Antibiotic Resistance and Virulence Phenotypes of Recent Bacterial Strains Isolated from Urinary Tract Infections in Elderly Patients with Prostatic Disease
Abstract
:1. Introduction
2. Results
2.1. Prevalence of Uropathogenic Bacteria Associated with Urinary Tract Infections in Older Patients with Benign Prostatic Hyperplasia
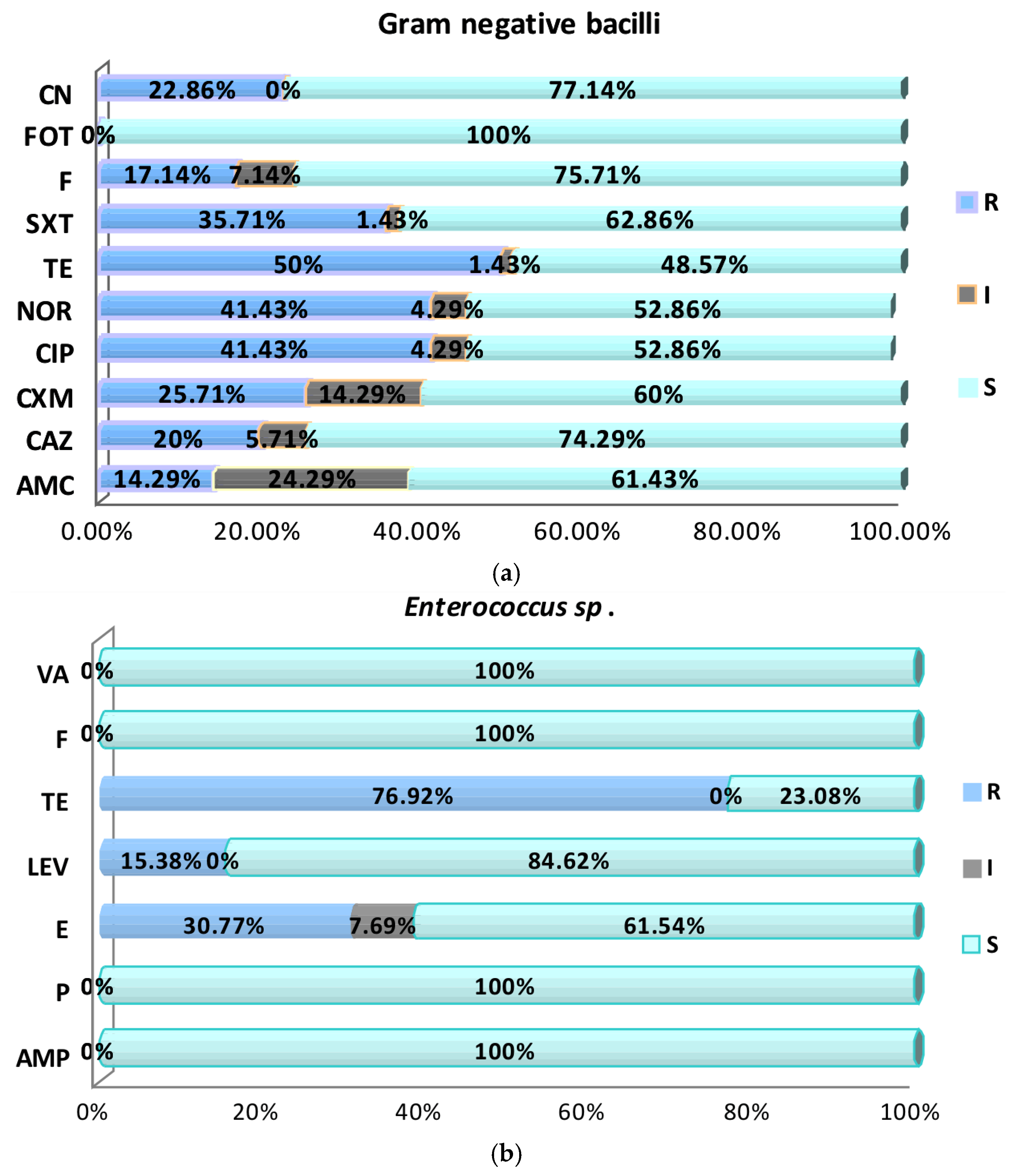
2.2. Antimicrobial Susceptibility
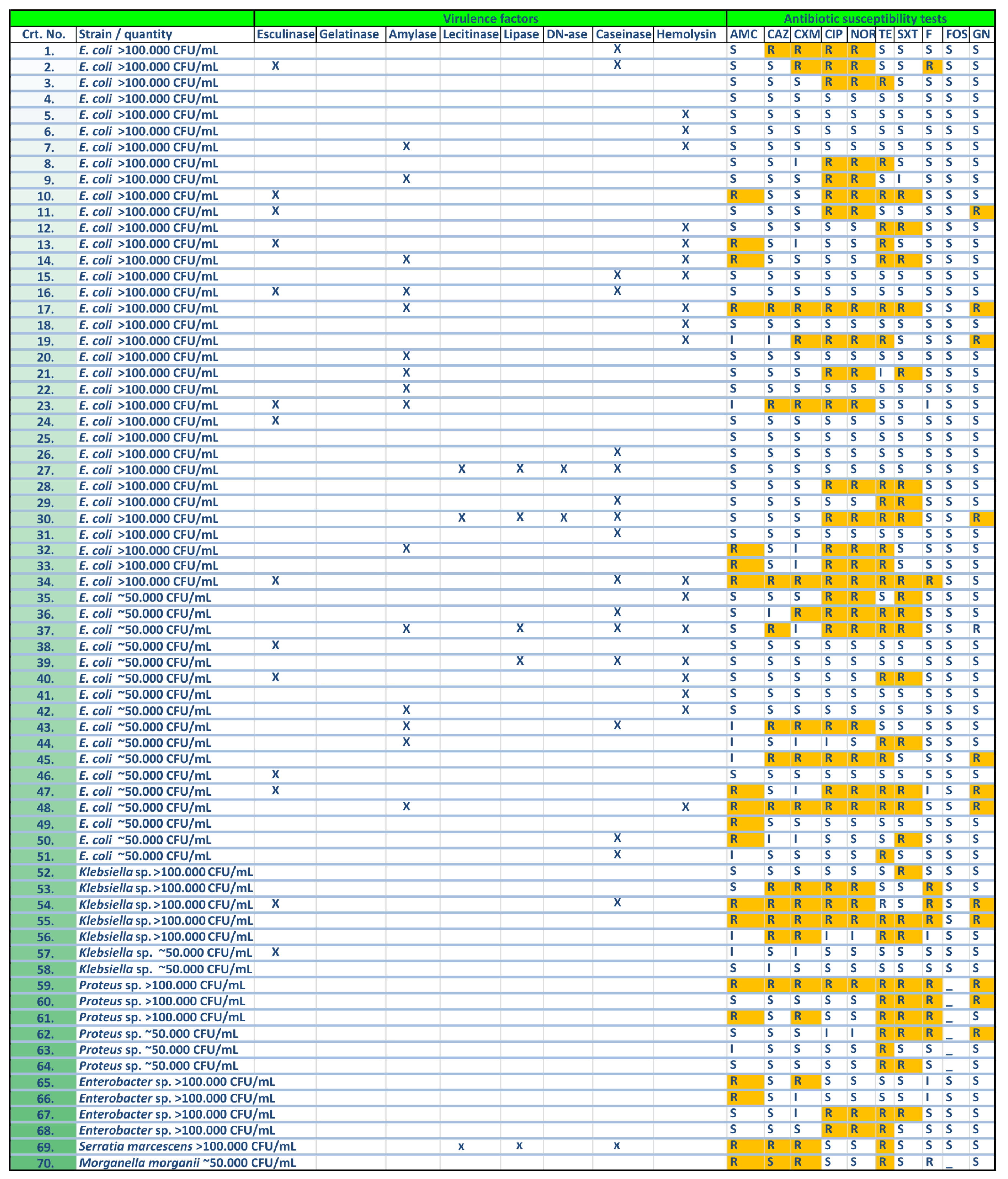
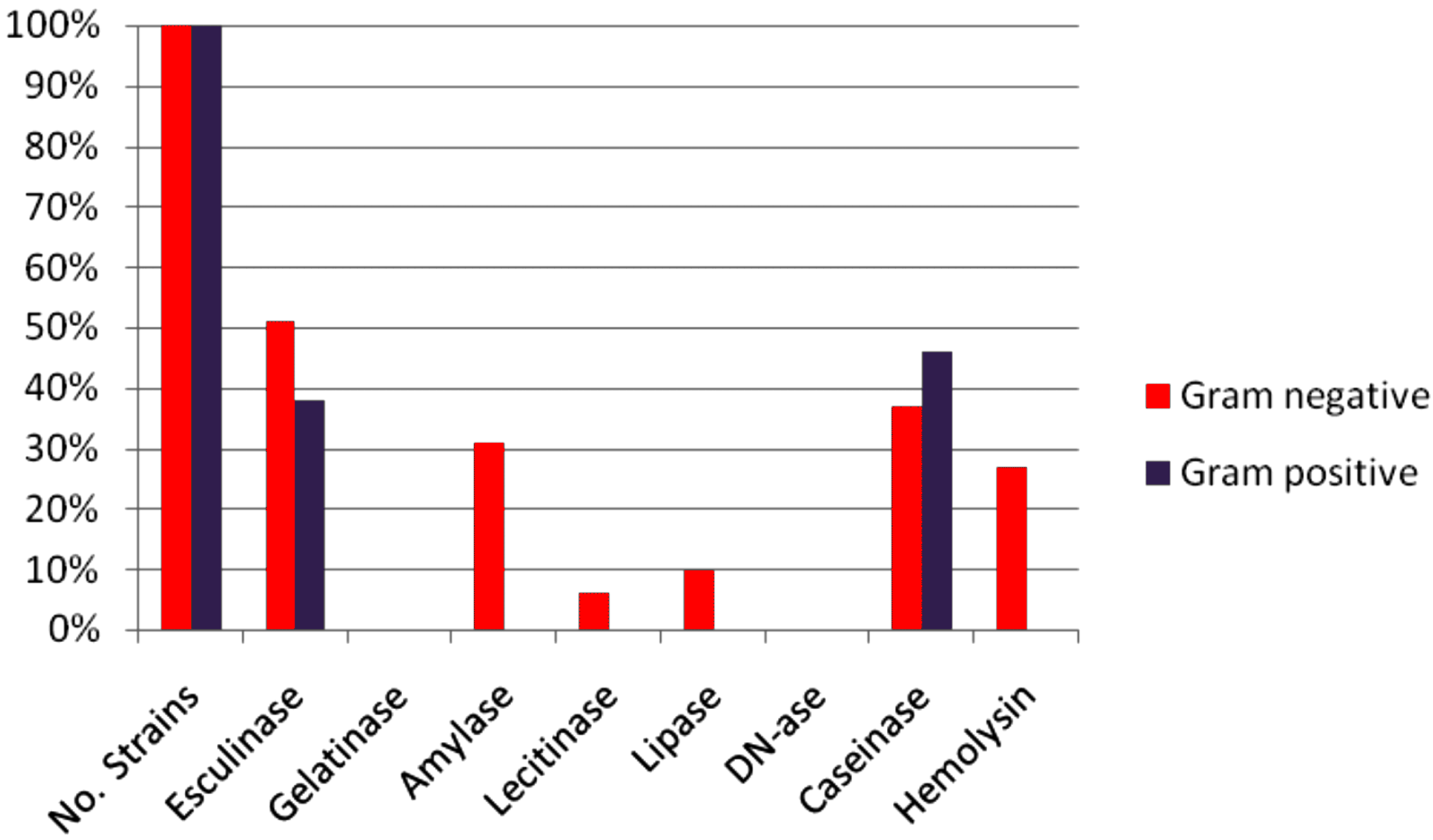
2.3. Virulence Factors Expression
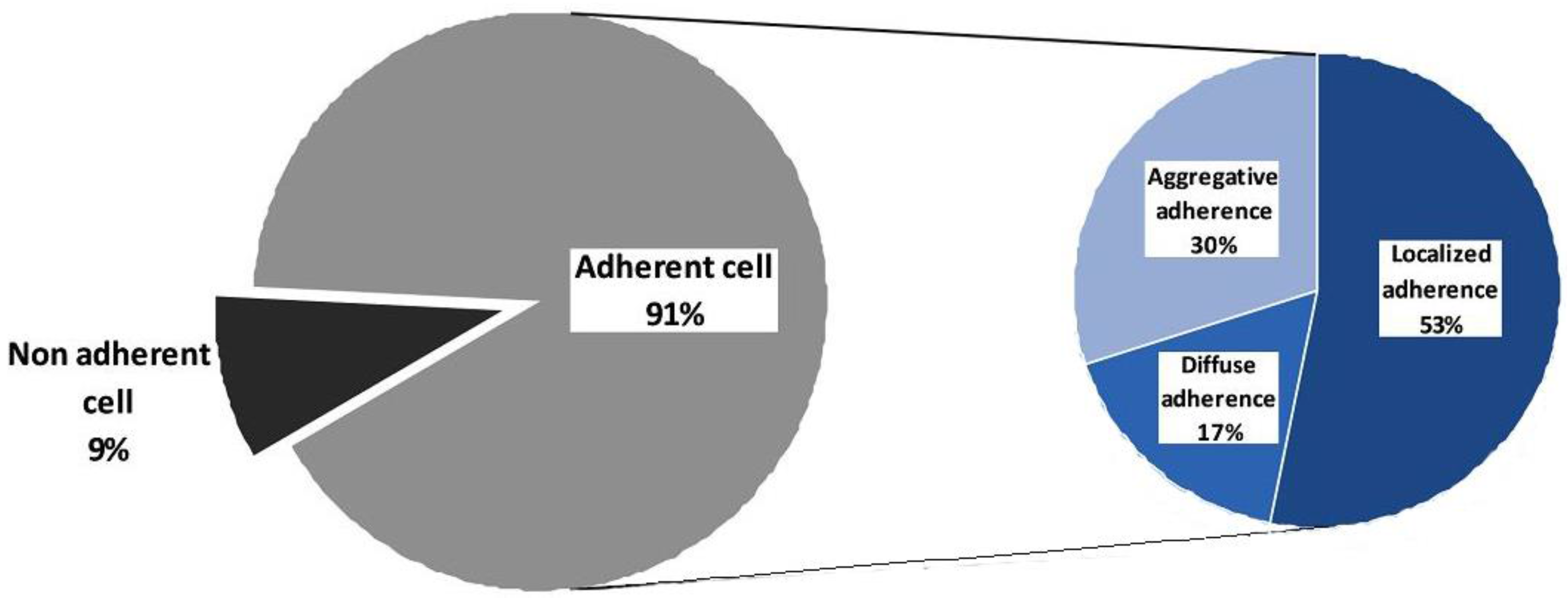
2.3.1. Cell Associated Virulence Factors
2.3.2. Soluble Virulence Factors
3. Discussion
4. Materials and Methods
4.1. Bacterial Cultures and Antimicrobial Susceptibility Testing
4.2. Investigation of Cell-Associated and Soluble Virulence Microbial Factors
4.2.1. Adherence Assay
4.2.2. Soluble Virulence Factor Production
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, E.; Bhusal, Y.; Horwitz, D.; Shelburne, S.A., 3rd; Trautner, B.W. Overtreatment of enterococcal bacteriuria. Arch. Intern. Med. 2012, 172, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Soto, S.M. Importance of biofilms in urinary tract infections: New therapeutic approaches. Adv. Biol. 2014, 2014, 543974. [Google Scholar] [CrossRef]
- Perletti, G.; Marras, E.; Wagenlehner, F.M.; Magri, V. Antimicrobial therapy for chronic bacterial prostatitis. Cochrane Database Syst. Rev. 2013, 8, CD009071. [Google Scholar] [CrossRef]
- Swaminathan, S.; Alangaden, G.J. Treatment of resistant enterococcal urinary tract infections. Curr. Infect. Dis. Rep. 2010, 12, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Raveh, D.; Rosenzweig, I.; Rudensky, B.; Wiener-Well, Y.; Yinnon, A.M. Risk factors for bacteriuria due to Pseudomonas aeruginosa or Enterococcus spp in patients hospitalized via the emergency department. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Wisell, K.T.; Kahlmeter, G.; Giske, C.G. Trimethoprim and enterococci in urinary tract infections: New perspectives on an old issue. J. Antimicrob. Chemother. 2008, 62, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Popescu, G.A.; Pistol, A.; Şerban, R. Consumul de Antibiotice, Rezistența Microbiană și Infecții Nosocomiale în România—2012. Bucharest, Romania, 2015; pp. 5–37. Available online: http://www.cnscbt.ro/index.php/analiza-date-supraveghere/infectii-nosocomiale-1/525-consumul-de-antibiotice-rezistenta-microbiana-si-infectii-nosocomiale-in-romania-2012/file (accessed on 8 May 2017).
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial resistance surveillance in Europe 2015. In Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); ECDC: Stockholm, Sweden, 2017; Available online: http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-europe-2015.pdf (accessed on 8 May 2017).
- Wagenlehner, F.M.; Weidner, W.; Pilatz, A.; Naber, K.G. Urinary tract infections and bacterial prostatitis in men. Curr. Opin. Infect. Dis. 2014, 27, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Bradway, C.; Bixby, M.B.; Hirschman, K.B.; McCauley, K.; Naylor, M.D. Case study: Transitional care for a patient with benign prostatic hyperplasia and recurrent urinary tract infections. Urol. Nurs. 2013, 33, 177–200. [Google Scholar]
- Zhang, S.J.; Qian, H.N.; Zhao, Y.; Sun, K.; Wang, H.Q.; Liang, G.Q.; Li, F.H.; Li, Z. Relationship between age and prostate size. Asian J. Androl. 2013, 15, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Bronsema, D.A.; Adams, J.R.; Pallares, R.; Wenzel, R.P. Secular trends in rates and etiology of nosocomial urinary tract infections at a university hospital. J. Urol. 1993, 150, 414–416. [Google Scholar] [PubMed]
- Beveridge, L.A.; Davey, P.G.; Phillips, G.; McMurdo, M.E. Optimal management of urinary tract infections in older people. Clin. Interv. Aging 2011, 6, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Woodford, H.J.; George, J. Diagnosis and management of urinary tract infection in hospitalized older people. J. Am. Geriatr. Soc. 2009, 57, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Gopal Rao, G.; Patel, M. Urinary tract infection in hospitalized elderly patients in the United Kingdom: The importance of making an accurate diagnosis in the post broad-spectrum antibiotic era. J. Antimicrob. Chemother. 2009, 63, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [PubMed]
- Hummers-Pradier, E.; Ohse, A.M.; Koch, M.; Heizmann, W.R.; Kochen, M.M. Urinary tract infection in men. Int. J. Clin. Pharmacol. Ther. 2004, 42, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, A.J.; Nicolle, L.E. Urinary tract infections in older men. N. Engl. J. Med. 2016, 374, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Daoud, Z.; Salem Sokhn, E.; Masri, K.; Cheaito, K.; Haidar-Ahmad, N.; Matar, G.M.; Doron, S. Escherichia coli isolated from urinary tract infections of Lebanese patients between 2005 and 2012: Epidemiology and profiles of resistance. Front. Med. 2015, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. The development of the BSAC standardized method of disc diffusion testing. J. Antimicrob. Chemother. 2001, 48, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Mangiarotti, P.; Pizzini, C.; Fanos, V. Antibiotic prophylaxis in children with relapsing urinary tract infections: Review. J. Chemother. 2000, 12, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E. Urinary tract pathogens in complicated infection and in elderly individuals. J. Infect. Dis. 2001, 183, S5–S8. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.L.; Gaido, L. Laboratory diagnosis of urinary tract infections in adult patients. Clin. Infect. Dis. 2004, 38, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Routh, J.C.; Alt, A.L.; Ashley, R.A.; Kramer, S.A.; Boyce, T.G. Increasing prevalence and associated risk factors for methicillin resistant Staphylococcus aureus bacteriuria. J. Urol. 2009, 181, 1694–1698. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Perrelli, E.; Towle, V.; Van Ness, P.H.; Juthani-Mehta, M. Antimicrobial susceptibility of bacteria isolated from urine samples obtained from nursing home residents. Infect. Control Hosp. Epidemiol. 2009, 30, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.; AMMI Canada Guidelines Committee. Complicated urinary tract infection in adults. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 349–360. [Google Scholar] [PubMed]
- Pallett, A.; Hand, K. Complicated urinary tract infections: Practical solutions for the treatment of multiresistant Gram-negative bacteria. J. Antimicrob. Chemother. 2010, 65, iii25–iii33. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E. Resistant pathogens in urinary tract infections. J. Am. Geriatr. Soc. 2002, 50, S230–S235. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E. Urinary tract infection: Traditional pharmacologic therapies. Am. J. Med. 2002, 113, 35S–44S. [Google Scholar] [CrossRef]
- Sundqvist, M.; Kahlmeter, G. ‘Pre-emptive culturing’ will improve the chance of ‘getting it right’ when empirical therapy of urinary tract infections fails. J. Antimicrob. Chemother. 2009, 64, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Wimmerstedt, A.; Kahlmeter, G. Associated antimicrobial resistance in Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumoniae and Streptococcus pyogenes. Clin. Microbiol. Infect. 2008, 14, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Andreu, A.; Alós, J.I.; Gobernado, M.; Marco, F.; de la Rosa, M.; García-Rodríguez, J.A.; Grupo Cooperativo Español para el Estudio de la Sensibilidad Antimicrobiana de los Patógenos Urinarios. Etiology and antimicrobial susceptibility among uropathogens causing community-acquired lower urinary tract infections: A nationwide surveillance study. Enferm. Infecc. Microbiol. Clin. 2005, 23, 4–9. [Google Scholar] [PubMed]
- Alós, J.I.; Serrano, M.G.; Gómez-Garcés, J.L.; Perianes, J. Antibiotic resistance of Escherichia coli from community-acquired urinary tract infections in relation to demographic and clinical data. Clin. Microbiol. Infect. 2005, 11, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Pitout, J.D. Multiresistant Enterobacteriaceae.: New threat of an old problem. Expert Rev. Anti-Infect. Ther. 2008, 6, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Pitout, J.D.; Church, D.L.; Gregson, D.B.; Chow, B.L.; McCracken, M.; Mulvey, M.R.; Laupland, K.B. Molecular epidemiology of CTX-M-producing Escherichia coli in the Calgary Health Region: Emergence of CTX-M-15-producing isolates. Antimicrob. Agents Chemother. 2007, 51, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Winokur, P.L.; Canton, R.; Casellas, J.M.; Legakis, N. Variations in the prevalence of strains expressing an extended-spectrum β-lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific Region. Clin. Infect. Dis. 2001, 32, S94–S103. [Google Scholar] [CrossRef] [PubMed]
- Itokazu, G.S.; Quinn, J.P.; Bell-Dixon, C.; Kahan, F.M.; Weinstein, R.A. Antimicrobial resistance rates among aerobic Gram-negative bacilli recovered from patients in intensive care units: Evaluation of a national postmarketing surveillance program. Clin. Infect. Dis. 1996, 23, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Rolhion, N.; Carvalho, F.A.; Darfeuille-Michaud, A. OmpC and the sigma(E) regulatory pathway are involved in adhesion and invasion of the Crohn’s disease-associated Escherichia Coli strain LF82. Mol. Microbiol. 2007, 63, 1684–1700. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Yan, J.J.; Lei, H.Y.; Teng, C.H.; Wang, M.C.; Tseng, C.C.; Wu, J.J. Loss of outer membrane protein C in Escherichia coli contributes to both antibiotic resistance and escaping antibody-dependent bactericidal activity. Infect. Immun. 2012, 80, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Chart, H.; Scotland, S.M.; Willshaw, G.A.; Rowe, B. HEp-2 adhesion and the expression of a 94 kDa outer-membrane protein by strains of Escherichia coli belonging to the enteropathogenic serogroups. J. Gen. Microbiol. 1988, 134, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Bekhit, A.; Fukamachi, T.; Saito, H.; Kobayashi, H. The role of OmpC and OmpF in acidic resistance in Escherichia coli. Biol. Pharm. Bull. 2011, 34, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Fito-Boncompte, L.; Chapalain, A.; Bouffartigues, E.; Chaker, H.; Lesouhaitier, O.; Gicquel, G.; Bazire, A.; Madi, A.; Connil, N.; Véron, W.; et al. Full virulence of Pseudomonas aeruginosa requires OprF. Infect. Immun. 2011, 79, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Arhin, A.; Boucher, C. The outer membrane protein OprQ and adherence of Pseudomonas aeruginosa to human fibronectin. Microbiology 2010, 156, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Pitout, J.D.; Laupland, K.B.; Church, D.L.; Menard, M.L.; Johnson, J.R. Virulence factors of Escherichia coli isolates that produce CTX-M-type extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 2005, 49, 4667–4670. [Google Scholar] [CrossRef] [PubMed]
- Peirano, G.; Schreckenberger, P.C.; Pitout, J.D. Characteristics of NDM-1-producing Escherichia coli isolates that belong to the successful and virulent clone ST131. Antimicrob. Agents Chemother. 2011, 55, 2986–2988. [Google Scholar] [CrossRef] [PubMed]
- Rijavec, M.; Müller-Premru, M.; Zakotnik, B.; Zgur-Bertok, D. Virulence factors and biofilm production among Escherichia coli strains causing bacteraemia of urinary tract origin. J. Med. Microbiol. 2008, 57, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Simon, K.; Ruiz, J.; Horcajada, J.P.; Velasco, M.; Barranco, M.; Moreno, A.; Mensa, J. Are quinolone-resistant uropathogenic Escherichia coli less virulent? J. Infect. Dis. 2002, 186, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Hunstad, D.A.; Justice, S.S. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu. Rev. Microbiol. 2010, 64, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.G.; Palermo, J.J.; Schilling, J.D.; Roth, R.; Heuser, J.; Hultgren, S.J. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 2003, 301, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Blango, M.G.; Mulvey, M.A. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob. Agents Chemother. 2010, 54, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 1991, 4, 80–128. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaeger, T.A.; Dobrindt, U.; Hacker, J. Virulence factors of uropathogens. Curr. Opin. Urol. 2002, 12, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.A. Adhesion and entry of uropathogenic Escherichia coli. Cell. Microbiol. 2002, 4, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Favre-Bonte, S.; Darfeuille-Michaud, A.; Forestier, C. Aggregative adherence of Klebsiella pneumoniae to human intestine-407 cells. Infect. Immun. 1995, 63, 1318–1328. [Google Scholar] [PubMed]
- Yamamoto, T.; Endo, S.; Yokota, T.; Echeverria, P. Characteristics of adherence of enteroaggregative Escherichia coli to human and animal mucosa. Infect. Immun. 1991, 59, 3722–3739. [Google Scholar] [PubMed]
- Torres, A.G.; Zhou, X.; Kaper, J.B. Adherence of diarrheagenic Escherichia coli strains to epithelial cells. Infect. Immun. 2005, 73, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Feng, Y.; Wheatcroft, R.; Chambers, J.; Gong, J.; Gyles, C.L. Adherence of Escherichia coli O157:H7 to epithelial cells in vitro and in pig gut loops is affected by bacterial culture conditions. Can. J. Vet. Res. 2011, 75, 81–88. [Google Scholar] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Cunha, M.L.R.S. Bacterial biofilms with emphasis on coagulase-negative staphylococci. J. Venom. Anim. Toxins Incl. Trop. Dis. 2008, 14, 572–596. [Google Scholar] [CrossRef]
- Hynes, W.L.; Walton, S.L. Hyaluronidases of Gram-positive bacteria. FEMS Microbiol. Lett. 2000, 183, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.W.; Schurr, M.J.; LeBlanc, C.L.; Ramamurthy, R.; Buchanan, K.L.; Nickerson, C.A. Mechanisms of bacterial pathogenicity. Postgrad. Med. J. 2002, 78, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Wiles, T.J.; Kulesus, R.R.; Mulvey, M.A. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp. Mol. Pathol. 2008, 85, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.C.; Rasmussen, S.B.; Grande, K.K.; Conran, R.M.; O’Brien, A.D. Hemolysin of uropathogenic Escherichia coli evokes extensive shedding of the uroepithelium and hemorrhage in bladder tissue within the first 24 hours after intraurethral inoculation of mice. Infect. Immun. 2008, 76, 2978–2990. [Google Scholar] [CrossRef] [PubMed]
- Los, F.C.O.; Randis, T.M.; Aroian, R.V.; Ratner, A.J. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 2013, 77, 173–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, N.; Chen, Z. Recurrent urinary tract infections caused by multidrug-resistant uropathogenic Escherichia coli: Implications for diagnosis and treatment. Eur. Urol. 2013, 63, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.J.; Mendonca, N. Association between antimicrobial resistance and virulence in Escherichia coli. Virulence 2012, 3, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Available online: http://clsi.org/ (accessed on 12 January 2016).
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [PubMed]
- Israil, A.M.; Delcaru, C.; Balotescu Chifiriuc, M.C. Impact of different parameters upon the expression of certain virulence factors of nonhalophilic and halophilic Vibrio strains. Rom. Biotechnol. Lett. 2009, 14, 4545–4559. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delcaru, C.; Podgoreanu, P.; Alexandru, I.; Popescu, N.; Măruţescu, L.; Bleotu, C.; Mogoşanu, G.D.; Chifiriuc, M.C.; Gluck, M.; Lazăr, V. Antibiotic Resistance and Virulence Phenotypes of Recent Bacterial Strains Isolated from Urinary Tract Infections in Elderly Patients with Prostatic Disease. Pathogens 2017, 6, 22. https://doi.org/10.3390/pathogens6020022
Delcaru C, Podgoreanu P, Alexandru I, Popescu N, Măruţescu L, Bleotu C, Mogoşanu GD, Chifiriuc MC, Gluck M, Lazăr V. Antibiotic Resistance and Virulence Phenotypes of Recent Bacterial Strains Isolated from Urinary Tract Infections in Elderly Patients with Prostatic Disease. Pathogens. 2017; 6(2):22. https://doi.org/10.3390/pathogens6020022
Chicago/Turabian StyleDelcaru, Cristina, Paulina Podgoreanu, Ionela Alexandru, Nela Popescu, Luminiţa Măruţescu, Coralia Bleotu, George Dan Mogoşanu, Mariana Carmen Chifiriuc, Marinela Gluck, and Veronica Lazăr. 2017. "Antibiotic Resistance and Virulence Phenotypes of Recent Bacterial Strains Isolated from Urinary Tract Infections in Elderly Patients with Prostatic Disease" Pathogens 6, no. 2: 22. https://doi.org/10.3390/pathogens6020022