Liposomal and Deoxycholate Amphotericin B Formulations: Effectiveness against Biofilm Infections of Candida spp.
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Material and Methods
4.1. Organisms and Growth Conditions
4.2. Antifungal Drugs
4.3. Antifungal Susceptibility Tests
4.4. Planktonic Susceptibility Evaluation
4.4.1. Minimum Inhibitory Concentrations (MICs)
4.4.2. Minimum Fungicidal Concentration (MFC)
4.5. Biofilm Structure, Susceptibility Evaluation and Biomass Reduction Analysis
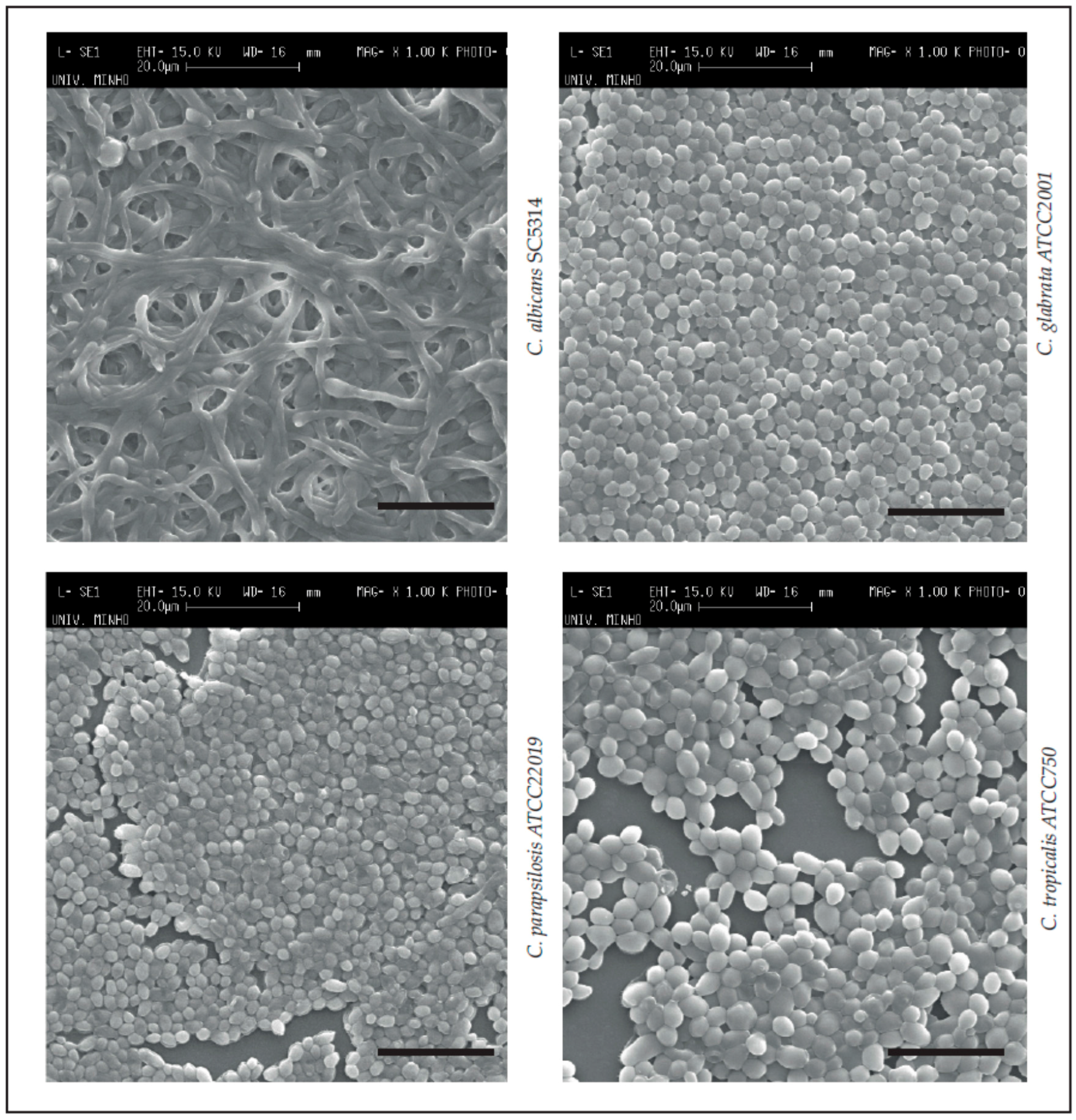
4.5.1. Scanning Electronic Microscopy (Biofilm Structure Visualization)
4.5.2. Minimum Biofilm Eradication Concentration (MBEC)
4.5.3. Biofilm Total Biomass Quantification—Crystal Violet Staining
4.6. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rodrigues, C.; Rodrigues, M.; Silva, S.; Henriques, M. Candida glabrata Biofilms: How Far Have We Come? J. Fungi 2017, 3, 11. [Google Scholar] [CrossRef]
- Silva, S.; Rodrigues, C.; Araújo, D.; Rodrigues, M.; Henriques, M. Candida Species Biofilms’ Antifungal Resistance. J. Fungi 2017, 3, 8. [Google Scholar] [CrossRef]
- Lass-Flörl, C. The changing face of epidemiology of invasive fungal disease in Europe. Mycoses 2009, 52, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Tomičić, Z.; Zupan, J.; Matos, T.; Raspor, P. Probiotic yeast Saccharomyces boulardii (nom. nud.) modulates adhesive properties of Candida glabrata. Med. Mycol. 2016, 54, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Kwa, A.L.; Cheng, S.; Du, C.; Clancy, C.J. The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob. Agents Chemother. 2012, 56, 4862–4869. [Google Scholar] [CrossRef] [PubMed]
- Douglas, L.J. Candida biofilms and their role in infection. Trends Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; She, X.; Merenstein, D.; Wang, C.; Hamilton, P.; Blackmon, A.; Hu, H.; Calderone, R.; Li, D. Fluconazole Resistance Patterns in Candida Species that Colonize Women with HIV Infection. Curr. Ther. Res. Clin. Exp. 2014, 76, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Srinivasan, A.; Uppuluri, P.; Ramasubramanian, A.K.; López-Ribot, J.L. Antifungal therapy with an emphasis on biofilms. Curr. Opin. Pharmacol. 2013, 13, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Delattin, N.; Cammue, B.P.A.; Thevissen, K. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med. Chem. 2014, 6, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Lemke, A.; Kiderlen, A.F.; Kayser, O. Amphotericin B. Appl. Microbiol. Biotechnol. 2005, 68, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Baginski, M.; Czub, J. Amphotericin B and its new derivatives-mode of action. Curr. Drug Metab. 2009, 10, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Botero, M.C.; Puentes-Herrera, M.; Cortés, J.A. Lipid formulations of amphotericin. Rev. Chil. Infectol. 2014, 31, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Stephens, N.; Rawlings, B.; Caffrey, P. Streptomyces nodosus host strains optimized for polyene glycosylation engineering. Biosci. Biotechnol. Biochem. 2012, 76, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Storm, G.; van Etten, E. Biopharmaceutical aspects of lipid formulations of amphotericin B. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 16, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Laniado-Laborin, R.; Cabrales-Vargas, M. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Azanza, P.; Ramon, J.; Jose, B. Anfotericina B forma liposomal: Un perfil farmacocinético exclusivo. Una historia incabada. Rev. Esp. Quimioter. 2012, 25, 17–24. [Google Scholar]
- Kshirsagar, N.; Pandya, S.; Kirodian, G.; Sanath, S. Liposomal drug delivery system from laboratory to clinic. J. Postgrad. Med. 2005, 51 (Suppl. 1), S5–S15. [Google Scholar] [PubMed]
- Adler-Moore, J.; Proffitt, R. Amphotericin B lipid preparations: What are the differences? Clin. Microbiol. Infect. 2008, 14 (Suppl. 4), 25–36. [Google Scholar] [CrossRef] [PubMed]
- Hamill, R. Amphotericin B formulations: A comparative review of efficacy and toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. Amphotericin B colloidal dispersion. Expert Opin. Pharmacother. 2000, 1, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, A.; Dupont, B. Lipid formulations of amphotericin B: Where are we today? J. Mycol. Med. 2005, 14, 230–238. [Google Scholar] [CrossRef]
- Berenguer, J.; Rodriguez-Tudela, J.; Richard, C.; Alvarez, M.; Sanz, M.; Gaztelurrutia, L.; Ayats, J.; Martinez-Suarez, J.; Scedosporium prolificans Spanish Study Group. Deep infections caused by Scedosporium prolificans. A report on 16 cases in Spain and a review of the literature. Medicine 1997, 76, 256–265. [Google Scholar] [CrossRef]
- Boutati, E.; Anaissie, E. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: Ten years’ experience at a cancer center and implications for management. Blood 1997, 90, 999–1008. [Google Scholar] [PubMed]
- Tritz, D.; Woods, G. Fatal disseminated infection with Aspergillus terreus in immunocompromised hosts. Clin. Infect. Dis. 1993, 16, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Montagna, M.T.; Lovero, G.; Coretti, C.; De Giglio, O.; Martinelli, D.; Bedini, A.; Delia, M.; Rosato, A.; Codeluppi, M.; Caggiano, G. In vitro activities of amphotericin B deoxycholate and liposomal amphotericin B against 604 clinical yeast isolates. J. Med. Microbiol. 2014, 63, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Moen, M.D.; Lyseng-Williamson, K.A.; Scott, L.J. Liposomal amphotericin B: A review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections. Drugs 2009, 69, 361–392. [Google Scholar] [CrossRef] [PubMed]
- Faria-Ramos, I.; Neves-Maia, J.; Ricardo, E.; Santos-Antunes, J.; Silva, A.T.; Costa-de-Oliveira, S.; Cantón, E.; Rodrigues, A.G.; Pina-Vaz, C. Species distribution and in vitro antifungal susceptibility profiles of yeast isolates from invasive infections during a Portuguese multicenter survey. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Krogh-Madsen, M.; Arendrup, M.C.; Heslet, L.; Knudsen, J.D. Amphotericin B and Caspofungin Resistance in Candida glabrata Isolates Recovered from a Critically Ill Patient. Clin. Infect. Dis. 2006, 42, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Prażyńska, M.; Gospodarek, E. In vitro effect of amphotericin B on Candida albicans, Candida glabrata and Candida parapsilosis biofilm formation. Mycopathologia 2014, 177, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kiraz, N.; Oz, Y. Species distribution and in vitro antifungal susceptibility of clinical Candida isolates from a university hospital in Turkey over a 5-year period. Med. Mycol. 2011, 49, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kawai, A.; Yamagishi, Y.; Mikamo, H. In vitro efficacy of liposomal amphotericin B, micafungin and fluconazole against non-albicans Candida species biofilms. J. Infect. Chemother. 2015, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.V.; Silva, L.B.; de Oliveira, D.B.C.; da Silva, P.R.; Ferreira-Paim, K.; Andrade-Silva, L.E.; Silva-Vergara, M.L.; Andrade, A.A. Species Distribution, Virulence Factors, and Antifungal Susceptibility Among Candida parapsilosis Complex Isolates Recovered from Clinical Specimens. Mycopathologia 2015, 180, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Kanani, A.; Zaini, F.; Kordbacheh, P.; Falahati, M.; Rezaie, S.; Daie, R.; Farahyar, S.; Safara, M.; Fateh, R.; Faghihloo, E.; et al. Identification of Azole Resistance Markers in Clinical Isolates of Candida tropicalis Using cDNA-AFLP Method. J. Clin. Lab. Anal. 2015, 272, 266–272. [Google Scholar]
- Nabili, M.; Abdollahi Gohar, A.; Badali, H.; Mohammadi, R.; Moazeni, M. Amino acid substitutions in Erg11p of azole-resistant Candida glabrata: Possible effective substitutions and homology modelling. J. Glob. Antimicrob. Resist. 2016, 5, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; Pappas, P.G. Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2016, 30, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Rhomberg, P.R.; Jones, R.N.; Castanheira, M. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J. Antimicrob. Chemother. 2016, 101, 2868–2873. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.; Posteraro, B.; Lass-Flörl, C. Antifungal drug resistance among Candida species: Mechanisms and clinical impact. Mycoses 2015, 58 (Suppl. 2), 2–13. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Roman, E.; Sanchez-Fresneda, R.; Casas, C.; Herrero, E.; Arguelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The Production of Reactive Oxygen Species Is a Universal Action Mechanism of Amphotericin B against Pathogenic Yeasts and Contributes to the Fungicidal Effect of This Drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef] [PubMed]
- Neofytos, D.; Lombardi, L.R.; Shields, R.K.; Ostrander, D.; Warren, L.; Nguyen, M.H.; Thompson, C.B.; Marr, K.A. Administration of voriconazole in patients with renal dysfunction. Clin. Infect. Dis. 2012, 54, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Linder, N.; Klinger, G.; Shalit, I.; Levy, I.; Ashkenazi, S.; Haski, G.; Levit, O.; Sirota, L. Treatment of candidaemia in premature infants: Comparison of three amphotericin B preparations. J. Antimicrob. Chemother. 2003, 52, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Zeichner, L.; Marr, K.A.; Rex, J.H.; Cohen, S.H. Amphotericin B: Time for a new “gold standard”. Clin. Infect. Dis. 2003, 37, 415–425. [Google Scholar] [PubMed]
- Safdar, A.; Ma, J.; Saliba, F.; Dupont, B.; Wingard, J.; Hachem, R.; Mattiuzzi, G.; Chandrasekar, P.; Kontoyiannis, D.; Rolston, K.; et al. Drug-induced nephrotoxicity caused by amphotericin B lipid complex and liposomal amphotericin B: A review and meta-analysis. Medicine 2010, 89, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Bjarnsholt, T. The complexity of microbial biofilm research—An introduction to the 3rd Thematic Issue on Biofilms. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing Antifungal Agents Breakpoint Tables for Interpretation of MICs, Version 8.1. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/Antifungal_breakpoints_v_8.1_March_2017.pdf (accessed on 1 March 2017).
- Fielding, R.; Smith, P.; Wang, L.; Porter, J.; Guo, L. Comparative pharmacokinetics of amphotericin B after administration of a novel colloidal delivery system, ABCD, and a conventional formulation to rats. Antimicrob. Agents Chemother. 1991, 35, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Muñoz, A.; Quinds, G.; Tur, C.; Ruesga, M.; Miranda, Y.; del Valle, O.; Cossum, P.; Wallace, T. In Vitro antifungal activity of liposomal nystatin in comparison with nystatin, amphotericin B cholesteryl sulphate, liposomal amphotericin B, amphotericin B lipid complex, amphotericin B desoxycholate, fluconazole and itraconazole. J. Antimicrob. Chemother. 1999, 44, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G.M.; Elizondo, M.; Ayala, J. Trends in species distribution and susceptibility of bloodstream isolates of Candida collected in Monterrey, Mexico, to seven antifungal agents: Results of a 3-year (2004 to 2007) surveillance study. J. Clin. Microbiol. 2008, 46, 2902–2905. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.M.; Bader, O.; Parker, J.E.; Weig, M.; Gross, U.; Warrilow, A.G.S.; Kelly, D.E.; Kelly, S.L. Two clinical isolates of Candida glabrata exhibiting reduced sensitivity to amphotericin B both harbor mutations in ERG2. Antimicrob. Agents Chemother. 2012, 56, 6417–6421. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.M.; Parker, J.E.; Bader, O.; Weig, M.; Gross, U.; Warrilow, A.G.S.; Kelly, D.E.; Kelly, S.L. Facultative sterol uptake in an ergosterol-deficient clinical isolate of candida glabrata harboring a missense mutation in ERG11 and exhibiting cross-resistance to azoles and amphotericin B. Antimicrob. Agents Chemother. 2012, 56, 4223–4232. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Hollis, R.J.; Jones, R.N.; Diekema, D.J. In Vitro Activities of Ravuconazole and Voriconazole Compared with Those of Four Approved Systemic Antifungal Agents against 6, 970 Clinical Isolates of Candida spp. Antimicrob. Agents Chemother. 2002, 46, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Groll, A.H.; Giri, N.; Petraitis, V.; Petraitiene, R.; Candelario, M.; Bacher, J.S.; Piscitelli, S.C.; Walsh, T.J. Comparative Efficacy and Distribution of Lipid Formulations of Amphotericin B in Experimental Candida albicans Infection of the Central Nervous System. J. Infect. Dis. 2000, 182, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Anaissie, E.; Paetznick, V.L.; Proffitt, R.; Adler-Moore, J.; Bodey, G. Comparison of the in vitro antifungal activity of free and liposome-encapsulated amphotericin B. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Jessup, C.; Reyes, G.; Fothergill, A.; McCarthy, D.; Rinaldi, M.; Messer, S.; Pfaller, M.; Ghannoum, M. A head-on comparison of the in vitro antifungal activity of conventional and lipid-based amphotericin B: A multicenter study. J. Chemother. 2000, 12, 22–29. [Google Scholar] [PubMed]
- Lass-Flörl, C.; Mayr, A.; Perkhofer, S.; Hinterberger, G.; Hausdorfer, J.; Speth, C.; Fille, M. Activities of antifungal agents against yeasts and filamentous fungi: Assessment according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob. Agents Chemother. 2008, 52, 3637–3641. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.I.; Hooshmand-Rad, R. Treatment of Candida infections with amphotericin B lipid complex. Clin. Infect. Dis. 2005, 40 (Suppl. 6), S384–S391. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Z.; Chu, H.; Guo, J.; Jiang, G.; Qi, Q. Candida albicans Amphotericin B-Tolerant Persister Formation is Closely Related to Surface Adhesion. Mycopathologia 2016, 181, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Boucherit, Z.; Seksek, O.; Bolard, J. Dormancy of Candida albicans cells in the presence of the polyene antibiotic amphotericin B: Simple demonstration by flow cytometry. Med. Mycol. 2007, 45, 525–533. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, M.D.; Kumamoto, C.A.; Lewis, K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006, 50, 3839–3846. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, M.; Qingguo, Q.; Lewis, K. Patients with long-term oral carriage harbor high-persister mutants of Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhaheri, R.S.R.S.; Douglas, L.J.J. Absence of amphotericin B-tolerant persister cells in biofilms of some Candida species. Antimicrob. Agents Chemother. 2008, 52, 1884–1887. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhaheri, R.S.; Douglas, L.J. Apoptosis in Candida biofilms exposed to amphotericin B. J. Med. Microbiol. 2010, 59, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Bink, A.; Vandenbosch, D.; Coenye, T.; Nelis, H.; Cammue, B.P.A.; Thevissen, K. Superoxide dismutases are involved in Candida albicans biofilm persistence against miconazole. Antimicrob. Agents Chemother. 2011, 55, 4033–4037. [Google Scholar] [CrossRef] [PubMed]
- Dawson, C.C.; Intapa, C.; Jabra-Rizk, M.A. “Persisters”: Survival at the cellular level. PLoS Pathog. 2011, 7, e1002121. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Chaturvedi, A.K.; Srinivasan, A.; Banerjee, M.; Ramasubramaniam, A.K.; Köhler, J.R.; Kadosh, D.; Lopez-Ribot, J.L. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog 2010, 6, e1000828. [Google Scholar] [CrossRef] [PubMed]
- Lal, P.; Sharma, D.; Pruthi, P.; Pruthi, V. Exopolysaccharide analysis of biofilm-forming Candida albicans. J. Appl. Microbiol. 2010, 109, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, E.; Silva, S.; Rodrigues, C.F.; Alves, C.T.; Azeredo, J.; Henriques, M. Effects of fluconazole on Candida glabrata biofilms and its relationship with ABC transporter gene expression. Biofouling 2014, 30, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Hawser, S.P.; Douglas, L.J. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 1995, 39, 2128–2131. [Google Scholar] [CrossRef] [PubMed]
- Taff, H.T.; Nett, J.E.; Andes, D.R. Comparative analysis of Candida biofilm quantitation assays. Med. Mycol. 2012, 50, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Heffner, D.K.; Franklin, W.A. Endocarditis caused by Torulopsis glabrata. Am. J. Clin. Pathol. 1978, 70, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Fiori, B.; Trecarichi, E.M.; Posteraro, P.; Losito, A.R.; de Luca, A.; Sanguinetti, M.; Fadda, G.; Cauda, R.; Posteraro, B.; et al. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS ONE 2012, 7, e33705. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.V.M.; Rozental, S. Biofilm formation as a pathogenicity factor of medically important fungi. In Fungal Pathogenicity; InTech: Rijeka, Croatia, 2016; pp. 1–24. [Google Scholar]
- López-Ribot, J.L. Candida albicans biofilms: More than filamentation. Curr. Biol. 2005, 15, R453–R455. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.F.; Taff, H.T.; Cuevas, M.A.; Reinicke, E.L.; Sanchez, H.; Andes, D.R. Role of matrix β-1,3-glucan in antifungal resistance of non-albicans Candida biofilms. Antimicrob. Agents Chemother. 2013, 57, 1918–1920. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Silva, S.; Azeredo, J.; Henrique, M.; Henriques, M. Detection and Quantification of Fluconazole Within Candida glabrata Biofilms. Mycopathologia 2015, 179, 391–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingard, J.; White, M.; Anaissie, E.; Raffalli, J.; Goodman, J.; Arrieta, A.; L Amph/ABLC Collaborative Study Group. A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. Clin. Infect. Dis. 2000, 64, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Astellas Pharma US, Inc. AmBisome (Amphotericin B) Liposome for Injection; Astellas Pharma US, Inc.: Northbrook, IL, USA, 2012. [Google Scholar]
- Vandeputte, P.; Tronchin, G.; Larcher, G.; Ernoult, E.; Bergès, T.; Chabasse, D.; Bouchara, J.-P. A nonsense mutation in the ERG6 gene leads to reduced susceptibility to polyenes in a clinical isolate of Candida glabrata. Antimicrob. Agents Chemother. 2008, 52, 3701–3709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandeputte, P.; Tronchin, G.; Bergès, T.; Hennequin, C.; Chabasse, D.; Bouchara, J.-P. Reduced susceptibility to polyenes associated with a missense mutation in the ERG6 gene in a clinical isolate of Candida glabrata with pseudohyphal growth. Antimicrob. Agents Chemother. 2007, 51, 982–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, S.; Sanguinetti, M.; De Bernardis, F.; Torelli, R.; Posteraro, B.; Vandeputte, P.; Sanglard, D. Loss of mitochondrial functions associated with azole resistance in Candida glabrata results in enhanced virulence in mice. Antimicrob. Agents Chemother. 2011, 55, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Shin, J.H.; Jung, S.I.; Park, K.H.; Cho, D.; Kee, S.J.; Shin, M.G.; Suh, S.P.; Ryang, D.W. Species-specific differences in the susceptibilities of biofilms formed by Candida bloodstream isolates to echinocandin antifungals. Antimicrob. Agents Chemother. 2007, 51, 1520–1523. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal Susceptibility of Candida Biofilms: Unique Efficacy of Amphotericin B Lipid Formulations and Echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; ZhangGuan, X.; Zhu, Z.; Yao, X.; Yang, Y.; Jiang, Y.; Cao, Y. Enhancement of the antibiofilm activity of amphotericin B by polyamine biosynthesis inhibitors. Int. J. Antimicrob. Agents 2014, 46, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Luiz, R.L.F.; Vila, T.V.M.; de Mello, J.C.P.; Nakamura, C.V.; Rozental, S.; Ishida, K. Proanthocyanidins polymeric tannin from Stryphnodendron adstringens are active against Candida albicans biofilms. BMC Complement. Altern. Med. 2015, 15, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvasnickova, E.; Matatkova, O.; Cejkova, A.; Masak, J. Evaluation of baicalein, chitosan and usnic acid effect on Candida parapsilosis and Candida krusei biofilm using a Cellavista device. J. Microbiol. Methods 2015, 118, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Mahl, C.D.; Behling, C.S.; Hackenhaar, F.S.; de Carvalho E Silva, M.N.; Putti, J.; Salomon, T.B.; Alves, S.H.; Fuentefria, A.; Benfato, M.S. Induction of ROS generation by fluconazole in Candida glabrata: Activation of antioxidant enzymes and oxidative DNA damage. Diagn. Microbiol. Infect. Dis. 2015, 82, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Zambrano, L.; Escribano, P.; Bouza, E.; Guinea, J. Comparison of the antifungal activity of micafungin and amphotericin B against Candida tropicalis biofilms. J. Antimicrob. Chemother. 2016, 71, 2498–2501. [Google Scholar] [CrossRef] [PubMed]
- Zahran, K.M.; Agban, M.N.; Ahmed, S.H.; Hassan, E.A.; Sabet, M.A. Patterns of candida biofilm on intrauterine devices. J. Med. Microbiol. 2015, 64, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Shanmughapriya, S.; Sornakumari, H.; Lency, A.; Kavitha, S.; Natarajaseenivasan, K. Synergistic effect of amphotericin B and tyrosol on biofilm formed by Candida krusei and Candida tropicalis from intrauterine device users. Med. Mycol. 2014, 52, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Maiolo, E.M.; Tafin, U.F.; Borens, O.; Trampuz, A. Activities of fluconazole, caspofungin, anidulafungin, and amphotericin B on planktonic and biofilm candida species determined by microcalorimetry. Antimicrob. Agents Chemother. 2014, 58, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Aslan, H.; Gulmez, D. Investigation of the correlation between biofilm forming ability of urinary Candida isolates with the use of urinary catheters and change of antifungal susceptibility in the presence of biofilm. Mikrobiyol. Buleni 2016, 50, 256–265. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Wang, Y.; Jin, L.; Abiko, Y.; Samaranayake, L.P.; Jayampath Seneviratne, C.; Wang, Y.; Jin, L.; Abiko, Y.; Samaranayake, L.P. Proteomics of drug resistance in candida glabrata biofilms. Proteomics 2010, 10, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Silva, S.; Azeredo, J.; Henriques, M. Candida glabrata’s recurrent infections: Biofilm formation during Amphotericin B treatment. Lett. Appl. Microbiol. 2016, 63, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.H.; Melake, N.A.; Somily, A.M.; Zakaria, A.S.; Baddour, M.M.; Mahmoud, A.Z.; Arthington-Skaggs, B.A.; Jradi, H.; Desai, T.; Morrison, C.J.; et al. The effect of antifungal combination on transcripts of a subset of drug-resistance genes in clinical isolates of Candida species induced biofilms. Antimicrob. Agents Chemother. 2015, 23, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Kohno, S. ER stress response mechanisms in the pathogenic yeast Candida glabrata and their roles in virulence. Virulence 2014, 5, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, K.D.; Suci, P.A.; Miller, R.L.; Nelson, R.D.; Tyler, B.J. A Small Subpopulation of Blastospores in Candida albicans Biofilms Exhibit Resistance to Amphotericin B Associated with Differential Regulation of Ergosterol and B-1,6-Glucan Pathway Genes. Antimicrob. Agents Chemother. 2006, 50, 3708–3716. [Google Scholar]
- Arendrup, M.C.; Arikan, S.; Barchiesi, F.; Bille, J.; Dannaoui, E.; Denning, D.W.; Donnelly, J.P.; Fegeler, W.; Moore, C.; Richardson, M.; et al. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia—Forming moulds. Clin. Microbiol. Infect. 2008, 14, 982–984. [Google Scholar]
- Alastruey-Izquierdo, A.; Cuenca-Estrella, M. EUCAST and CLSI: How to assess in vitro susceptibility and clinical resistance. Curr. Fungal Infect. Rep. 2012, 6, 229–234. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Gonçalves, B.; Rodrigues, M.E.; Silva, S.; Azeredo, J.; Henriques, M. The Effectiveness of Voriconazole in Therapy of Candida glabrata’s Biofilms Oral Infections and Its Influence on the Matrix Composition and Gene Expression. Mycopathologia 2017, 182, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Henriques, M. Oral mucositis caused by Candida glabrata biofilms: Failure of the concomitant use of fluconazole and ascorbic acid. Ther. Adv. Infect. Dis. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
| Species | MIC (mg/L) | MFC (mg/L) | ||
|---|---|---|---|---|
| AmB-Deox | AmB-L | AmB-Deox | AmB-L | |
| Candida albicans SC5314 | 0.25 | 0.5 | 1 | 1.5 |
| Candida glabrata ATCC2001 | 0.25 | 1 | 1 | 1.5 |
| Candida parapsilosis ATCC22019 | 0.25 | 1 | 1 | 1.5 |
| Candida tropicalis ATCC750 | 0.5 | 1 | 1 | 1.5 |
| Species | MBEC (mg/L) | |||
|---|---|---|---|---|
| AmB-Deox | % of Maximum Permitted Dose # | AmB-L | % of Maximum Permitted Dose # | |
| Candida albicans SC5314 | 2 | 11.90 | 3 | 3.57 *** |
| Candida glabrata ATCC2001 | 4 | 23.81 | ≥8 | ≥9.52 *** |
| Candida parapsilosis ATCC22019 | 2 | 11.90 | 2 | 2.38 *** |
| Candida tropicalis ATCC750 | 2 | 11.90 | ≥8 | ≥9.52 *** |
| Species | [Drug] mg/L for Biofilm Reduction Closer to 50% | |||
|---|---|---|---|---|
| [AmB-Deox] | % Max Biofilm Reduction | [AmB-L] | % Max Biofilm Reduction | |
| Candida albicans SC5314 | 0.25 | 68.56 | 0.5 | 70.72 |
| Candida glabrata ATCC2001 | 1.5 | 51.56 | 1.5 | 48.86 |
| Candida parapsilosis ATCC22019 | 1.5 | 34.64 | 1 | 43.78 |
| Candida tropicalis ATCC750 | 0.25 | 89.58 | 1 | 50.66 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, C.F.; Henriques, M. Liposomal and Deoxycholate Amphotericin B Formulations: Effectiveness against Biofilm Infections of Candida spp. Pathogens 2017, 6, 62. https://doi.org/10.3390/pathogens6040062
Rodrigues CF, Henriques M. Liposomal and Deoxycholate Amphotericin B Formulations: Effectiveness against Biofilm Infections of Candida spp. Pathogens. 2017; 6(4):62. https://doi.org/10.3390/pathogens6040062
Chicago/Turabian StyleRodrigues, Célia F., and Mariana Henriques. 2017. "Liposomal and Deoxycholate Amphotericin B Formulations: Effectiveness against Biofilm Infections of Candida spp." Pathogens 6, no. 4: 62. https://doi.org/10.3390/pathogens6040062






