Evaluation of the Immunomodulatory Properties of Streptococcus suis and Group B Streptococcus Capsular Polysaccharides on the Humoral Response
Abstract
:1. Introduction
2. Results
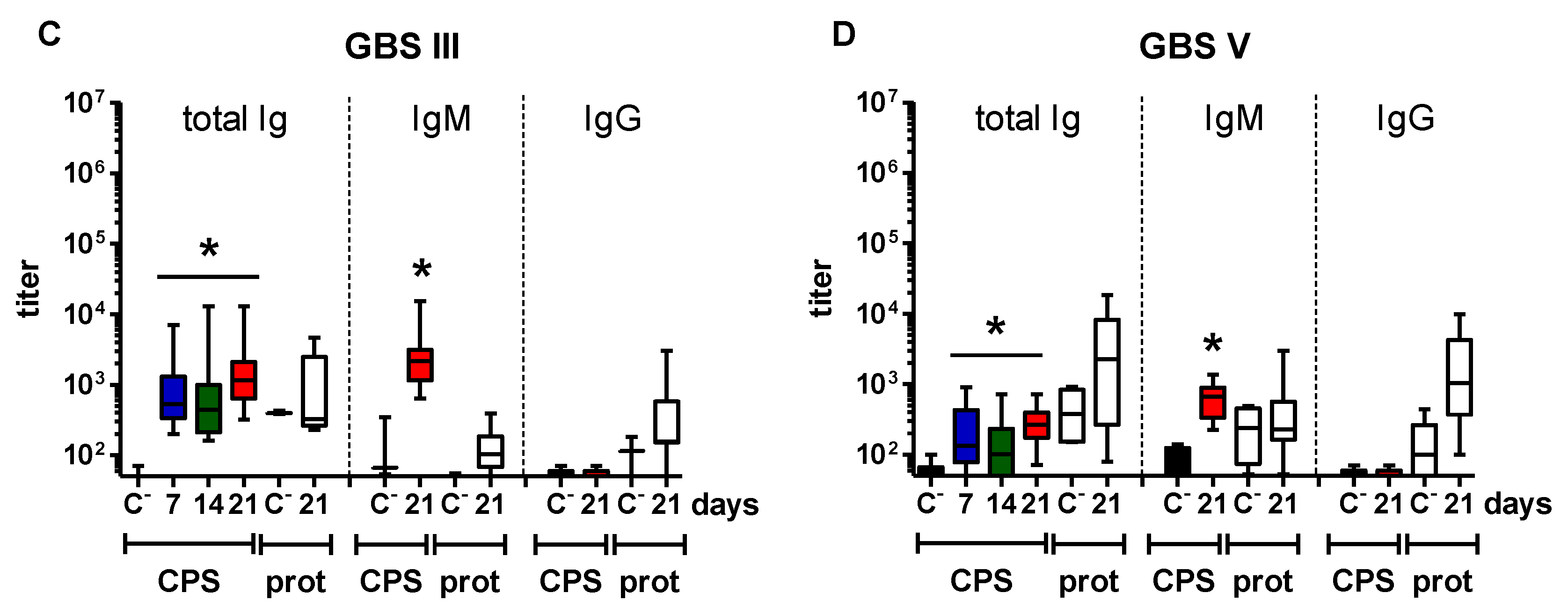
2.1. Distinct Features of the CPS-Specific Ab Response between Mice Infected with Live S. suis and GBS
2.2. Features of the CPS-Specific Ab Response in Mice Immunized with Purified S. suis and GBS CPSs
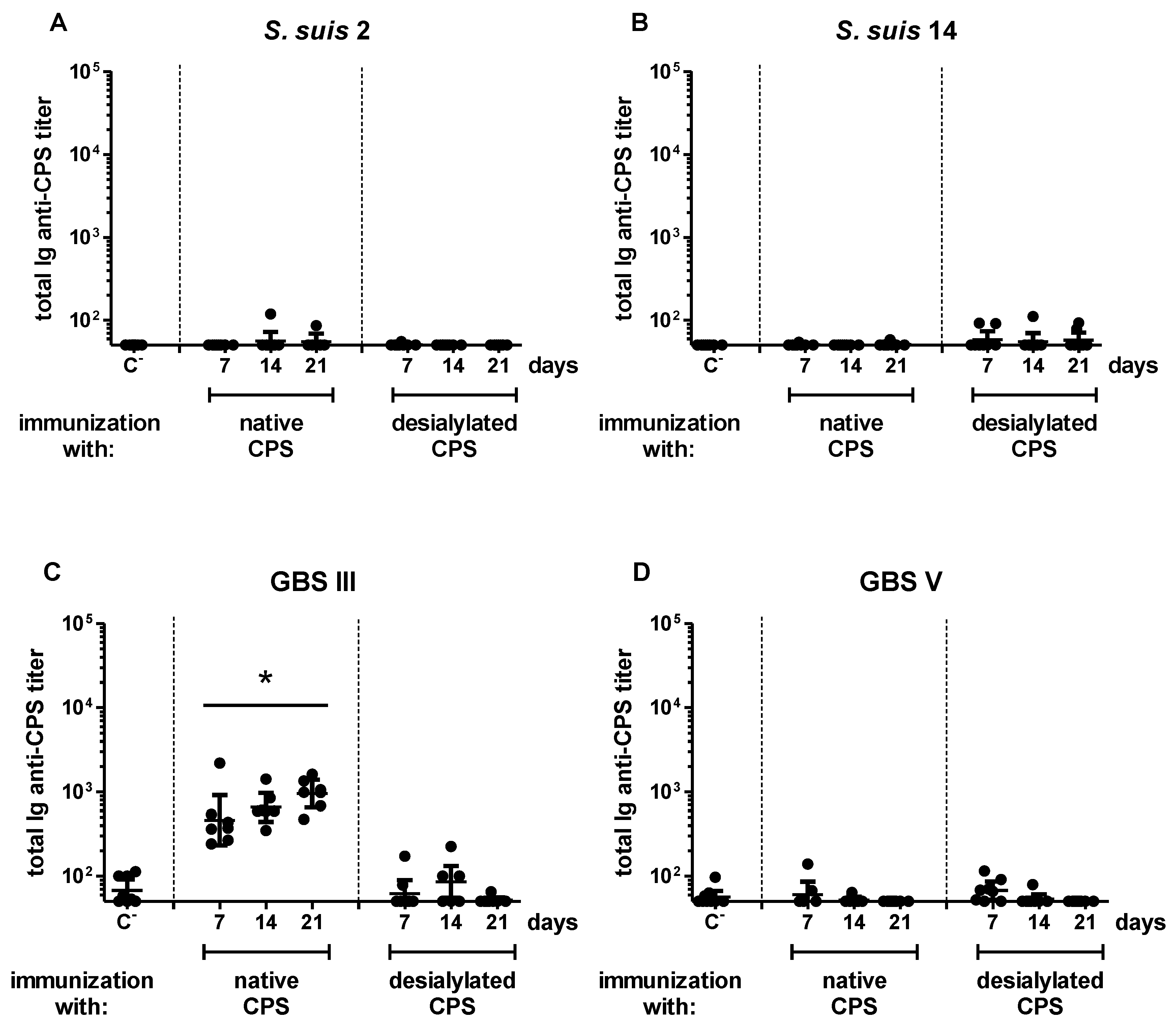
2.3. Influence of Sialic Acid on the Features of the CPS-Specific Ab Response in Mice Immunized with Purified S. suis and GBS CPSs
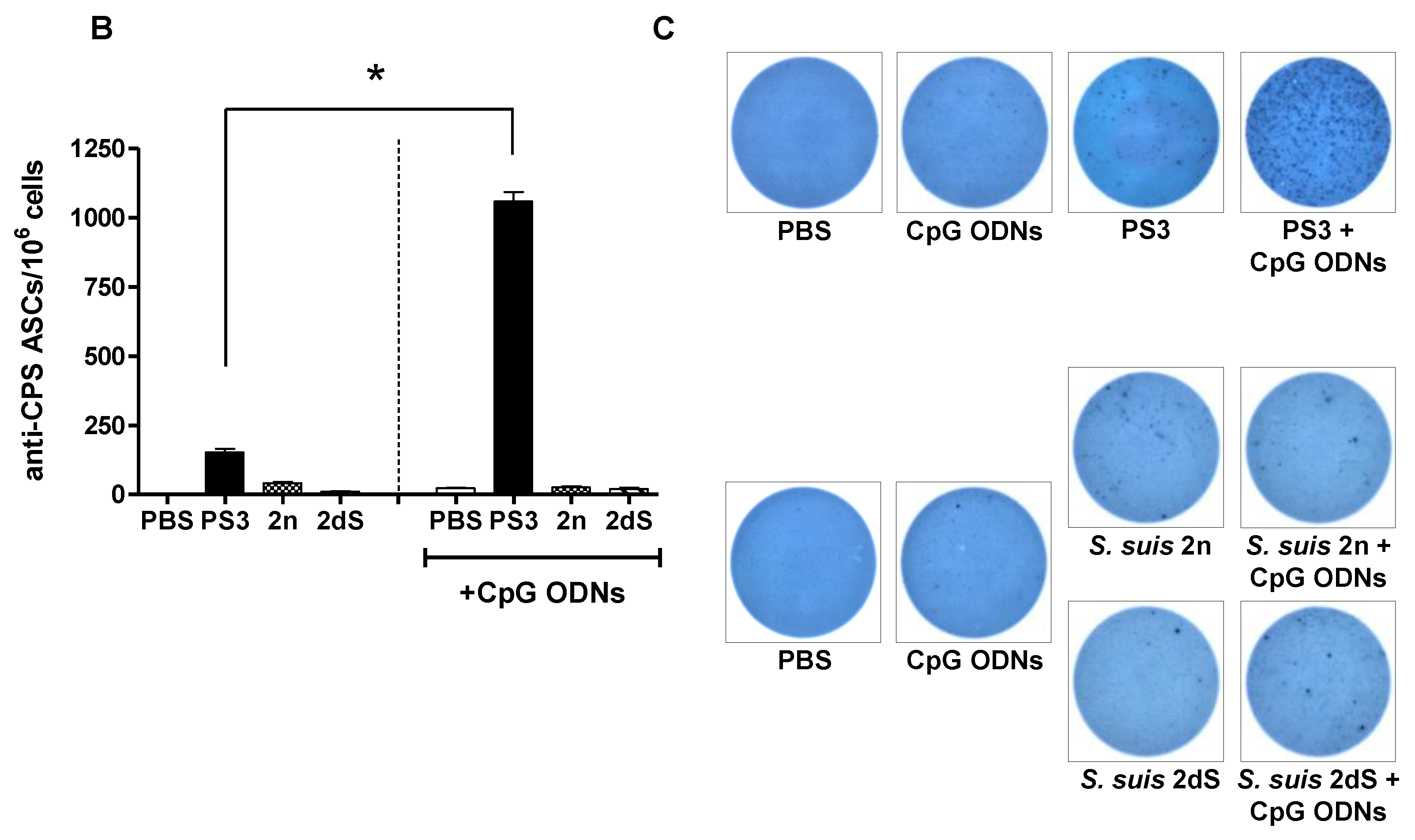
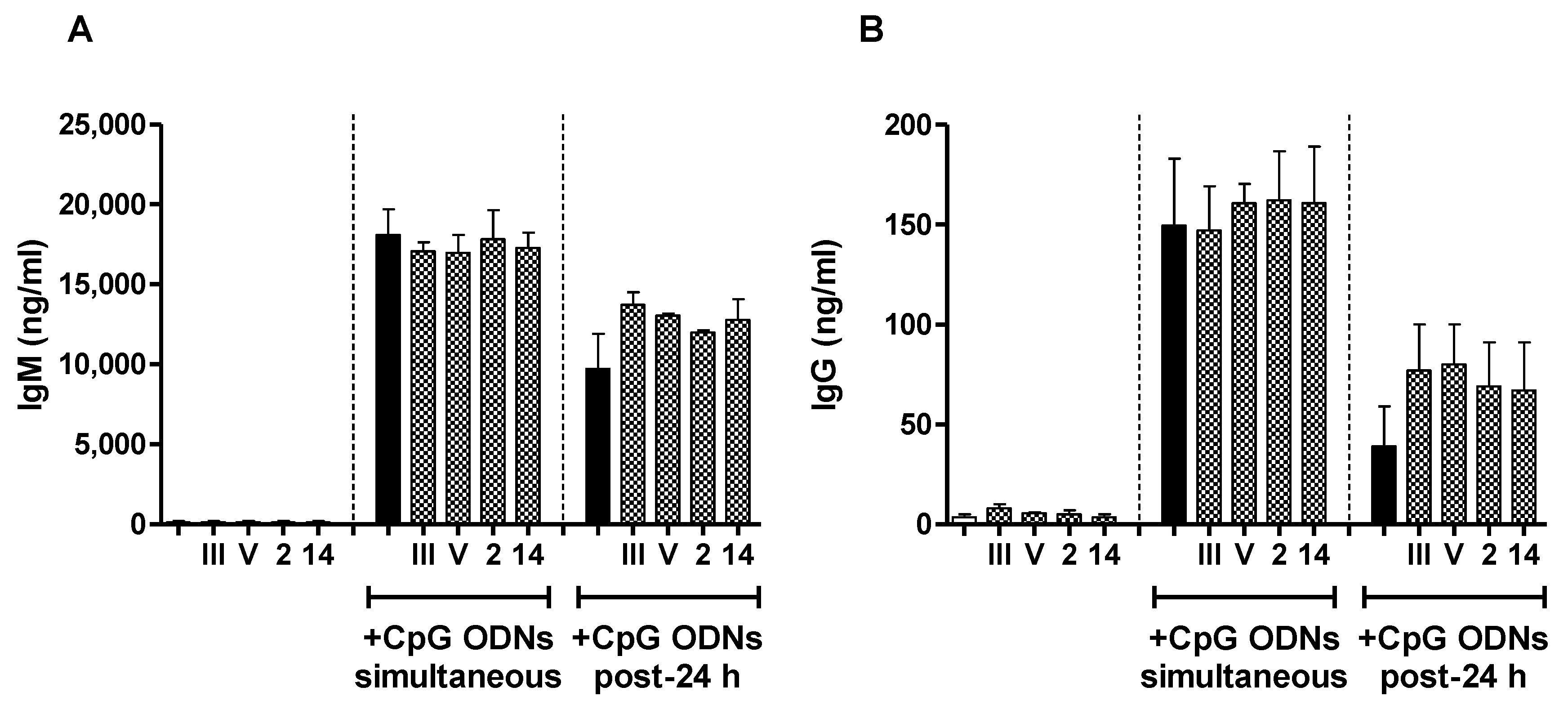
2.4. In Vivo Effect of Exogenous TLR Agonists on S. suis Type 2 CPS-Specific Ab Response
2.5. In Vitro Study of the Immunomodulatory Effect of Purified S. suis and GBS CPSs
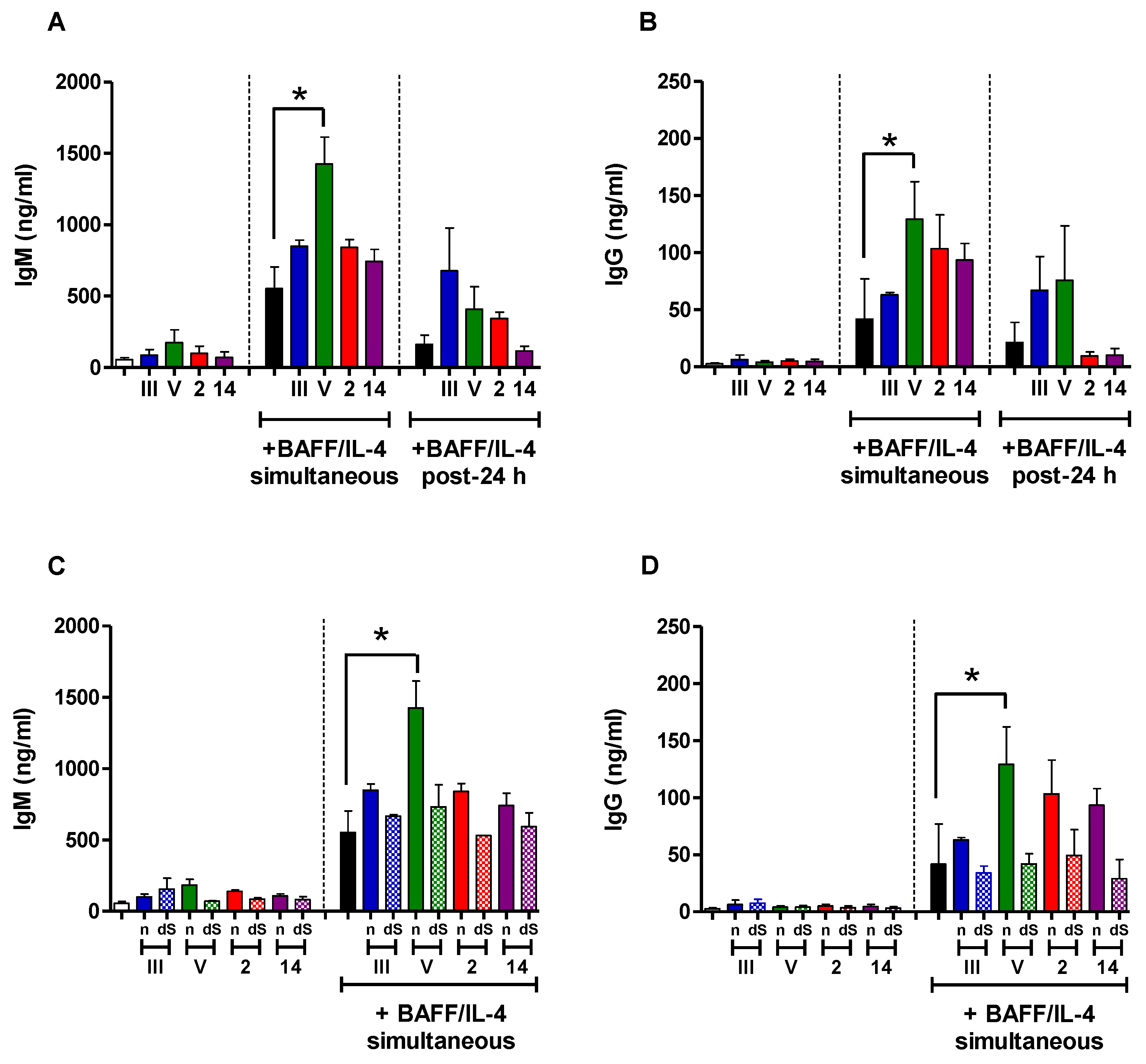
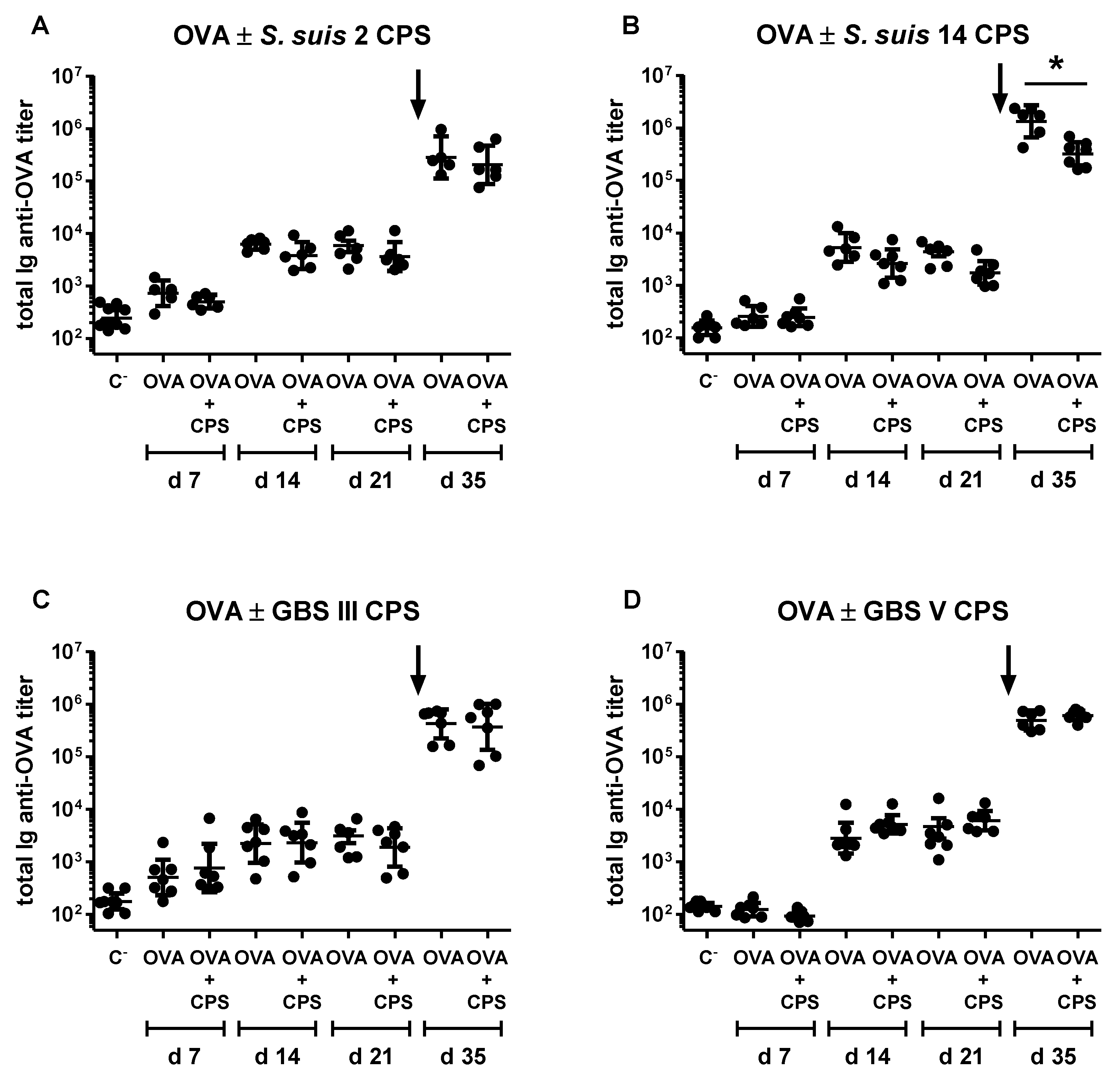
2.6. Study of the Immunomodulatory Effect of Purified S. suis and GBS CPSs on a T Cell-Dependent Response
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Bacterial Strains and Growth Conditions
4.3. CPS Purification and Desialylation
4.4. Bacterial Infections
4.5. Immunizations
4.6. In Vitro B Cell Stimulation Assay
4.7. ELISA
4.7.1. Samples from In Vivo Assays
4.7.2. Samples from In Vitro Assays
4.8. ELISpot
4.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A

References
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent—An update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014, 3, e45. [Google Scholar] [CrossRef] [PubMed]
- Le Doare, K.; Heath, P.T. An overview of global GBS epidemiology. Vaccine 2013, 31 (Suppl. S4), D7–D12. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.E.; Damman, M.; van der Velde, J.; Wagenaar, F.; Wisselink, H.J.; Stockhofe-Zurwieden, N.; Smits, M.A. Identification and characterization of the cps locus of Streptococcus suis serotype 2: The capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 1999, 67, 1750–1756. [Google Scholar] [PubMed]
- Rubens, C.E.; Wessels, M.R.; Heggen, L.M.; Kasper, D.L. Transposon mutagenesis of type III group B Streptococcus: Correlation of capsule expression with virulence. Proc. Natl. Acad. Sci. USA 1987, 84, 7208–7212. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Auger, J.P.; Segura, M.; Fittipaldi, N.; Takamatsu, D.; Okura, M.; Gottschalk, M. Role of the capsular polysaccharide as a virulence factor for Streptococcus suis serotype 14. Can. J. Vet. Res. 2015, 79, 141–146. [Google Scholar] [PubMed]
- Lecours, M.P.; Segura, M.; Lachance, C.; Mussa, T.; Surprenant, C.; Montoya, M.; Gottschalk, M. Characterization of porcine dendritic cell response to Streptococcus suis. Vet. Res. 2011, 42, 72. [Google Scholar] [CrossRef] [PubMed]
- Lecours, M.P.; Gottschalk, M.; Houde, M.; Lemire, P.; Fittipaldi, N.; Segura, M. Critical role for Streptococcus suis cell wall modifications and suilysin in resistance to complement-dependent killing by dendritic cells. J. Infect. Dis. 2011, 204, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; Gottschalk, M.; Olivier, M. Encapsulated Streptococcus suis inhibits activation of signaling pathways involved in phagocytosis. Infect. Immun. 2004, 72, 5322–5330. [Google Scholar] [CrossRef] [PubMed]
- Lemire, P.; Houde, M.; Lecours, M.P.; Fittipaldi, N.; Segura, M. Role of capsular polysaccharide in group B Streptococccus interactions with dendritic cells. Microbes Infect. 2012, 14, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Lemire, P.; Roy, D.; Fittipaldi, N.; Okura, M.; Takamatsu, D.; Bergman, E.; Segura, M. Implication of TLR- but not of NOD2-signaling pathways in dendritic cell activation by group B Streptococcus serotypes III and V. PLoS ONE 2014, 9, e113940. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.A.; Cleroux, P.; Gottschalk, M. Streptococcus suis and group B Streptococcus differ in their interactions with murine macrophages. FEMS Immunol. Med. Microbiol. 1998, 21, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Wessels, M.R.; Pozsgay, V.; Kasper, D.L.; Jennings, H.J. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of type III group B Streptococcus. A revised structure for the type III group B streptococcal polysaccharide antigen. J. Biol. Chem. 1987, 262, 8262–8267. [Google Scholar] [PubMed]
- Wessels, M.R.; DiFabio, J.L.; Benedi, V.J.; Kasper, D.L.; Michon, F.; Brisson, J.R.; Jelinkova, J.; Jennings, H.J. Structural determination and immunochemical characterization of the type V group B Streptococcus capsular polysaccharide. J. Biol. Chem. 1991, 266, 6714–6719. [Google Scholar] [PubMed]
- Van Calsteren, M.R.; Gagnon, F.; Lacouture, S.; Fittipaldi, N.; Gottschalk, M. Structure determination of Streptococcus suis serotype 2 capsular polysaccharide. Biochem. Cell. Biol. 2010, 88, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Van Calsteren, M.R.; Gagnon, F.; Calzas, C.; Goyette-Desjardins, G.; Okura, M.; Takamatsu, D.; Gottschalk, M.; Segura, M. Structure determination of Streptococcus suis serotype 14 capsular polysaccharide. Biochem. Cell. Biol. 2013, 91, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.B.; Kasper, D.L.; Pangburn, M.K.; Wessels, M.R. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect. Immun. 1992, 60, 3986–3993. [Google Scholar] [PubMed]
- Cress, B.F.; Englaender, J.A.; He, W.; Kasper, D.; Linhardt, R.J.; Koffas, M.A. Masquerading microbial pathogens: Capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol. Rev. 2014, 38, 660–697. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Netravali, I.A.; Cariappa, A.; Mattoo, H. Siglecs and immune regulation. Annu. Rev. Immunol. 2012, 30, 357–392. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Nizet, V. The interplay between siglecs and sialylated pathogens. Glycobiology 2014, 24, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Jones, C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An. Acad. Bras. Cienc. 2005, 77, 293–324. [Google Scholar] [CrossRef] [PubMed]
- Snapper, C.M. Mechanisms underlying in vivo polysaccharide-specific immunoglobulin responses to intact extracellular bacteria. Ann. N. Y. Acad. Sci. 2012, 1253, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Unkeless, J.C.; Scigliano, E.; Freedman, V.H. Structure and function of human and murine receptors for IgG. Annu. Rev. Immunol. 1988, 6, 251–281. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, T.E.; Kolberg, J.; Aase, A.; Herstad, T.K.; Hoiby, E.A. The four mouse IgG isotypes differ extensively in bactericidal and opsonophagocytic activity when reacting with the P1.16 epitope on the outer membrane PorA protein of Neisseria meningitidis. Scand. J. Immunol. 2004, 59, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Baums, C.G.; Kock, C.; Beineke, A.; Bennecke, K.; Goethe, R.; Schroder, C.; Waldmann, K.H.; Valentin-Weigand, P. Streptococcus suis bacterin and subunit vaccine immunogenicities and protective efficacies against serotypes 2 and 9. Clin. Vaccine Immunol. 2009, 16, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Charland, N.; Jacques, M.; Lacouture, S.; Gottschalk, M. Characterization and protective activity of a monoclonal antibody against a capsular epitope shared by Streptococcus suis serotypes 1, 2 and 1/2. Microbiology 1997, 143, 3607–3614. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T. An update on vaccination against group B Streptococcus. Expert Rev. Vaccines 2011, 10, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Mond, J.J.; Lees, A.; Snapper, C.M. T cell-independent antigens type 2. Annu. Rev. Immunol. 1995, 13, 655–692. [Google Scholar] [CrossRef] [PubMed]
- Vos, Q.; Lees, A.; Wu, Z.Q.; Snapper, C.M.; Mond, J.J. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol. Rev. 2000, 176, 154–170. [Google Scholar] [PubMed]
- Litinskiy, M.B.; Nardelli, B.; Hilbert, D.M.; He, B.; Schaffer, A.; Casali, P.; Cerutti, A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 2002, 3, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Schiemann, B.; Gommerman, J.L.; Vora, K.; Cachero, T.G.; Shulga-Morskaya, S.; Dobles, M.; Frew, E.; Scott, M.L. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 2001, 293, 2111–2114. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, B.; Belvedere, O.; Roschke, V.; Moore, P.A.; Olsen, H.S.; Migone, T.S.; Sosnovtseva, S.; Carrell, J.A.; Feng, P.; Giri, J.G.; et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood 2001, 97, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Kanswal, S.; Katsenelson, N.; Selvapandiyan, A.; Bram, R.J.; Akkoyunlu, M. Deficient TACI expression on B lymphocytes of newborn mice leads to defective Ig secretion in response to BAFF or APRIl. J. Immunol. 2008, 181, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, J.; Bozzotti, P.; Tougne, C.; Davis, H.L.; Lambert, P.H.; Krieg, A.M.; Siegrist, C.A. Adjuvant effects of CpG oligodeoxynucleotides on responses against T-independent type 2 antigens. Immunology 2001, 102, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Taillardet, M.; Haffar, G.; Mondiere, P.; Asensio, M.J.; Gheit, H.; Burdin, N.; Defrance, T.; Genestier, L. The thymus-independent immunity conferred by a pneumococcal polysaccharide is mediated by long-lived plasma cells. Blood 2009, 114, 4432–4440. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.E.; van den Dobbelsteen, G.P.; Oomen, L.A.; de Weers, O.; van Buren, L.; Beurret, M.; Poolman, J.T.; Hoogerhout, P. Immunogenicity of Streptococcus pneumoniae type 6B and 14 polysaccharide-tetanus toxoid conjugates and the effect of uncoupled polysaccharide on the antigen-specific immune response. Vaccine 1998, 16, 1941–1949. [Google Scholar] [CrossRef]
- Peeters, C.C.; Tenbergen-Meekes, A.M.; Poolman, J.T.; Zegers, B.J.; Rijkers, G.T. Immunogenicity of a Streptococcus pneumoniae type 4 polysaccharide-protein conjugate vaccine is decreased by admixture of high doses of free saccharide. Vaccine 1992, 10, 833–840. [Google Scholar] [CrossRef]
- Kocabas, C.; Katsenelson, N.; Kanswal, S.; Kennedy, M.N.; Cui, X.; Blake, M.S.; Segal, D.M.; Akkoyunlu, M. Neisseria meningitidis type C capsular polysaccharide inhibits lipooligosaccharide-induced cell activation by binding to CD14. Cell Microbiol. 2007, 9, 1297–1310. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, U.; Goldblatt, D. Pneumococcal polysaccharides interact with human dendritic cells. Infect. Immun. 2006, 74, 1890–1895. [Google Scholar] [CrossRef] [PubMed]
- Calzas, C.; Goyette-Desjardins, G.; Lemire, P.; Gagnon, F.; Lachance, C.; van Calsteren, M.R.; Segura, M. Group B Streptococcus and Streptococcus suis capsular polysaccharides induce chemokine production by dendritic cells via Toll-like receptor 2- and MyD88-dependent and -independent pathways. Infect. Immun. 2013, 81, 3106–3118. [Google Scholar] [CrossRef] [PubMed]
- Lemire, P.; Calzas, C.; Segura, M. The NOD2 receptor does not play a major role in the pathogenesis of group B Streptococcus in mice. Microb. Pathog. 2013, 65, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Calzas, C.; Lemire, P.; Auray, G.; Gerdts, V.; Gottschalk, M.; Segura, M. Antibody response specific to the capsular polysaccharide is impaired in Streptococcus suis serotype 2-infected animals. Infect. Immun. 2015, 83, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Defrance, T.; Taillardet, M.; Genestier, L. T cell-independent B cell memory. Curr. Opin. Immunol. 2011, 23, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, D.O.; Mentele, L.M.; Rubens, C.E. Sialylation of group B streptococcal capsular polysaccharide is mediated by cpsk and is required for optimal capsule polymerization and expression. J. Bacteriol. 2005, 187, 4615–4626. [Google Scholar] [CrossRef] [PubMed]
- Lecours, M.P.; Fittipaldi, N.; Takamatsu, D.; Okura, M.; Segura, M.; Goyette-Desjardins, G.; van Calsteren, M.R.; Gottschalk, M. Sialylation of Streptococcus suis serotype 2 is essential for capsule expression but is not responsible for the main capsular epitope. Microbes Infect. 2012, 14, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Outeirino, J.; Kadirvelraj, R.; Woods, R.J. Structural elucidation of type III group B Streptococcus capsular polysaccharide using molecular dynamics simulations: The role of sialic acid. Carbohydr. Res. 2005, 340, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Taillardet, M.; Haffar, G.; Mondiere, P.; Asensio, M.J.; Pleau-Pison, T.; Burdin, N.; Defrance, T.; Genestier, L. Toll-like receptor agonists allow generation of long-lasting antipneumococcal humoral immunity in response to a plain polysaccharidic vaccine. J. Infect. Dis. 2010, 202, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Garcia de Vinuesa, C.; O’Leary, P.; Sze, D.M.; Toellner, K.M.; MacLennan, I.C. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur. J. Immunol. 1999, 29, 1314–1323. [Google Scholar] [CrossRef]
- Martin, F.; Oliver, A.M.; Kearney, J.F. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 2001, 14, 617–629. [Google Scholar] [CrossRef]
- Chattopadhyay, G.; Chen, Q.; Colino, J.; Lees, A.; Snapper, C.M. Intact bacteria inhibit the induction of humoral immune responses to bacterial-derived and heterologous soluble T cell-dependent antigens. J. Immunol. 2009, 182, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, M.; Carrasco-Marin, E.; Alvarez-Dominguez, C.; Outschoorn, I.M.; Leyva-Cobian, F. Inhibitory effects of thymus-independent type 2 antigens on MHC class II-restricted antigen presentation: Comparative analysis of carbohydrate structures and the antigen presenting cell. Cell. Immunol. 1997, 176, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yauch, L.E.; Lam, J.S.; Levitz, S.M. Direct inhibition of T-cell responses by the Cryptococcus capsular polysaccharide glucuronoxylomannan. PLoS Pathog. 2006, 2, e120. [Google Scholar] [CrossRef] [PubMed]
- Segura, M. Streptococcus suis vaccines: Candidate antigens and progress. Expert Rev. Vaccines 2015, 14, 1587–1608. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Feldman, R.G. Vaccination against group B Streptococcus. Expert Rev. Vaccines 2005, 4, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Goyette-Desjardins, G.; Calzas, C.; Shiao, T.C.; Neubauer, A.; Kempker, J.; Roy, R.; Gottschalk, M.; Segura, M. Protection against Streptococcus suis serotype 2 infection using a capsular polysaccharide glycoconjugate vaccine. Infect. Immun. 2016, 84, 2059–2075. [Google Scholar] [CrossRef] [PubMed]
- Guttormsen, H.K.; Paoletti, L.C.; Mansfield, K.G.; Jachymek, W.; Jennings, H.J.; Kasper, D.L. Rational chemical design of the carbohydrate in a glycoconjugate vaccine enhances IgM-to-IgG switching. Proc. Natl. Acad. Sci. USA 2008, 105, 5903–5908. [Google Scholar] [CrossRef] [PubMed]
- Del Campo Sepulveda, E.M.; Altman, E.; Kobisch, M.; D’Allaire, S.; Gottschalk, M. Detection of antibodies against Streptococcus suis capsular type 2 using a purified capsular polysaccharide antigen-based indirect ELISA. Vet. Microbiol. 1996, 52, 113–125. [Google Scholar] [CrossRef]
- Houde, M.; Gottschalk, M.; Gagnon, F.; van Calsteren, M.R.; Segura, M. Streptococcus suis capsular polysaccharide inhibits phagocytosis through destabilization of lipid microdomains and prevents lactosylceramide-dependent recognition. Infect. Immun. 2012, 80, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Lees, A.; Snapper, C.M. Differential regulation of IgG anti-capsular polysaccharide and antiprotein responses to intact Streptococcus pneumoniae in the presence of cognate CD4+ T cell help. J. Immunol. 2004, 172, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Arjunaraja, S.; Massari, P.; Wetzler, L.M.; Lees, A.; Colino, J.; Snapper, C.M. The nature of an in vivo anti-capsular polysaccharide response is markedly influenced by the composition and/or architecture of the bacterial subcapsular domain. J. Immunol. 2012, 188, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Arjunaraja, S.; Paoletti, L.C.; Snapper, C.M. Structurally identical capsular polysaccharide expressed by intact group B Streptococcus versus Streptococcus pneumoniae elicits distinct murine polysaccharide-specific IgG responses in vivo. J. Immunol. 2012, 188, 5238–5246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cannons, J.L.; Paton, J.C.; Akiba, H.; Schwartzberg, P.L.; Snapper, C.M. A novel ICOS-independent, but CD28- and SAP-dependent, pathway of T cell-dependent, polysaccharide-specific humoral immunity in response to intact Streptococcus pneumoniae versus pneumococcal conjugate vaccine. J. Immunol. 2008, 181, 8258–8266. [Google Scholar] [CrossRef] [PubMed]
- Duong, B.H.; Tian, H.; Ota, T.; Completo, G.; Han, S.; Vela, J.L.; Ota, M.; Kubitz, M.; Bovin, N.; Paulson, J.C.; et al. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J. Exp. Med. 2010, 207, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Von Hunolstein, C.; Teloni, R.; Mariotti, S.; Recchia, S.; Orefici, G.; Nisini, R. Synthetic oligodeoxynucleotide containing CpG motif induces an anti-polysaccharide type 1-like immune response after immunization of mice with Haemophilus influenzae type b conjugate vaccine. Int. Immunol. 2000, 12, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kanswal, S.; Katsenelson, N.; Allman, W.; Uslu, K.; Blake, M.S.; Akkoyunlu, M. Suppressive effect of bacterial polysaccharides on BAFF system is responsible for their poor immunogenicity. J. Immunol. 2011, 186, 2430–2443. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R.; McMillan, S.J.; Richards, H.E. CD33-related siglecs as potential modulators of inflammatory responses. Ann. N. Y. Acad. Sci. 2012, 1253, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, L.; Carsetti, R.; Ocker, B.; Kohler, G.; Lamers, M.C. CD22 is a negative regulator of B-cell receptor signalling. Curr. Biol. 1997, 7, 133–143. [Google Scholar] [CrossRef]
- Hoffmann, A.; Kerr, S.; Jellusova, J.; Zhang, J.; Weisel, F.; Wellmann, U.; Winkler, T.H.; Kneitz, B.; Crocker, P.R.; Nitschke, L. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat. Immunol. 2007, 8, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Cariappa, A.; Takematsu, H.; Liu, H.; Diaz, S.; Haider, K.; Boboila, C.; Kalloo, G.; Connole, M.; Shi, H.N.; Varki, N.; et al. B cell antigen receptor signal strength and peripheral B cell development are regulated by a 9-O-acetyl sialic acid esterase. J. Exp. Med. 2009, 206, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Jennings, H.J.; Roy, R.; Gamian, A. Induction of meningococcal group B polysaccharide-specific IgG antibodies in mice by using an N-propionylated B polysaccharide-tetanus toxoid conjugate vaccine. J. Immunol. 1986, 137, 1708–1713. [Google Scholar] [PubMed]
- Genestier, L.; Taillardet, M.; Mondiere, P.; Gheit, H.; Bella, C.; Defrance, T. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J. Immunol. 2007, 178, 7779–7786. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Santamaria, R.; Xu, W.; Cols, M.; Chen, K.; Puga, I.; Shan, M.; Xiong, H.; Bussel, J.B.; Chiu, A.; et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat. Immunol. 2010, 11, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Animal Welfare Committee of the Université de Montréal. Guide for the Care and Use of Laboratory Animals; Animal Welfare Committee of the Université de Montréal: Montréal, QC, Canada, 2012. [Google Scholar]
- Slater, J.D.; Allen, A.G.; May, J.P.; Bolitho, S.; Lindsay, H.; Maskell, D.J. Mutagenesis of Streptococcus equi and Streptococcus suis by transposon Tn917. Vet. Microbiol. 2003, 93, 197–206. [Google Scholar] [CrossRef]
- Gottschalk, M.; Higgins, R.; Jacques, M.; Mittal, K.R.; Henrichsen, J. Description of 14 new capsular types of Streptococcus suis. J. Clin. Microbiol. 1989, 27, 2633–2636. [Google Scholar] [PubMed]
- Kuypers, J.M.; Heggen, L.M.; Rubens, C.E. Molecular analysis of a region of the group B Streptococcus chromosome involved in type III capsule expression. Infect. Immun. 1989, 57, 3058–3065. [Google Scholar] [PubMed]
- Stills, H.F., Jr. Adjuvants and antibody production: Dispelling the myths associated with Freund’s complete and other adjuvants. ILAR J. 2005, 46, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Michon, F.; Uitz, C.; Sarkar, A.; D’Ambra, A.J.; Laude-Sharp, M.; Moore, S.; Fusco, P.C. Group B streptococcal type II and III conjugate vaccines: Physicochemical properties that influence immunogenicity. Clin. Vaccine Immunol. 2006, 13, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Goyette-Desjardins, G.; van Calsteren, M.R.; Takamatsu, D.; Okura, M.; Fittipaldi, N.; Gottschalk, M.; Segura, M. Novel biogenetic approach to study sialic acid-linkage modifications in group B Streptococcus and Streptococcus suis capsular polysaccharide structures. In Proceedings of the 7e Symposium du FQRNT-Centre de Recherche en Infectiologie Porcine et Avicole (CRIPA), St-Hyacinthe, QC, Canada, 3–4 June 2014. [Google Scholar]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzas, C.; Taillardet, M.; Fourati, I.S.; Roy, D.; Gottschalk, M.; Soudeyns, H.; Defrance, T.; Segura, M. Evaluation of the Immunomodulatory Properties of Streptococcus suis and Group B Streptococcus Capsular Polysaccharides on the Humoral Response. Pathogens 2017, 6, 16. https://doi.org/10.3390/pathogens6020016
Calzas C, Taillardet M, Fourati IS, Roy D, Gottschalk M, Soudeyns H, Defrance T, Segura M. Evaluation of the Immunomodulatory Properties of Streptococcus suis and Group B Streptococcus Capsular Polysaccharides on the Humoral Response. Pathogens. 2017; 6(2):16. https://doi.org/10.3390/pathogens6020016
Chicago/Turabian StyleCalzas, Cynthia, Morgan Taillardet, Insaf Salem Fourati, David Roy, Marcelo Gottschalk, Hugo Soudeyns, Thierry Defrance, and Mariela Segura. 2017. "Evaluation of the Immunomodulatory Properties of Streptococcus suis and Group B Streptococcus Capsular Polysaccharides on the Humoral Response" Pathogens 6, no. 2: 16. https://doi.org/10.3390/pathogens6020016





