Microbial Biofilms in Urinary Tract Infections and Prostatitis: Etiology, Pathogenicity, and Combating strategies
Abstract
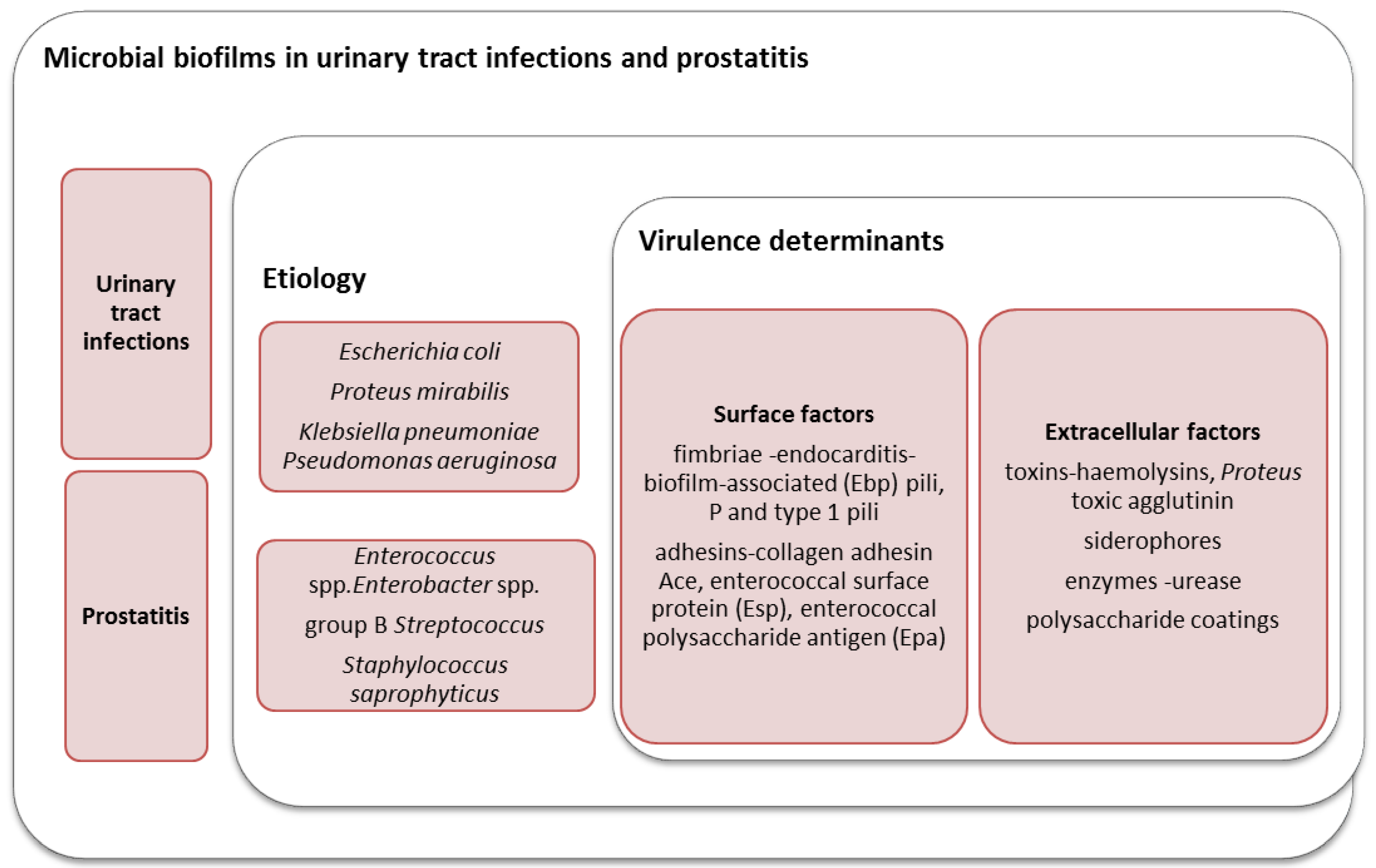
:1. Introduction
Medical Biofilms: Definition, Development Stages, and Properties
2. The Role of Microbial Biofilms in the Etiology of UTIs in Women
3. The Role of Biofilms in Prostatitis and Urethritis in Men
4. Catheter Associated Infections
5. Antimicrobial Strategies for Fighting Against Urinary Tract Biofilms
5.1. Antibiotics
5.2. Natural Antimicrobial Compounds
5.3. Nanoparticles
5.4. Antimicrobial Coatings
5.5. Enzyme Inhibitors
5.6. Bacteriophages
5.7. Quorum Sensing Inhibitors
5.8. Other Antibiofilm Strategies
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roshni Amalaradjou, M.A.; Venkitanarayanan, K. Recent Advances in the Field of Urinary Tract Infections. In Role of Bacterial Biofilms in Catheter-Associted Urinary Tract Infections (CAUTI) and Strategies for Their Control; Nelius, T., Ed.; INTECH: Vienna, Austria, 2013; p. 184. [Google Scholar]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med. 2002, 113 (Suppl. 1A), S5–S13. [Google Scholar] [CrossRef]
- Nicolle, L.E. Urinary tract infection in long-term-care facility residents. Clin. Infect. Dis. 2000, 31, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaeger, T.A.; Dobrindt, U.; Hacker, J. Pathogenicity islands of uropathogenic E. coli and the evolution of virulence. Int. J. Antimicrob. Agents 2002, 19, 517–521. [Google Scholar] [CrossRef]
- Emody, L.; Kerenyi, M.; Nagy, G. Virulence factors of uropathogenic Escherichia coli. Int. J. Antimicrob. Agents 2003, 2, 29–33. [Google Scholar] [CrossRef]
- Arisoy, M.; Aysev, D.; Ekim, M.; Ozel, D.; Kose, S.K.; Ozso, E.D.; Akar, N. Detection of virulence factors of Escherichia coli from children by multiplex polymerase chain reaction. Int. J. Clin. Pract. 2006, 60, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Anghel, I.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Anghel, A.G.; Chifiriuc, M.C. Biohybrid Nanostructured Iron Oxide Nanoparticles and Satureja hortensis to Prevent Fungal Biofilm Development. Int. J. Mol. Sci. 2013, 14, 18110–18123. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V.; Bezirtzoglou, E. Microbial biofilms. Available online: http://www.eolss.net/Sample-Chapters/C03/E6-59-89-00.pdf (accessed on 30 November 2016).
- Israil, A.M.; Chifiriuc, M.C. Interbacteriana Phenomenon of Communication: New Concepts in Anti-Infective Therapy; Asclepius: Bucharest, Romania, 2009; pp. 110–115. [Google Scholar]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, J.H.; Gornbein, J.; Heidari, A.; Dien, S.L.; Lau, V.H.; Chahal, P.; Churchill, B.M.; Haake, D.A. Uropathogens and Host Characteristics. J. Clin. Microbiol. 2008, 46, 3980–3986. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Romling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lamothe, R.; Mitchell, G.; Gattuso, M.; Diarra, M.S.; Malouin, F.; Bouarab, K. Plant antimicrobial agents and their effects on plant and human pathogens. Int. J. Mol. Sci. 2009, 10, 3400–3419. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; van der Mei, H.C. How do bacteria know they are on a surface and regulate their response to an adhering state? PLoS Pathog. 2012, 8, e1002440. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Elsevier 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilm: Microbial Life on Surface. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Rutter, P.R.; Vincent, B. Microbial Adhesion to Surfaces; Ellis Horwood: London, UK, 1980. [Google Scholar]
- Liu, Y.; Yang, S.; Xu, H.; Qin, L.; Tay, J.H. The influence of cell and substratum surface hydrophobicities on microbial attachment. J. Biotechnol. 2004, 110, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, M.C.; Mihaescu, G.; Lazar, V. Medical Microbiology and Virology; University of Bucharest Publishing House: Bucharest, Romania, 2011; pp. 745–752. [Google Scholar]
- Chifiriuc, M.C.; Grumezescu, A.M.; Lazar, V. Quorum Sensing Inhibitors from the Sea: Lessons from Marine Symbiotic Relationships. Curr. Org. Chem. 2014, 18, 823–839. [Google Scholar] [CrossRef]
- Lazar, V.; Chifiriuc, M.C. Mechanisms and experimental models for the assessment of biofilms phenotypic resistance/tolerance. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex Research Center: Badajoz, Spain, 2011. [Google Scholar]
- Limban, C.; Chifiriuc, C.; Grumezescu, A.M. Thiourea Derivatives as Antimicrobials: Synthesis, Biological Activity and Potentiation by Nanotechnological Solutions; Lap Lambert Academic: Bucharest, Romania, 2013. [Google Scholar]
- Lawrence, J.R.; Scharf, B.; Packroff, G.; Neu, T.R. Microscale evaluation of the effects of grazing by invertebrates with contrasting feeding modes on river biofilm architecture and composition. Microb. Ecol. 2002, 44, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.C.; Morgenroth, E. Monitoring biofilm detachment under dynamic changes in shear stress using laser-based particle size analysis and mass fractionation. Water Sci. Technol. 2003, 47, 69–76. [Google Scholar] [PubMed]
- Ymele-Leki, P.; Ross, J.M. Erosion from Staphylococcus aureus biofilms grown under physiologically relevant fluid shear forces yields bacterial cells with reduced avidity to collagen. Appl. Environ. Microbiol. 2007, 73, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, Y.; Ikeda, Y.; Yamashiro, Y.; Takahata, M.; Todo, Y.; Narita, H. In vitro and in vivo antibacterial activities of T-3761, a new quinolone derivative. Antimicrob. Agents Chemother. 1993, 37, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Tanaka, M.; Ito, E.; Kurosaka, Y.; Murakami, Y.; Onodera, K.; Akasaka, T.; Sato, K. In vitro and in vivo antibacterial activities of DK-507k, a novel fluoroquinolone. Antimicrob. Agents Chemother. 2003, 47, 3750–3759. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, S.; Takahashi, Y.; Murata, M.; Domon, H.; Furuya, N.; Ishii, Y.; Matsumoto, T.; Ohno, A.; Tateda, K.; Miyazaki, S.; et al. The in vivo activity of olamufloxacin (HSR-903) in systemic and urinary tract infections in mice. J. Antimicrob. Chemother. 2001, 48, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Kadurugamuwa, J.L.; Modi, K.; Yu, J.; Francis, K.P.; Purchio, T.; Contag, P.R. Noninvasive biophotonic imaging for monitoring of catheter-associated urinary tract infections and therapy in mice. Infect. Immun. 2005, 73, 3878–3887. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Pinkner, J.S.; Caparon, M.G.; Hultgren, S.J. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci. Transl. Med. 2014, 6, 254ra127. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, C.E.; Mobley, H.L. Merging mythology and morphology: The multifaceted lifestyle of Proteus mirabilis. Nat. Rev. Microbiol. 2012, 10, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Alamuri, P.; Mobley, H.L. A novel autotransporter of uropathogenic Proteus mirabilis is both a cytotoxin and an agglutinin. Mol. Microbiol. 2008, 68, 997–1017. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.; Murray, B.E. The rise of the Enterococcus: Beyond vancomycin resistance. Nat. Rev. Microbiol. 2012, 10, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Soto, S.M. Importance of Biofilms in Urinary Tract Infections: New Therapeutic Approaches. Adv. Biol. 2014, 13, 543974. [Google Scholar] [CrossRef]
- Francolini, I.; Donelli, G. Prevention and control of biofilm-based medical-device-related infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal microbiome: Rethinking health and disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Ravel, J. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirkka, V.K.; Stephen, P.; Baroja, M.L.; Kingsley, A.; Kate, C.; Kristine, C.; Gregor, R. Abnormal Immunological Profile and Vaginal Microbiota in Women Prone to Urinary Tract Infections. Clin. Vaccine Immunol. 2009, 16, 29–36. [Google Scholar]
- Murray, T.S.; Ledizet, M.; Kazmierczak, B.I. Swarming motility, secretion of type 3 effectors and biofilm formation phenotypes exhibited within a large cohort of Pseudomonas aeruginosa clinical isolates. J. Med. Microbiol. 2010, 59 Pt 5, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Bhusal, Y.; Horwitz, D.; Shelburne, S.A., 3rd; Trautner, B.W. Overtreatment of Enterococcal Bacteriuria. Arch. Intern. Med. 2012, 172, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Luthje, P.; Hirschberg, A.; Brauner, A. Estrogenic action on innate defense mechanisms in the urinary tract. Maturitas 2014, 77, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.J., Jr.; Mende, K.; Beckius, M.L.; Akers, K.S.; Romano, D.R.; Wenke, J.C.; Murray, C.K. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect. Dis. 2013, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.M.; Shirtliff, M.E. Proteus mirabilis biofilms and catheter-associated urinary tract infections. Virulence 2011, 2, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Sabbuba, N.A.; Stickler, D.J.; Mahenthiralingam, E.; Painter, D.J.; Parkin, J.; Feneley, R.C. Genotyping demonstrates that the strains of Proteus mirabilis from bladder stones and catheter encrustations of patients undergoing long-term bladder catheterization are identical. J. Urol. 2004, 171, 1925–1928. [Google Scholar] [CrossRef] [PubMed]
- Baron, S. Medical Microbiology, 4th ed.; University of Texas Medical: Galveston, TX, USA, 1996. [Google Scholar]
- Mobley, H.L.; Warren, J.W. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J. Clin. Microbiol. 1987, 25, 2216–2217. [Google Scholar] [PubMed]
- Ko, R.; Cadieux, P.A.; Dalsin, J.L.; Lee, B.P.; Elwood, C.N.; Razvi, H. First prize: Novel uropathogen-resistant coatings inspired by marine mussels. J. Endourol. 2008, 22, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, A.; Gangodawila, S.; Long, M.J.; Morris, N.S.; Blacklock, A.R.; Stickler, D.J. An electrified catheter to resist encrustation by Proteus mirabilis biofilm. J. Urol. 2005, 174, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Stickler, D.J.; Zimakoff, J. Complications of urinary tract infections associated with devices used for long-term bladder management. J. Hosp. Infect. 1994, 28, 177–194. [Google Scholar] [CrossRef]
- Swaminathan, S.; Alangaden, G.J. Treatment of resistant enterococcal urinary tract infections. Curr. Infect. Dis. Rep. 2010, 12, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Raveh, D.; Rosenzweig, I.; Rudensky, B.; Wiener-Well, Y.; Yinnon, A.M. Risk factors for bacteriuria due to Pseudomonas aeruginosa or Enterococcus spp. in patients hospitalized via the emergency department. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Wisell, K.T.; Kahlmeter, G.; Giske, C.G. Trimethoprim and enterococci in urinary tract infections: New perspectives on an old issue. J. Antimicrob. Chemother. 2008, 62, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Florian, M.E.W.; Naber, K.G.; Bschleipfer, T.; Brahler, E.; Weidner, W. Prostatitis and Male Pelvic Pain Syndrome. Dtsch. Arztebl. Int. 2009, 106, 175–183. [Google Scholar]
- Sharp, V.J.; Takacs, E.B.; Powell, C.R. Prostatitis: Diagnosis and Treatment. Am. Fam. Physician. 2010, 82, 397–406. [Google Scholar] [PubMed]
- Steenackers, H.P.; Parijs, I.; Foster, K.R.; Vanderleyden, J. Experimental evolution in biofilm populations. FEMS Microbiol. Rev. 2016, 40, 373–397. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.K.; Collins, J.J. Dispersing biofilms with engineered enzymatic bacteriophage. Proc. Natl. Acad. Sci. USA 2007, 104, 11197–11202. [Google Scholar] [CrossRef] [PubMed]
- Kunin, C.M. Urinary tract infections in females. Clin. Infect. Dis. 1994, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hidron, A.I.; Edwards, J.R.; Patel, J.; Horan, T.C.; Sievert, D.M.; Pollock, D.A.; Fridkin, S.K. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Annual Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 2008, 29, 996–1011. [Google Scholar] [CrossRef] [PubMed]
- Robino, L.; Scavone, P.; Araujo, L.; Algorta, G.; Zunino, P.; Vignoli, R. Detection of intracellular bacterial communities in a child with Escherichia coli recurrent urinary tract infections. Pathog. Dis. 2013, 68, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Ichimiya, T.; Takeoka, K.; Hiramatsu, K.; Hirai, K.; Yamasaki, T.; Nasu, M. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy 1996, 42, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Guiton, P.S.; Hung, C.S.; Hancock, L.E.; Caparon, M.G.; Hultgren, S.J. Enterococcal biofilm formation and virulence in an optimized murine model of foreign body-associated urinary tract infections. Infect. Immun. 2010, 78, 4166–4175. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, S.; Sharma, P.; Charma, D.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli biofilm: Development and therapeutic strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, M.; Darouiche, R.O. New strategies to prevent catheter-associated urinary tract infections. Nat. Rev. Urol. 2012, 9, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Hamill, T.M.; Gilmore, B.F.; Jones, D.S.; Gorman, S.P. Strategies for the development of the urinary catheter. Expert Rev. Med. Devices 2007, 4, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Grigore, M.; Chifiriuc, M.C.; Ditu, L.M. Antibiotice si Substante Chimioterapeutice Antimicrobiene; Editura Academiei Române: Bucuresti, Romania, 2008; p. 358. [Google Scholar]
- Sun, F.; Qu, F.; Ling, Y.; Mao, P.; Xia, P.; Chen, H.; Zhou, D. Biofilm-associated infections: Antibiotic resistance and novel therapeutic strategies. Future Microbiol. 2013, 8, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Mihaescu, G.; Chifiriuc, C.; Ditu, L.M. Microbiologie Generala; University din Bucuresti: Bucuresti, Romane, 2007; p. 552. [Google Scholar]
- Grabe, M.; Bartoletti, R.; Bjerklund Johansen, T.E.; Cai, T.; Çek, M.; Köves, B.; Naber, K.G.; Pickard, R.S.; Tenke, P.; Wagenlehner, F.; et al. Guidelines on Urological Infectious; European Association of Urology: Arnhem, The Netherlands, 2013. [Google Scholar]
- Wang, Q.; Sun, F.; Liu, Y.; Xiong, L.; Xie, L.; Xia, P. Enhancement of biofilm formation by subinhibitory concentrations of macrolides in icaADBC -positive and -negative clinical isolates of Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2010, 54, 2707–2711. [Google Scholar] [CrossRef] [PubMed]
- Hannan, S.; Ready, D.; Jasni, A.S.; Rogers, M.; Pratten, J.; Roberts, A.P. Transfer of antibiotic resistance by transformation with eDNA within oral biofilms. FEMS Immunol. Med. Microbiol. 2010, 59, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Pop, C.S.; Hussien, M.D.; Popa, M.; Mares, A.; Grumezescu, A.M.; Grigore, R.; Lazar, V.; Chifiriuc, M.C.; Sakizlian, M.; Bezirtzoglou, E.; et al. Metallic-Based, Micro and Nanostructures with Antimicrobial Activity. Curr. Top. Med. Chem. 2015, 15, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Bilcu, M.; Grumezescu, A.M.; Oprea, A.E.; Popescu, R.C.; Mogosanu, G.D.; Hristu, R.; Stanciu, G.A.; Mihailescu, D.F.; Lazar, V.; Bezirtzoglou, E.; et al. Efficiency of Vanilla, Patchouli and Ylang Ylang Essential Oils Stabilized by Iron Oxide@C-14 Nanostructures against Bacterial Adherence and Biofilms Formed by Staphylococcus aureus and Klebsiella pneumonia. Molecules 2014, 19, 17943–17956. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, M.C.; Diţu, L.M.; Oprea, E.; Liţescu, S.; Bucur, M.; Măruţescu, L.; Enache, G.; Saviuc, C.; Burlibaşa, M.; Trăistaru, T. In vitro study of the inhibitory activity of usnic acid on dental plaque biofilm. Roum. Arch. Microbiol. Immunol. 2009, 68, 215–222. [Google Scholar] [PubMed]
- Grumezescu, A.M.; Andronescu, E.; Ficai, A.; Grumezescu, V.; Bleotu, C.; Saviuc, C.; Mihaiescu, D.E.; Chifiriuc, C.M. In vitro activity of the new water-dispersible Fe3O4 usnic acid nanostructure against planktonic and sessile bacterial cells. J. Nanopart. Res. 2013, 15, 1766. [Google Scholar] [CrossRef]
- Cotar, A.; Ionescu, B.; Pelinescu, D.; Voidarou, C.; Lazar, V.; Bezirtzoglou, E.; Chifiriuc, M.C. Current Solutions for the Interception of Quorum Sensing in Staphylococcus aureus. Curr. Org. Chem. 2013, 17, 97–104. [Google Scholar] [CrossRef]
- Magdalena, L.; Ştefania, G.; Elena, E.; Ioana, I.; Alexandra, B.; Grigore, M.; Luminita, M.; Mariana, C.C. Silver-titanium dioxide nanocomposites as effective antimicrobial and antibiofilm agents. J. Nanopart. Res. 2013, 16, 2203. [Google Scholar]
- Mariana, C.C.; Alexandru, M.G.; Veronica, L.; Alexandra, B.; Stefanos, T.; Raluca, G.; Serban, B. Contribution of Antimicrobial Peptides to the Development of New and Efficient Antimicrobial Strategies. Curr. Proteom. 2014, 11, 98–107. [Google Scholar]
- Grumezescu, A.M.; Chifiriuc, C.M. Prevention of Microbial Biofilms—The Contribution of Micro and Nanostructured Materials. Curr. Med. Chem. 2014, 21, 3311. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Ellis, B.; Lam, K.; Johnson, F.; Khoury, A.E. American Society for Microbiology, Mechanism of Electrical Enhancement of Efficacy of Antibiotics in Killing Biofilm Bacteria. Antimicrob. Agents Chemopher. 1994, 38, 2803–2809. [Google Scholar] [CrossRef]
- Beaulac, C.; Sachetelli, S.; Lagace, J. In Vitro bactericidal efficacy of sub-MIC concentrations of liposome-encapsulated antibiotic against Gram-negative and Gram-positive bacteria. J. Antimicrob. Chemother. 1998, 41, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Choong, S.; Whitfield, H. Biofilms and their role in infections in urology. BJU Int. 2000, 86, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Tenke, P.; Kovacs, B.; Jackel, M.; Nagy, E. The role of biofilm infection in urology. World J. Urol. 2006, 24, 13–20. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delcaru, C.; Alexandru, I.; Podgoreanu, P.; Grosu, M.; Stavropoulos, E.; Chifiriuc, M.C.; Lazar, V. Microbial Biofilms in Urinary Tract Infections and Prostatitis: Etiology, Pathogenicity, and Combating strategies. Pathogens 2016, 5, 65. https://doi.org/10.3390/pathogens5040065
Delcaru C, Alexandru I, Podgoreanu P, Grosu M, Stavropoulos E, Chifiriuc MC, Lazar V. Microbial Biofilms in Urinary Tract Infections and Prostatitis: Etiology, Pathogenicity, and Combating strategies. Pathogens. 2016; 5(4):65. https://doi.org/10.3390/pathogens5040065
Chicago/Turabian StyleDelcaru, Cristina, Ionela Alexandru, Paulina Podgoreanu, Mirela Grosu, Elisabeth Stavropoulos, Mariana Carmen Chifiriuc, and Veronica Lazar. 2016. "Microbial Biofilms in Urinary Tract Infections and Prostatitis: Etiology, Pathogenicity, and Combating strategies" Pathogens 5, no. 4: 65. https://doi.org/10.3390/pathogens5040065




