
2. Results and Discussion
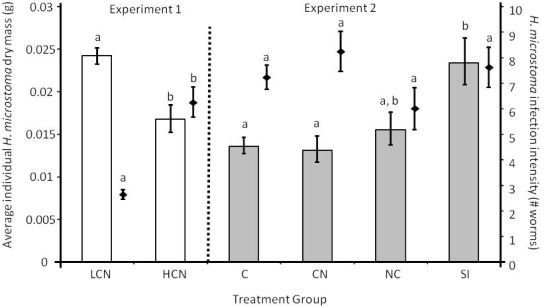
2.1. Parasite Life History
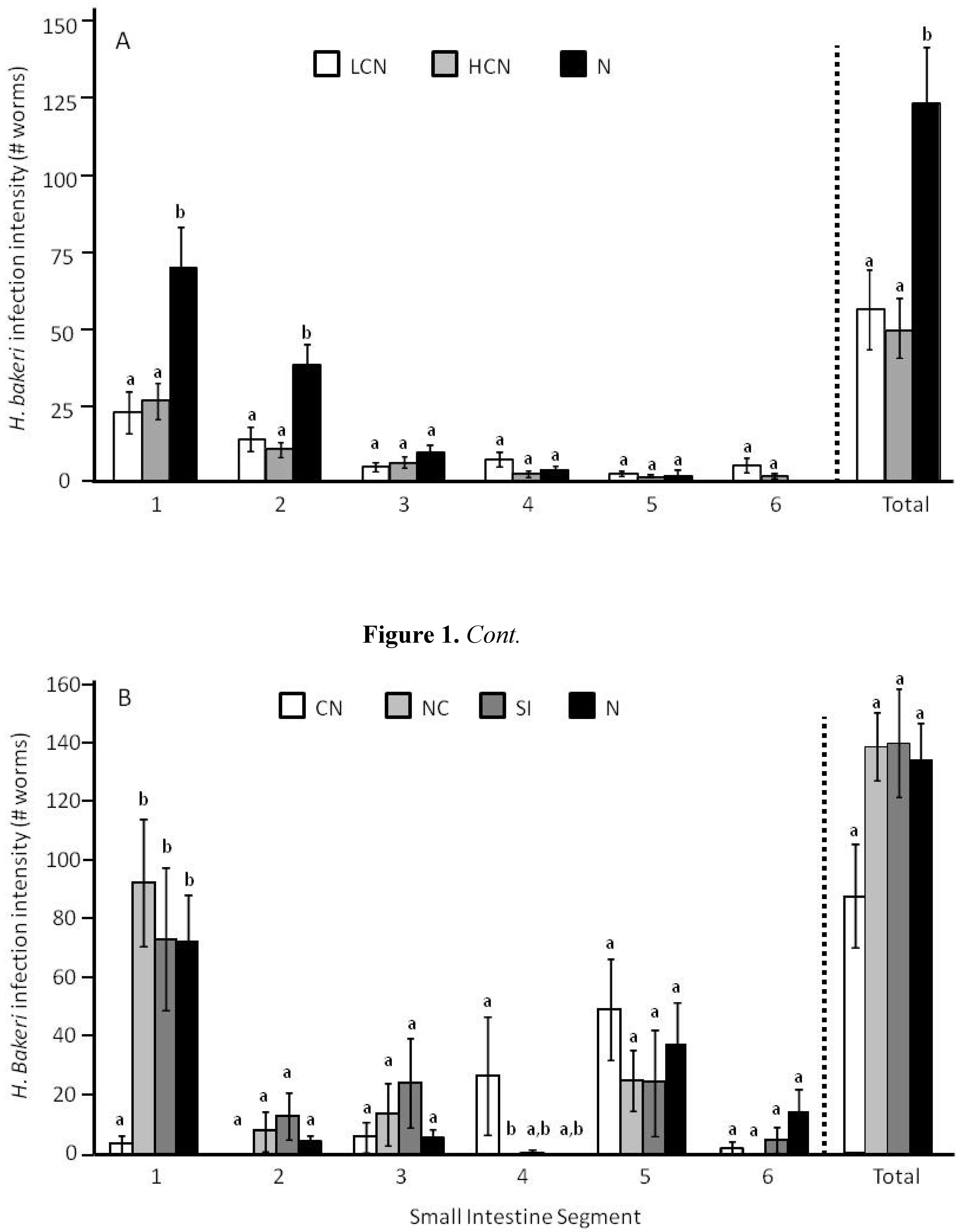
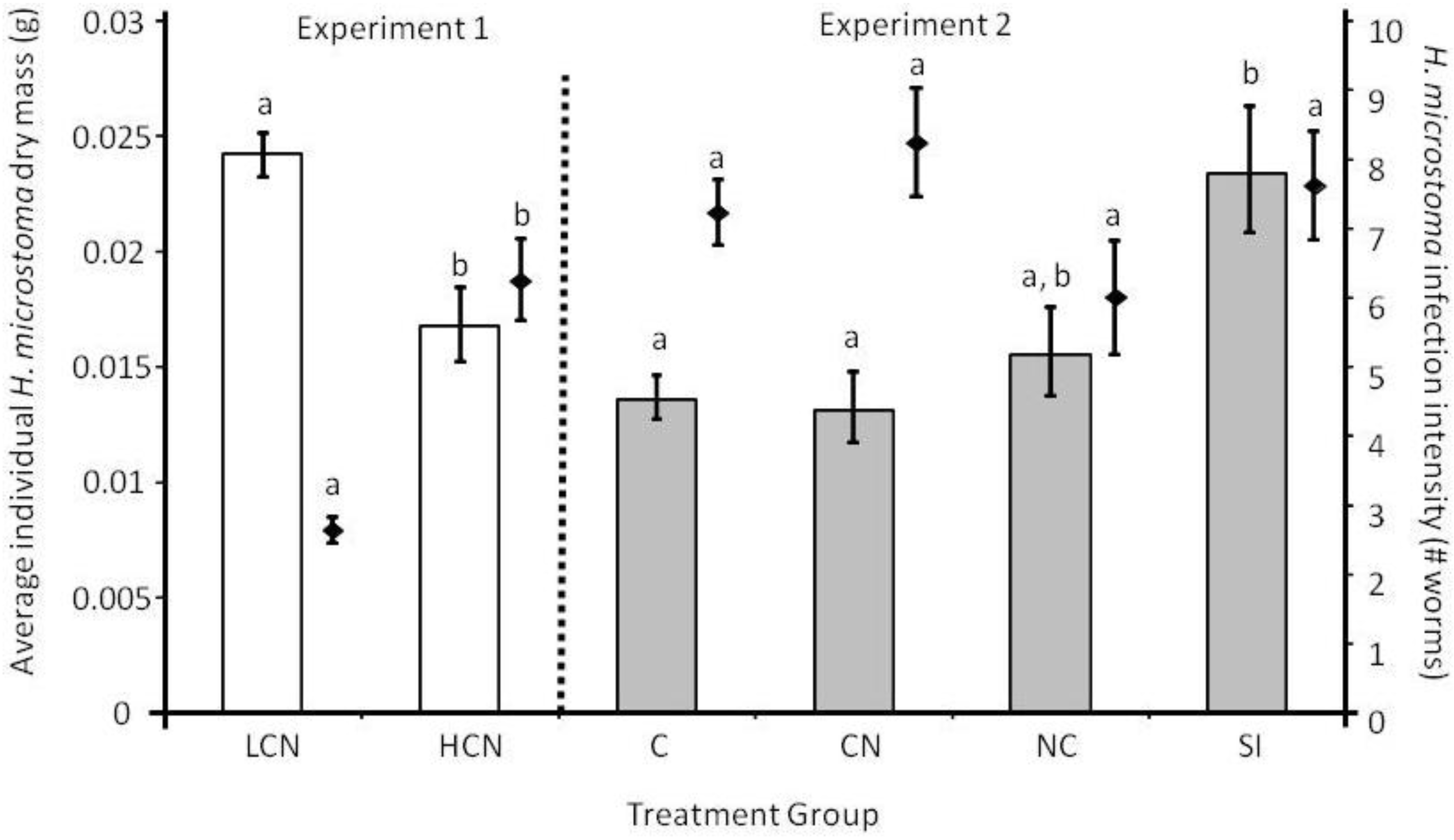
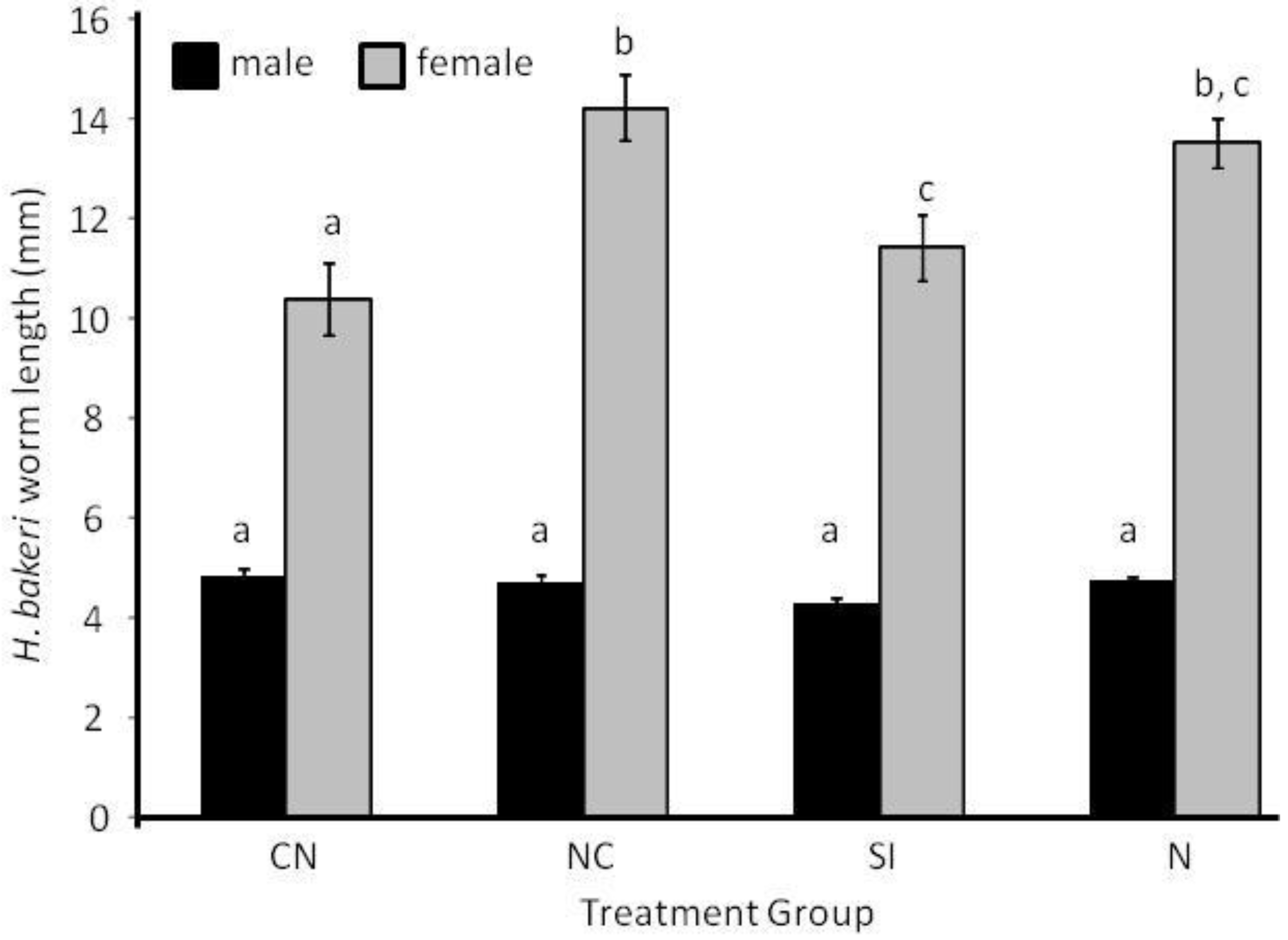
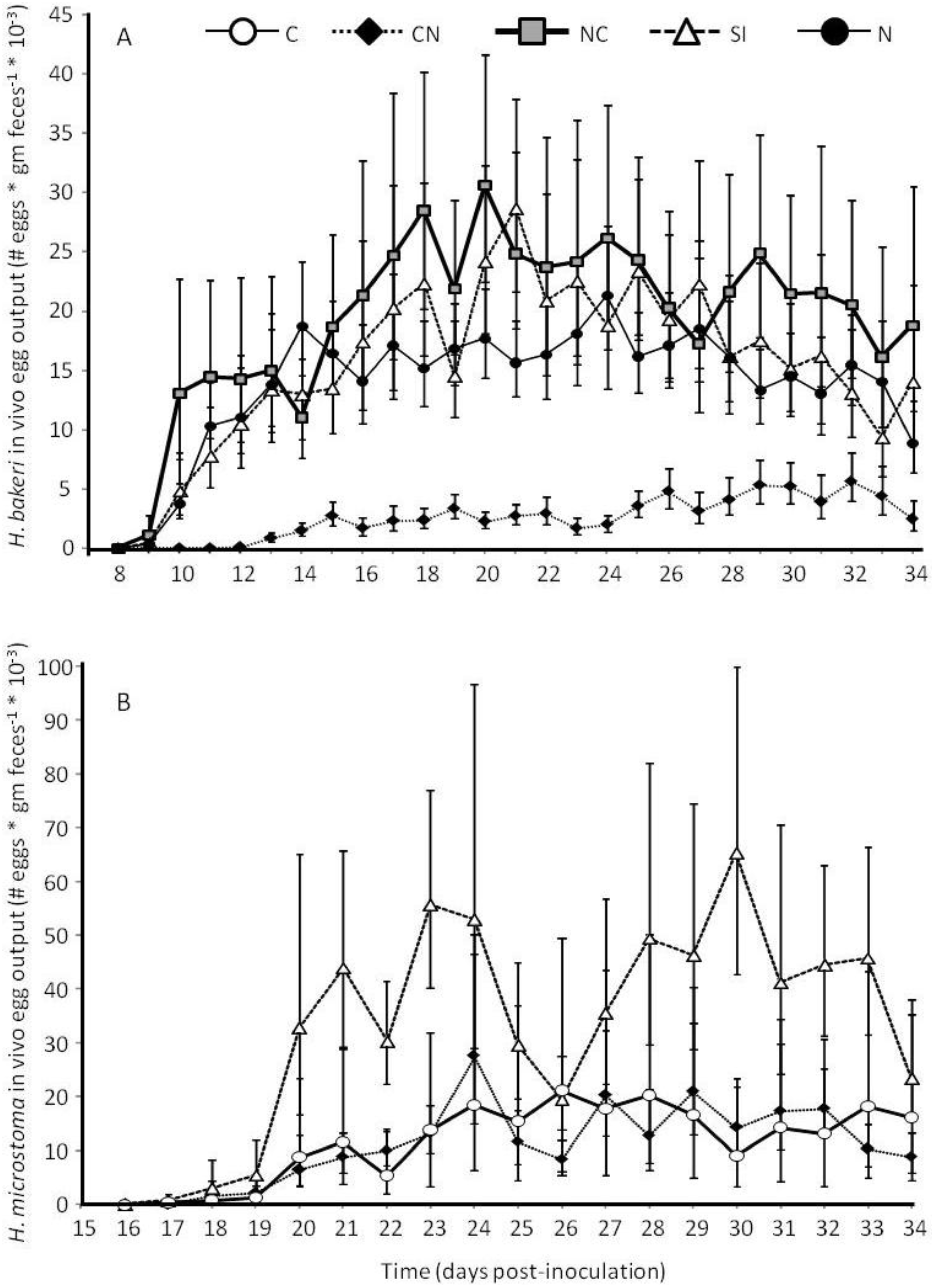
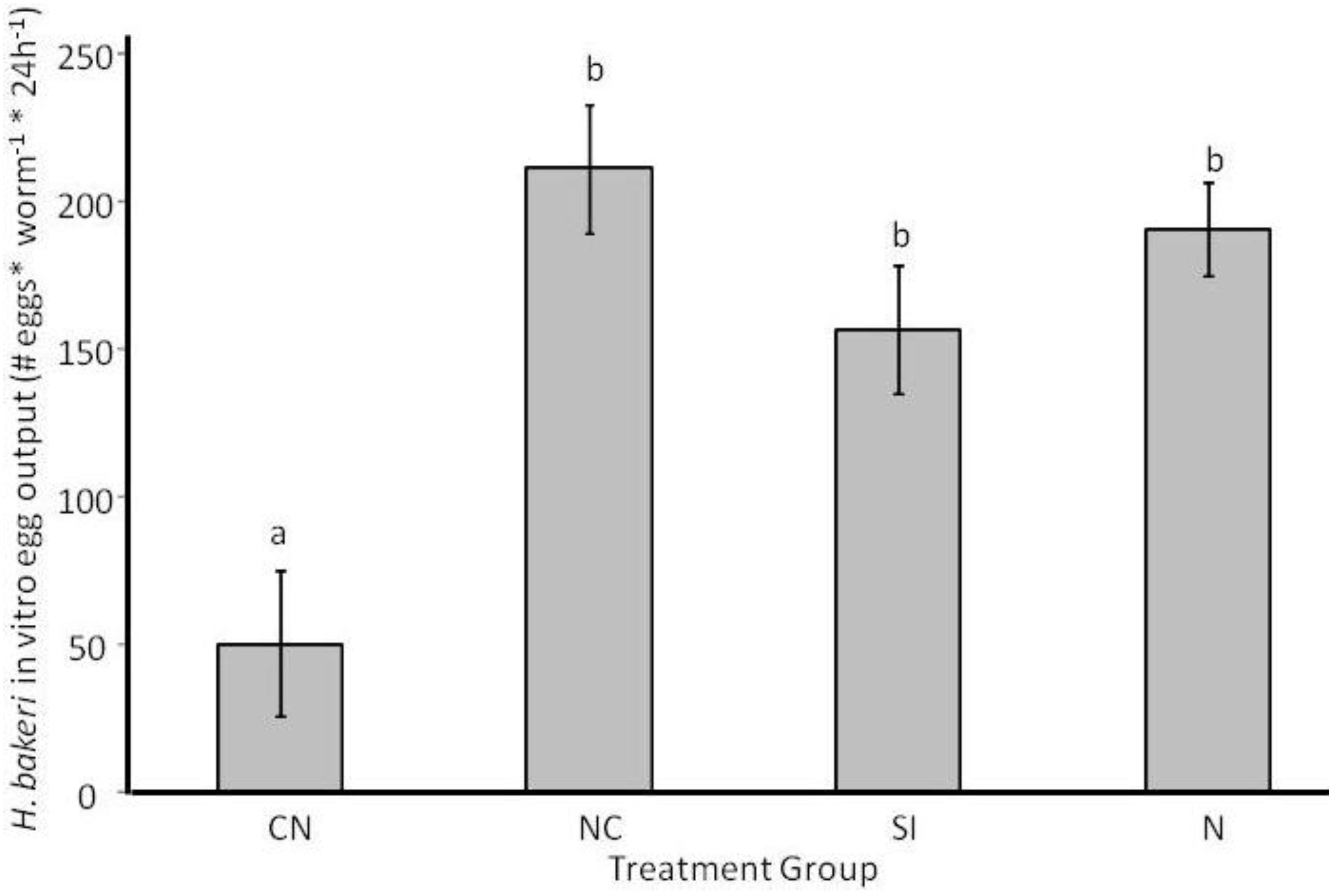
2.2. Host Morphology & Liver Function
| Initial body mass (g) | Final body mass (g) | Bile duct dry mass (g)a | Small intestine dry mass (g)b | Small intestine length (mm)b | |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| LCN | 30.1 ± 1.0 | 31.3 ± 1.0 | 0.023 ± 0.008 | 0.419 ± 0.019 | N/A c |
| HCN | 28.7 ± 0.9 | 29.6 ± 1.1 | 0.025 ± 0.005 | 0.399 ± 0.027 | N/A c |
| N | 28.8 ± 0.8 | 30.1 ± 0.6 | 0.007 ± 0.003 | 0.463 ± 0.097 | N/A c |
| Experiment 2 | |||||
| C | 29.7 ± 0.5 | 33.8 ± 0.4 | 0.026 ± 0.002 | 0.476d ± 0.007 | 442 ± 8 |
| CN | 28.8 ± 0.4 | 33.9 ± 0.5 | 0.026 ± 0.004 (U)/ 0.003 (L) | 0.556f ± 0.011 | 396 ± 14 |
| NC | 28.7 ± 0.7 | 32.2 ± 0.9 | 0.012 ± 0.002 | 0.490d,e ± 0.013 | 431 ± 16 |
| SI | 28.5 ± 0.8 | 31.9 ± 1.0 | 0.012 ± 0.002 | 0.528e,f ± 0.011 | 422 ± 14 |
| N | 29.5 ± 1.5 | 33.4 ± 1.8 | 0.003 ± 0.000 | 0.458d ± 0.008 | 426 ± 10 |
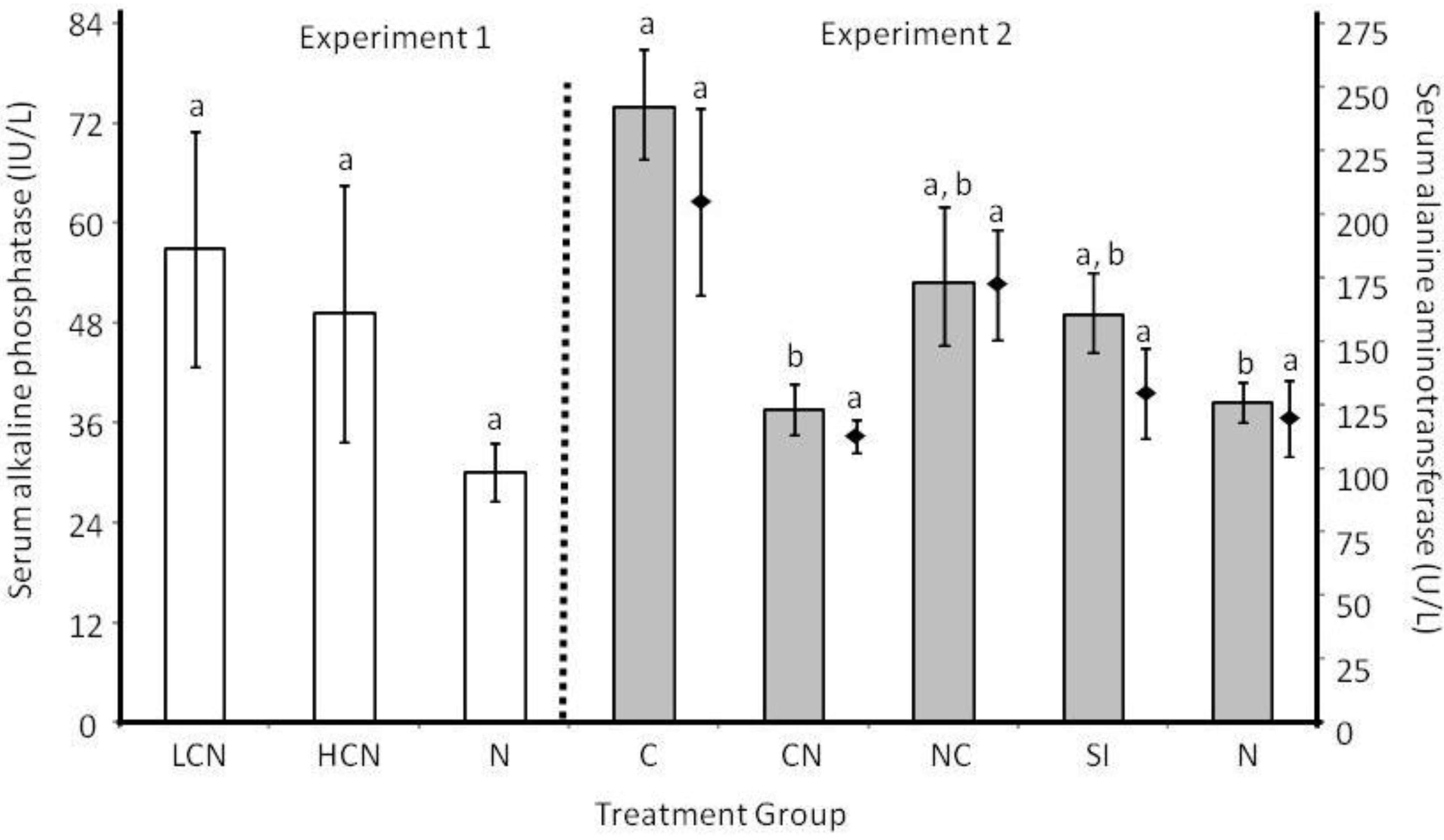
2.3. Discussion
2.3.1. Larval Establishment & Growth to Adult Size.
2.3.2. Adult Survival and Reproduction
2.3.3. Host Morphological and Physiological Responses
3. Experimental Methods
3.1. Experimental Design
3.1.1. Experiment 1
3.1.2. Experiment 2

3.2. Host Maintenance and Parasite Inoculations
3.3. Parasite Life History and Host Tissue Response Variables
3.4. Statistical Methods
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Cox, F.E.G. Concomitant infections, parasites and immune responses. Parasitol. 2001, 122, S23–S38. [Google Scholar] [CrossRef]
- Behnke, J.M.; Eira, C.; Rogan, M.; Gilbert, F.S.; Torres, J.; Miquel, J.; Lewis, J.W. Helminth species richness in wild wood mice, Apodemus sylvaticus, is enhanced by the presence of the intestinal nematode Heligmosomoides polygyrus. Parasitology 2009, 136, 793–804. [Google Scholar] [CrossRef]
- Khan, I.A.; Hakak, R.; Eberle, K.; Sayles, P.; Weiss, L.M.; Urban, J.F., Jr. Coinfection with Heligmosomoides polygyrus fails to establish CD8+ T-cell immunity against Toxoplasma gondii. Infect. Immunity 2008, 76, 1305–1313. [Google Scholar] [CrossRef]
- Bickle, Q.D.; Solum, J.; Helmby, H. Chronic intestinal nematode infection exacerbates experimental Schistosoma mansoni infection. Infect. Immunity 2008, 76, 5802–5809. [Google Scholar] [CrossRef]
- Behnke, J.M. Structure in parasite component communities in wild rodents: Predictability, stability, associations and interactions .... or pure randomness? Parasitology 2008, 135, 751–766. [Google Scholar] [PubMed]
- Ferrari, N.; Cattadori, I.M.; Rizzoli, A.; Hudson, P.J. Heligmosomoides polygyrus reduces infestation of Ixodes ricinus in free-living yellow-necked mice, Apodemus flavicollis. Parasitology 2009, 136, 305–316. [Google Scholar] [CrossRef]
- Holland, C. Interspecific effects between Moniliformis (Acanthocephala), Hymenolepis (Cestoda) and Nippostrongylus (Nematoda) in the laboratory rat. Parasitology 1987, 94, 567–581. [Google Scholar] [CrossRef]
- Behnke, J.M.; Sinski, E.; Wakelin, D. Primary infections with Babesia microti are not prolonged by concurrent Heligmosomoides polygyrus. Parasitol. Int. 1999, 48, 183–187. [Google Scholar] [CrossRef]
- De Souza, B.; Helmby, H. Concurrent gastro-intestinal nematode infection does not alter the development of experimental cerebral malaria. Microbes Infect. 2008, 10, 916–921. [Google Scholar] [CrossRef]
- Abruzzi, A.; Fried, B. Coinfection of Schistosoma (Trematoda) with bacteria, protozoa and helminths. Adv. Parasitol. 2011, 77, 1–85. [Google Scholar] [CrossRef]
- Rausch, S.; Held, J.; Stange, J.; Lendner, M.; Hepworth, M.R.; Klotz, C.; Lucius, R.; Pogonka, T.; Hartmann, S. A matter of timing: Early, not chronic phase intestinal nematode infection restrains control of a concurrent enteric protozoan infection. Eur. J. Immunol. 2010, 40, 2804–2815. [Google Scholar] [CrossRef]
- Balmer, O.; Stearns, S.C.; Schotzau, A.; Brun, R. Intraspecific competition between co-infecting parasite strains enhances host survival in African trypanosomes. Ecology 2009, 90, 3367–3378. [Google Scholar] [CrossRef]
- Andreassen, J.; Odaibo, A.B.; Christensen, N.O. Concurrent infections of the trematode Echinostoma caproni and the tapeworms Hymenolepis diminuta and Hymenolepis microstoma in mice. J. Parasitol. 1990, 76, 573–575. [Google Scholar] [CrossRef]
- Liesenfeld, O.; Dunay, I.R.; Erb, K.J. Infection with Toxoplasma gondii reduces established and developing Th2 responses induced by Nippostrongylus brasiliensis infection. Infect. Immun. 2004, 72, 3812–3822. [Google Scholar] [CrossRef]
- Maizels, R.M.; Balic, A.; Gomez-Escobar, N.; Nair, M.; Taylor, M.D.; Allen, J.E. Helminth parasites - masters of regulation. Immunol. Rev. 2004, 202, 89–116. [Google Scholar]
- Hewitson, J.P.; Grainger, J.R.; Maizels, R.M. Helminth immunoregulation: The role of parasite secreted proteins in modulating host immunity. Mol. Biochem. Parasitol. 2009, 167, 1–11. [Google Scholar] [CrossRef]
- McKay, D.M. The immune response to and immunomodulation by Hymenolepis diminuta. Parasitology 2010, 137, 385–394. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Jolles, A.E. From host immunity to pathogen invasion: The effects of helminth coinfection on the dynamics of microparasites. Integr. Compar. Biol. 2011, 51, 540–551. [Google Scholar] [CrossRef]
- Bazzone, L.E.; Smith, P.M.; Rutitzky, L.I.; Shainheit, M.G.; Urban, J.F.; Setiawan, T.; Blum, A.M.; Weinstock, J.V.; Stadecker, M.J. Coinfection with the intestinal nematode Heligmosomoides polygyrus markedly reduces hepatic egg-induced immunopathology and proinflammatory cytokines in mouse models of severe schistosomiasis. Infect. Immun. 2008, 76, 5164–5172. [Google Scholar] [CrossRef] [PubMed]
- Cable, J.; Harris, P.D.; Lewis, J.W.; Behnke, J.M. Molecular evidence that Heligmosomoides polygyrus from laboratory mice and wood mice are separate species. Parasitology 2006, 133, 111–122. [Google Scholar] [CrossRef]
- Patel, N.; Kreider, T.; Urban, J.F., Jr.; Gause, W.C. Characterisation of effector mechanisms at the host:parasite interface during the immune response to tissue-dwelling intestinal nematode parasites. Int. J. Parasitol. 2009, 39, 13–21. [Google Scholar] [CrossRef]
- Ben-Smith, A.; Lammas, D.A.; Behnke, J.M. The relative involvement of Th1 and Th2 associated immune responses in the expulsion of a primary infection of Heligmosomoides polygyrus in mice of differing response phenotype. J. Helminthol. 2003, 77, 133–146. [Google Scholar] [CrossRef]
- Rausch, S.; Huehn, J.; Kirchhoff, D.; Rzepecka, J.; Schnoeller, C.; Pillai, S.; Loddenkemper, C.; Scheffold, A.; Hamann, A.; Lucius, R.; Hartmann, S. Functional analysis of effector and regulatory T cells in a parasitic nematode infection. Infect. Immun. 2008, 76, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kreider, T.; Bowdridge, S.; Liu, Z.; Song, Y.; Gaydo, A.G.; Urban, J.F., Jr.; Gause, W.C. B cells have distinct roles in host protection against different nematode parasites. J. Immunol. 2010, 184, 5213–5223. [Google Scholar] [CrossRef]
- Moss, G.D. The nature of the immune response of the mouse to the bile duct cestode, Hymenolepis microstoma. Parasitology 1971, 62, 285–294. [Google Scholar] [CrossRef]
- Alghali, S.T.O. Hymenolepis citelli, H. diminuta and H. microstoma: Immunoglobulin-containing cells in the lamina propria of the mouse gut during primary and secondary infections. J. Helminthol. 1987, 61, 329–340. [Google Scholar] [CrossRef]
- Novak, M.; Nombrado, S. Mast cell responses to Hymenolepis microstoma infection in mice. J. Parasitol. 1988, 74, 81–88. [Google Scholar] [CrossRef]
- McLaughlan, P.E.; Roberts, H.C.; Loxton, N.J.; Wastling, J.M.; Newlands, G.F.J.; Chappell, L.H. Mucosal mast cell responses and release of mast cell protease-I in infections of mice with Hymenolepis diminuta and H. microstoma: Modulation by cyclosporin. Parasite Immunol. 1999, 21, 151–161. [Google Scholar] [CrossRef]
- Parvathi, J.; Karemungikar, A. Leucocyte variation, an insight of host defenses during hymenolepiasis and restoration with praziquantel. Indian J. Pharm. Sci. 2011, 73, 76–79. [Google Scholar] [CrossRef]
- Courtney, C.H.; Forrester, D.J. Interspecific interactions between Hymenolepis microstoma (Cestoda) and Heligmosomoides polygyrus (Nematoda) in mice. J. Parasitol. 1973, 59, 480–483. [Google Scholar] [CrossRef]
- Pappas, P.W. Hymenolepis microstoma: Correlation of histopathological host response and organ hypertrophy. Exp. Parasitol. 1976, 40, 320–329. [Google Scholar] [CrossRef]
- Sukhdeo, M.V.; Croll, N.A. The location of parasites within their hosts: Bile and the site selection behaviour of Nematospiroides dubius. Int. J. Parasitol. 1981, 11, 157–162. [Google Scholar] [CrossRef]
- Morimoto, M.; Utsumiya, K. Enhanced protection against Heligmosomoides polygyrus in IL-2 receptor beta-chain overexpressed transgenic mice with intestinal mastocytosis. J. Vet. Med. Sci. 2011, 73, 849–851. [Google Scholar] [CrossRef]
- Evans, W.S.; Novak, M.; Basilevsky, A. Effects of environmental temperature, sex, and infection with Hymenolepis microstoma on the liver and bile duct weights of mice. J. Parasitol. 1985, 71, 106–109. [Google Scholar] [CrossRef]
- Novak, M.; McTavish, G.; Evans, W.S. Hymenolepis microstoma: Histopathological changes in mice maintained at different environmental temperatures. J. Parasitol. 1985, 71, 735–741. [Google Scholar] [CrossRef]
- Hardison, W.G.; Weiner, R.G.; Hatoff, D.E.; Miyai, K. Similarities and differences between models of extrahepatic biliary obstruction and complete biliary retention without obstruction in the rat. Hepatology 1983, 3, 383–390. [Google Scholar] [PubMed]
- Kountouras, J.; Billing, B.H.; Scheuer, P.J. Prolonged bile duct obstruction: A new experimental model for cirrhosis in the rat. Br. J. Exp. Pathol. 1984, 65, 305–311. [Google Scholar] [PubMed]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Kristan, D.M.; Hammond, K.A. Parasite infection and caloric restriction induce physiological and morphological plasticity. Am. J. Physiol. Regul. Integr. Compar. Physiol. 2001, 281, R502–R510. [Google Scholar]
- Mettrick, D.F. Hymenolepis diminuta: pH changes in rat intestinal contents and worm migration. Exp. Parasitol. 1971, 29, 386–401. [Google Scholar] [CrossRef]
- Mettrick, D.F. Hymenolepis diminuta: The microbial fauna, nutritional gradients, and physicochemical characteristics of the small intestine of uninfected and parasitized rats. Can. J. Physiol. Pharmacol. 1971, 49, 972–984. [Google Scholar] [CrossRef]
- Li, P.; Lee, K.Y.; Chang, T.M.; Chey, W.Y. Mechanism of acid-induced release of secretin in rats. Presence of a secretin-releasing peptide. J. Clin. Investig. 1990, 86, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, D.; Gigliozzi, A.; Fraioli, F.; Romeo, R.; Papa, E.; Delle Monache, M.; Capocaccia, L. Hormonal regulation of bicarbonate secretion in the biliary epithelium. Yale J. Biol. Med. 1997, 70, 417–426. [Google Scholar] [PubMed]
- Cooreman, I.; DeRycke, P.H. Migratory behavior of Hymenolepis microstoma. Z. Parasitenkd. 1972, 39, 269–276. [Google Scholar] [CrossRef]
- Wang, A.; McKay, D.M. Immune modulation by a high molecular weight fraction from the rat tapeworm Hymenolepis diminuta. Parasitology 2005, 130, 575–585. [Google Scholar] [CrossRef]
- Johnston, M.J.; Wang, A.; Catarino, M.E.; Ball, L.; Phan, V.C.; MacDonald, J.A.; McKay, D.M. Extracts of the rat tapeworm, Hymenolepis diminuta, suppress macrophage activation in vitro and alleviate chemically induced colitis in mice. Infect. Immun. 2010, 78, 1364–1375. [Google Scholar] [CrossRef]
- Fakae, B.B.; Harrison, L.J.; Ross, C.A.; Sewell, M.M. Expression of acquired immunity to Heligmosomoides polygyrus in mice concurrently infected with Trypanosoma congolense. Int. J. Parasitol. 1997, 27, 1107–1114. [Google Scholar] [CrossRef]
- Howard, R.J.; Christie, P.R.; Wakelin, D.; Wilson, M.M.; Behnke, J.M. The effect of concurrent infection with Trichinella spiralis on Hymenolepis microstoma in mice. Parasitology 1978, 77, 273–279. [Google Scholar] [CrossRef]
- De Rycke, P.H. Development of the cestode Hymenolepis microstoma in Mus musculus. Z. Parasitenkd 1966, 27, 350–354. [Google Scholar] [PubMed]
- Bryant, V. The life cycle of Nematosprioides dubius, Baylis, 1926 (Nematoda: Heligmosomoidae). J. Helminthol. 1973, 47, 263–268. [Google Scholar] [CrossRef]
- Ben-Smith, A.; Lammas, D.A.; Behnke, J.M. Effect of oxygen radicals and differential expression of catalase and superoxide dismutase in adult Heligmosomoides polygyrus during primary infections in mice with differing response phenotypes. Parasite Immunol. 2002, 24, 119–129. [Google Scholar] [CrossRef]
- Keymer, A.E.; Hiorns, R.W. Faecal egg counts and nematode fecundity: Heligmosomoides polygyrus and laboratory mice. Parasitology 1986, 93, 189–203. [Google Scholar] [CrossRef]
- Skorping, A.; Read, A.F.; Keymer, A.E. Life history covariation in intestinal nematodes of mammals. Oikos 1991, 60, 365–372. [Google Scholar] [CrossRef]
- Read, C.P. Longevity of the tapeworm, Hymenolepis diminuta. J. Parasitol. 1967, 53, 1055–1056. [Google Scholar] [CrossRef]
- Pappas, P.W. Biochemical alterations in organs of mice indected with HymenolepisMicrostoma, the mouse bile duct tapeworm. J. Parasitol. 1978, 64, 265–272. [Google Scholar] [CrossRef]
- Simpson, G.F.; Gleason, L.N. Lesion formation in the livers of mice caused by metabolic products of Hymenolepis microstoma. J. Parasitol. 1975, 61, 152–154. [Google Scholar] [CrossRef]
- Mayer, L.P.; Pappas, P.W. Hymenolepis microstoma: Effect of the mouse bile duct tapeworm on the metabolic rate of CF-1 mice. Exp. Parasitol. 1976, 40, 48–51. [Google Scholar] [CrossRef]
- Kristan, D.M.; Hammond, K.A. Combined effects of cold exposure and sub-lethal intestinal parasites on host morphology and physiology. J. Exp. Biol. 2000, 203, 3495–3504. [Google Scholar] [PubMed]
- Hammond, K.A.; Konarzewski, M.; Torres, R.M.; Diamond, J. Metabolic ceilings under a combination of peak energy demands. Physiol. Zool. 1994, 67, 1479–1506. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Clark, P.R.; Ward, W.T.; Lang, S.A.; Saghbini, A.; Kristan, D.M. Order of Inoculation during Heligmosomoides bakeri and Hymenolepis microstoma Coinfection Alters Parasite Life History and Host Responses. Pathogens 2013, 2, 130-152. https://doi.org/10.3390/pathogens2010130
Clark PR, Ward WT, Lang SA, Saghbini A, Kristan DM. Order of Inoculation during Heligmosomoides bakeri and Hymenolepis microstoma Coinfection Alters Parasite Life History and Host Responses. Pathogens. 2013; 2(1):130-152. https://doi.org/10.3390/pathogens2010130
Chicago/Turabian StyleClark, Paul R., W. Timothy Ward, Samantha A. Lang, Alaa Saghbini, and Deborah M. Kristan. 2013. "Order of Inoculation during Heligmosomoides bakeri and Hymenolepis microstoma Coinfection Alters Parasite Life History and Host Responses" Pathogens 2, no. 1: 130-152. https://doi.org/10.3390/pathogens2010130