Staphylococcus aureus Regulator Sigma B is Important to Develop Chronic Infections in Hematogenous Murine Osteomyelitis Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains for Infection Models
2.2. Haematogenous S. aureus Osteomyelitis Model in Mice
2.3. Statistical Analysis
3. Results and Discussion
3.1. SigB Is Required for S. aureus Persistence in Bone and Kidney Tissue
3.2. Mutation of sigB Induced Several Abscess in Kidneys and Enhanced Bacterial Clearing
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Gordon, R.J.; Lowy, F.D. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 2008, 46 (Suppl. 5), S350–S359. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.A.; von Eiff, C.; Kahl, B.C.; Becker, K.; McNamara, P.; Herrmann, M.; Peters, G. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 2006, 4, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherr, L.; Bischoff, M.; Lattar, S.M.; Noto Llana, M.; Pfortner, H.; Niemann, S.; Geraci, J.; Van de Vyver, H.; Fraunholz, M.J.; Cheung, A.L.; et al. Sigma factor sigb is crucial to mediate staphylococcus aureus adaptation during chronic infections. PLoS Pathog. 2015, 11, e1004870. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.A.; Nair, S.P. Interaction of staphylococci with bone. Int. J. Med. Microbiol. 2010, 300, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Larkin, E.A.; Carman, R.J.; Krakauer, T.; Stiles, B.G. Staphylococcus aureus: The toxic presence of a pathogen extraordinaire. Curr. Med. Chem. 2009, 16, 4003–4019. [Google Scholar] [CrossRef] [PubMed]
- Grundmeier, M.; Tuchscherr, L.; Bruck, M.; Viemann, D.; Roth, J.; Willscher, E.; Becker, K.; Peters, G.; Loffler, B. Staphylococcal strains vary greatly in their ability to induce an inflammatory response in endothelial cells. J. Infect. Dis. 2010, 201, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Haslinger-Loffler, B.; Kahl, B.C.; Grundmeier, M.; Strangfeld, K.; Wagner, B.; Fischer, U.; Cheung, A.L.; Peters, G.; Schulze-Osthoff, K.; Sinha, B. Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells. Cell Microbiol. 2005, 7, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Horst, S.A.; Hoerr, V.; Beineke, A.; Kreis, C.; Tuchscherr, L.; Kalinka, J.; Lehne, S.; Schleicher, I.; Kohler, G.; Fuchs, T.; et al. A novel mouse model of Staphylococcus aureus chronic osteomyelitis that closely mimics the human infection: An integrated view of disease pathogenesis. Am. J. Pathol. 2012, 181, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherr, L.; Loffler, B. Staphylococcus aureus dynamically adapts global regulators and virulence factor expression in the course from acute to chronic infection. Curr. Genet. 2016, 62, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, M.; Entenza, J.M.; Giachino, P. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 2001, 183, 5171–5179. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Fugere, A.; Pepin Gaudreau, K.; Brouillette, E.; Frost, E.H.; Cantin, A.M.; Malouin, F. SigB is a dominant regulator of virulence in Staphylococcus aureus small-colony variants. PLoS ONE 2013, 8, e65018. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Pathogenesis of Staphylococcus aureus abscesses. Am. J. Pathol. 2015, 185, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Atwood, D.N.; Beenken, K.E.; Lantz, T.L.; Meeker, D.G.; Lynn, W.B.; Mills, W.B.; Spencer, H.J.; Smeltzer, M.S. Regulatory mutations impacting antibiotic susceptibility in an established staphylococcus aureus biofilm. Antimicrob. Agents. Chemother. 2016, 60, 1826–1829. [Google Scholar] [CrossRef] [PubMed]
- Riordan, J.T.; O’Leary, J.O.; Gustafson, J.E. Contributions of sigb and sara to distinct multiple antimicrobial resistance mechanisms of staphylococcus aureus. Int. J. Antimicrob. Agents 2006, 28, 54–61. [Google Scholar] [CrossRef] [PubMed]
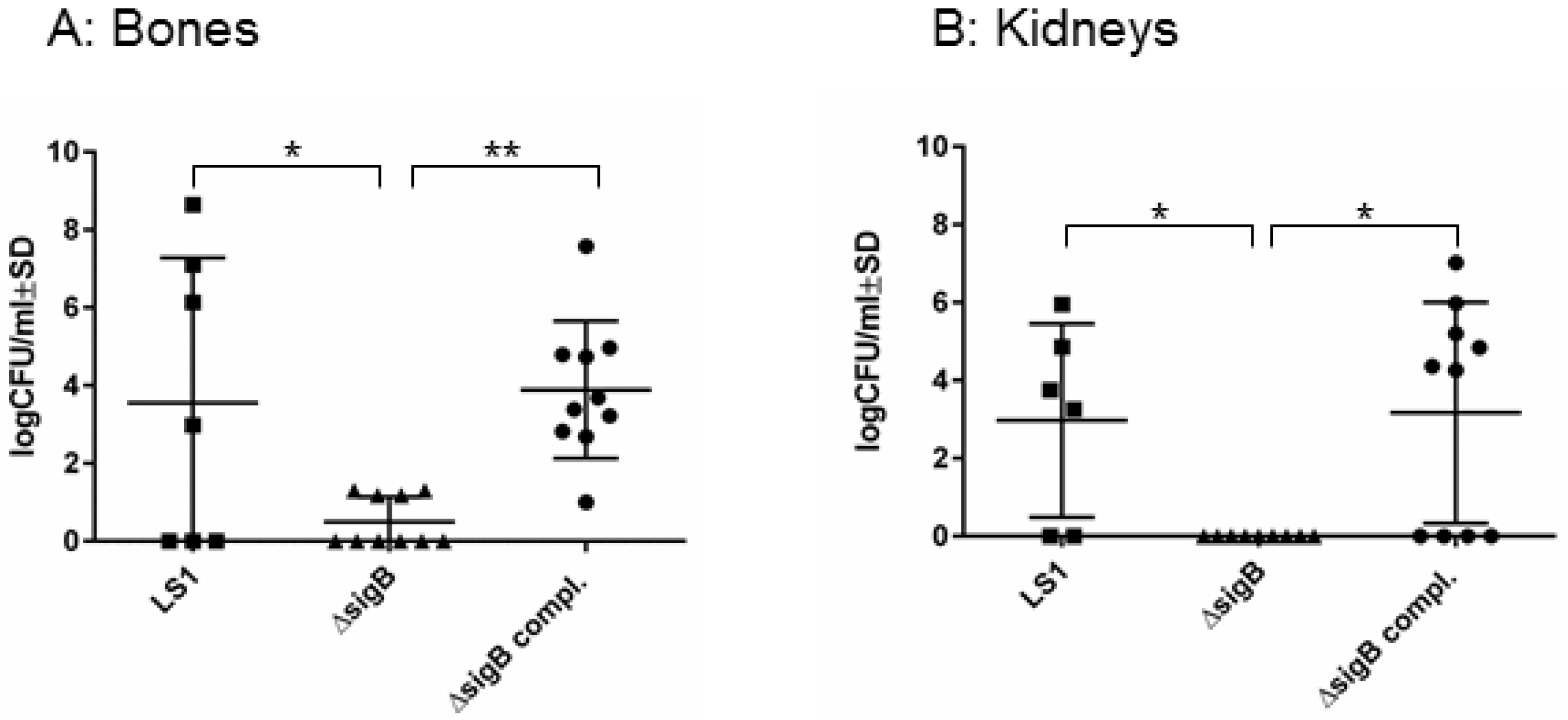

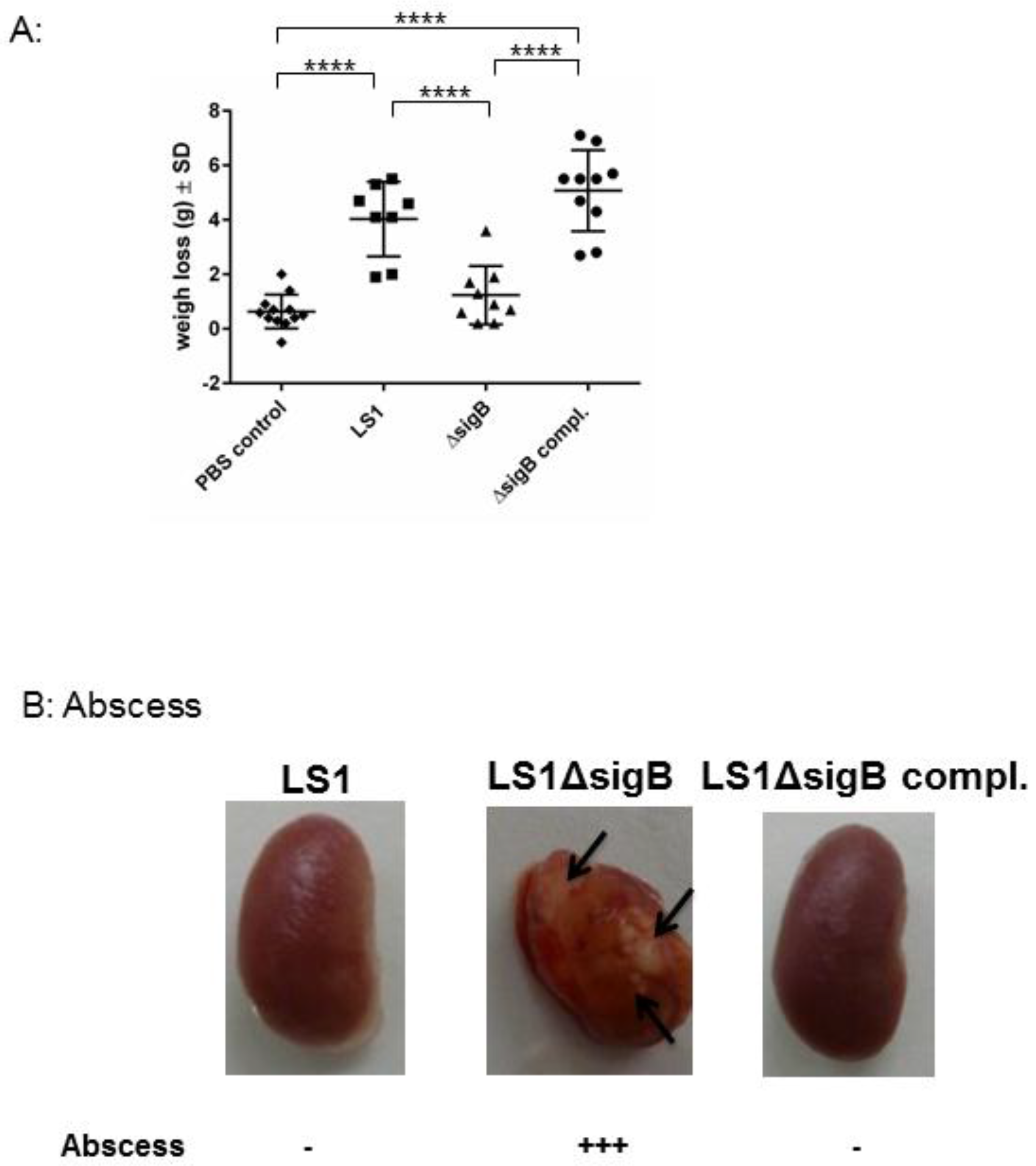
| Strain | Bone | Kidney |
|---|---|---|
| LS1 | 54.3% | 43.6% |
| LS1ΔsigB | 7.26% | 0% |
| LS1ΔsigB complemented | 55.7% | 46.4% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuchscherr, L.; Geraci, J.; Löffler, B. Staphylococcus aureus Regulator Sigma B is Important to Develop Chronic Infections in Hematogenous Murine Osteomyelitis Model. Pathogens 2017, 6, 31. https://doi.org/10.3390/pathogens6030031
Tuchscherr L, Geraci J, Löffler B. Staphylococcus aureus Regulator Sigma B is Important to Develop Chronic Infections in Hematogenous Murine Osteomyelitis Model. Pathogens. 2017; 6(3):31. https://doi.org/10.3390/pathogens6030031
Chicago/Turabian StyleTuchscherr, Lorena, Jennifer Geraci, and Bettina Löffler. 2017. "Staphylococcus aureus Regulator Sigma B is Important to Develop Chronic Infections in Hematogenous Murine Osteomyelitis Model" Pathogens 6, no. 3: 31. https://doi.org/10.3390/pathogens6030031






