What Is Our Current Understanding of PrPSc-Associated Neurotoxicity and Its Molecular Underpinnings?
Abstract
:1. Introduction
1.1. PrPC
1.2. PrPSc
1.3. The PRNP Gene
1.4. Human Prion Disease
1.5. Animal Prion Diseases
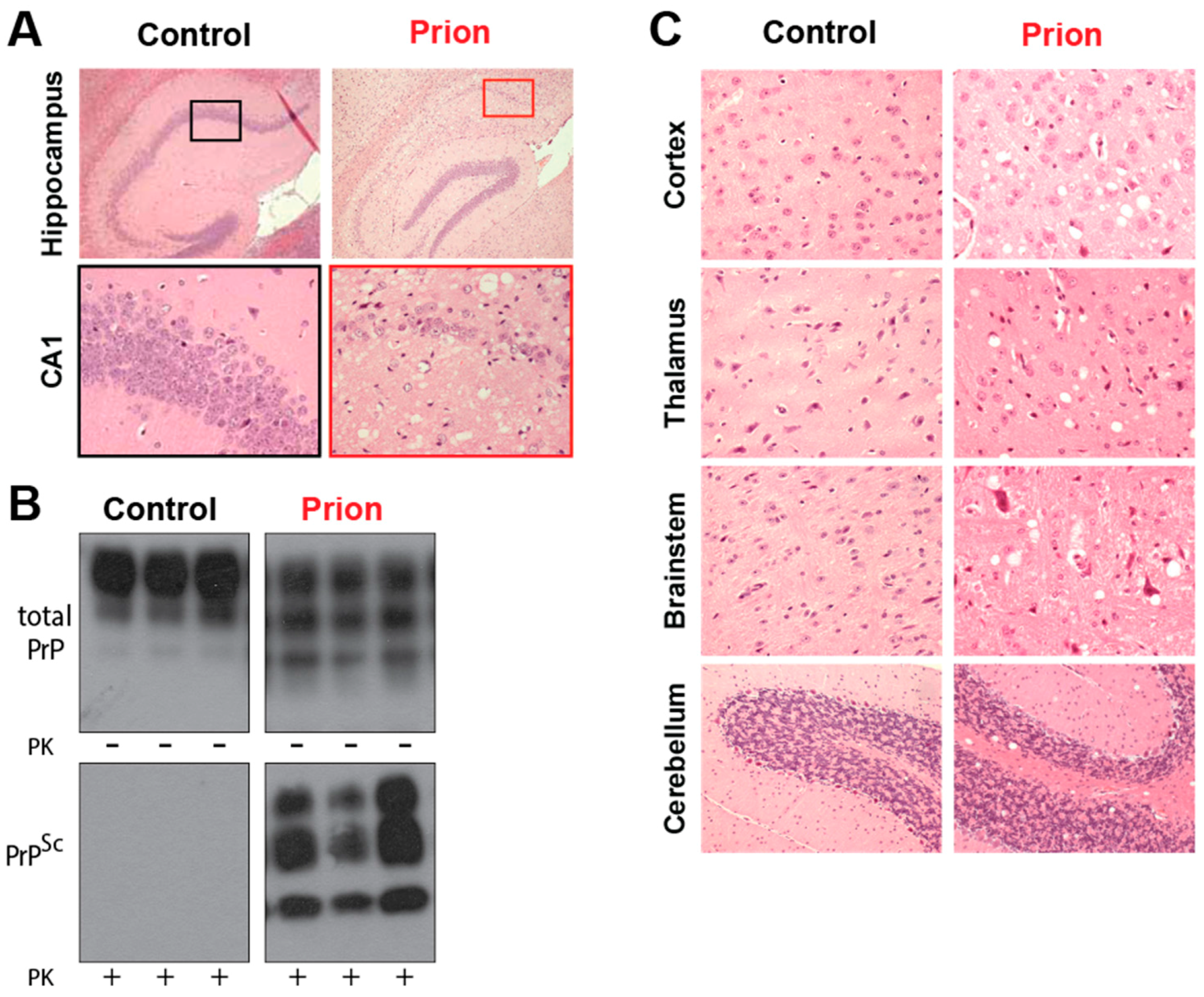
1.6. Selective Neuronal Vulnerability in Prion Diseases
2. Is the Prion Protein Directly Responsible for Prion Disease?
2.1. PrPC is Essential for Prion Disease
2.2. The Weak Links between PrPSc and Neurotoxicity
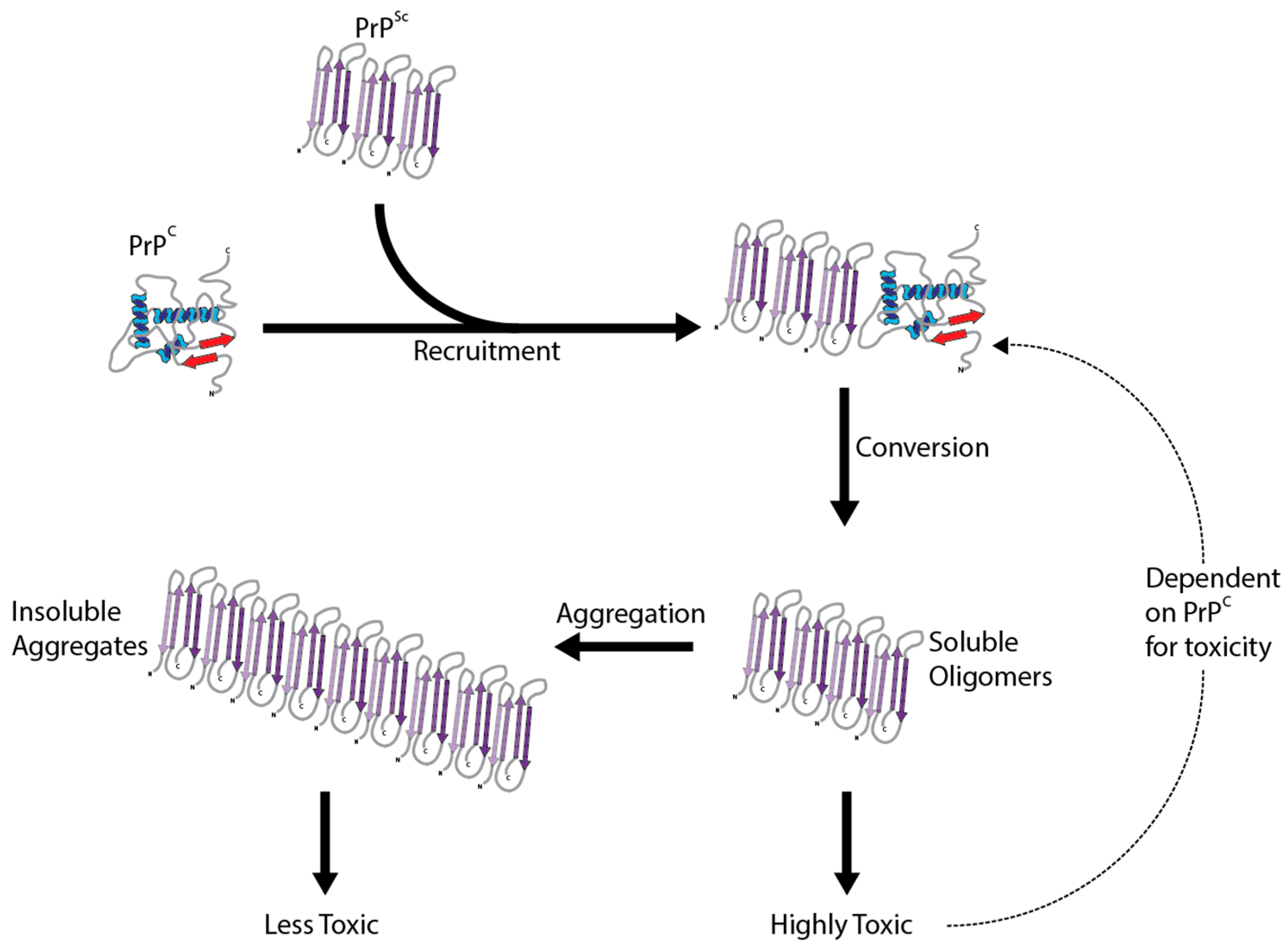
2.3. PrPSc Structure and Toxicity
3. The Molecular Underpinnings of PrPSc Toxicity
3.1. Autophagy
3.2. The Induction of Apoptosis
3.3. Proteasome/Ubiquitin Inhibition
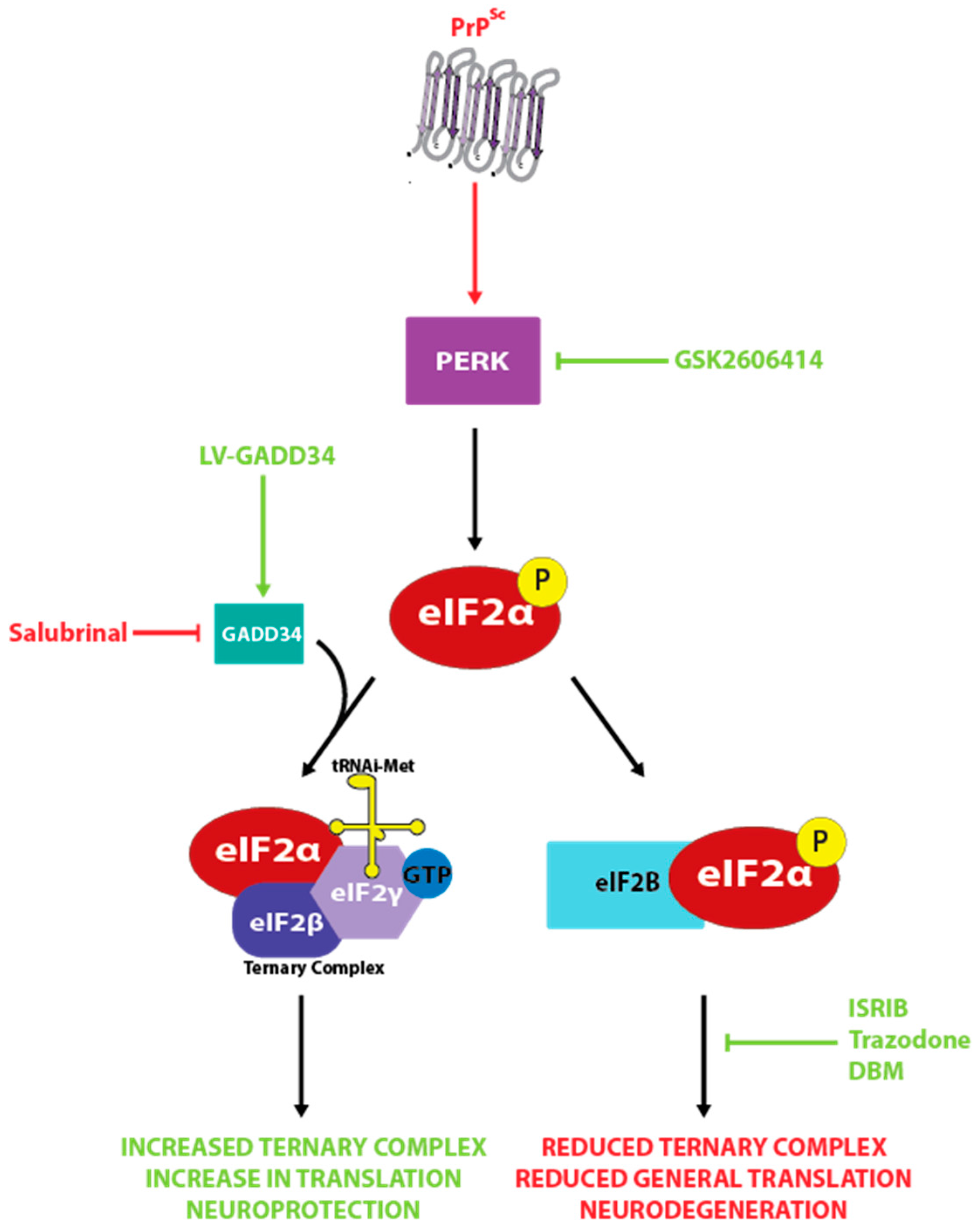
3.4. The Unfolded Protein Response
3.5. Oxidative Stress
3.6. Synaptic Dysfunction
3.7. Microglia
4. Discussion
Conflicts of Interest
References
- Rodriguez, J.A.; Jiang, L.; Eisenberg, D.S. Toward the atomic structure of PrPSc. Cold Spring Harb. Perspect. Biol. 2017, 9, a031336. [Google Scholar] [CrossRef] [PubMed]
- Castle, A.R.; Gill, A.C. Physiological functions of the cellular prion protein. Front. Mol. Biosci. 2017, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Stahl, N.; Borchelt, D.R.; Hsiao, K.; Prusiner, S.B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell 1987, 51, 229–240. [Google Scholar] [CrossRef]
- Peralta, O.A.; Huckle, W.R.; Eyestone, W.H. Developmental expression of the cellular prion protein (prp(c)) in bovine embryos. Mol. Reprod. Dev. 2012, 79, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Bamborough, P.; Wille, H.; Telling, G.C.; Yehiely, F.; Prusiner, S.B.; Cohen, F.E. Prion protein structure and scrapie replication: Theoretical, spectroscopic, and genetic investigations. Cold Spring Harb. Symp. Quant. Biol. 1996, 61, 495–509. [Google Scholar] [PubMed]
- Wulf, M.A.; Senatore, A.; Aguzzi, A. The biological function of the cellular prion protein: An update. BMC Biol. 2017, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Bremer, J.; Baumann, F.; Tiberi, C.; Wessig, C.; Fischer, H.; Schwarz, P.; Steele, A.D.; Toyka, K.V.; Nave, K.A.; Weis, J.; et al. Axonal prion protein is required for peripheral myelin maintenance. Nat. Neurosci. 2010, 13, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Qin, K.; Herms, J.W.; Madlung, A.; Manson, J.; Strome, R.; Fraser, P.E.; Kruck, T.; von Bohlen, A.; Schulz-Schaeffer, W.; et al. The cellular prion protein binds copper in vivo. Nature 1997, 390, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, C.; Takeuchi, A.M.; Nishimura, T.; Haraguchi, K.; Kubosaki, A.; Matsumoto, Y.; Saeki, K.; Matsumoto, Y.; Yokoyama, T.; Itohara, S.; et al. Prions prevent neuronal cell-line death. Nature 1999, 400, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Bounhar, Y.; Zhang, Y.; Goodyer, C.G.; LeBlanc, A. Prion protein protects human neurons against bax-mediated apoptosis. J. Biol. Chem. 2001, 276, 39145–39149. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Watt, N.T.; Walmsley, A.R.; Hooper, N.M. Tethering the n-terminus of the prion protein compromises the cellular response to oxidative stress. J. Neurochem. 2003, 84, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Mallucci, G.R.; Ratte, S.; Asante, E.A.; Linehan, J.; Gowland, I.; Jefferys, J.G.; Collinge, J. Post-natal knockout of prion protein alters hippocampal ca1 properties, but does not result in neurodegeneration. EMBO J. 2002, 21, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Katamine, S.; Shigematsu, K.; Nakatani, A.; Sakamoto, N.; Hasegawa, S.; Nakaoke, R.; Atarashi, R.; Kataoka, Y.; Miyamoto, T. Prion protein is necessary for latent learning and long-term memory retention. Cell. Mol. Neurobiol. 1997, 17, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alavez, M.; Conti, B.; Moroncini, G.; Criado, J.R. Contributions of neuronal prion protein on sleep recovery and stress response following sleep deprivation. Brain Res. 2007, 1158, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.H.; Hajj, G.N.; Muras, A.G.; Mancini, G.L.; Castro, R.M.; Ribeiro, K.C.; Brentani, R.R.; Linden, R.; Martins, V.R. Interaction of cellular prion and stress-inducible protein 1 promotes neuritogenesis and neuroprotection by distinct signaling pathways. J. Neurosci. 2005, 25, 11330–11339. [Google Scholar] [CrossRef] [PubMed]
- Loubet, D.; Dakowski, C.; Pietri, M.; Pradines, E.; Bernard, S.; Callebert, J.; Ardila-Osorio, H.; Mouillet-Richard, S.; Launay, J.M.; Kellermann, O.; et al. Neuritogenesis: The prion protein controls beta1 integrin signaling activity. FASEB J. 2012, 26, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.M.; Baldwin, M.; Nguyen, J.; Gasset, M.; Serban, A.; Groth, D.; Mehlhorn, I.; Huang, Z.; Fletterick, R.J.; Cohen, F.E.; et al. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. USA 1993, 90, 10962–10966. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.J.; Vazquez-Fernandez, E.; Onisko, B.; Requena, J.R. Proteinase k and the structure of PrPSc: The good, the bad and the ugly. Virus Res. 2015, 207, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Laurent, M. Prion diseases and the ‘protein only’ hypothesis: A theoretical dynamic study. Biochem. J. 1996, 318, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Castilla, J.; Saa, P.; Hetz, C.; Soto, C. In vitro generation of infectious scrapie prions. Cell 2005, 121, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.C.; Lebo, R.V.; Clawson, G.A.; Smuckler, E.A. Human prion protein cdna: Molecular cloning, chromosomal mapping, and biological implications. Science 1986, 233, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, H.A.; Stowring, L.E.; Westaway, D.; Stubblebine, W.H.; Prusiner, S.B.; Dearmond, S.J. Molecular cloning of a human prion protein cdna. DNA 1986, 5, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Collinge, J. Prion diseases of humans and animals: Their causes and molecular basis. Annu. Rev. Neurosci. 2001, 24, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Prusiner, S.B. Molecular biology of prion diseases. Science 1991, 252, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.S.; Dryden, A.J.; Hughes, J.T.; Collinge, J. Homozygous prion protein genotype predisposes to sporadic creutzfeldt-jakob disease. Nature 1991, 352, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Hizume, M.; Kobayashi, A.; Teruya, K.; Ohashi, H.; Ironside, J.W.; Mohri, S.; Kitamoto, T. Human prion protein (prp) 219k is converted to PrPSc but shows heterozygous inhibition in variant creutzfeldt-jakob disease infection. J. Biol. Chem. 2009, 284, 3603–3609. [Google Scholar] [CrossRef] [PubMed]
- Colby, D.W.; Prusiner, S.B. Prions. Cold Spring Harb. Perspect. Biol. 2011, 3, a006833. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Trabattoni, G.; Hainfellner, J.A.; Ironside, J.W.; Knight, R.S.; Budka, H. Mutations of the prion protein gene phenotypic spectrum. J. Neurol. 2002, 249, 1567–1582. [Google Scholar] [PubMed]
- Imran, M.; Mahmood, S. An overview of human prion diseases. Virol. J. 2011, 8, 559. [Google Scholar] [CrossRef] [PubMed]
- Gambetti, P.; Kong, Q.; Zou, W.; Parchi, P.; Chen, S.G. Sporadic and familial cjd: Classification and characterisation. Br. Med. Bull. 2003, 66, 213–239. [Google Scholar] [CrossRef] [PubMed]
- Gajdusek, D.C.; Zigas, V. Degenerative disease of the central nervous system in new guinea: The endemic occurrence of kuru in the native population. N. Engl. J. Med. 1957, 257, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, C.J., Jr.; Joy, A.; Heffner, R.; Franko, M.; Miyazaki, M.; Asher, D.M.; Parisi, J.E.; Brown, P.W.; Gajdusek, D.C. Clinical and pathological features and laboratory confirmation of creutzfeldt-jakob disease in a recipient of pituitary-derived human growth hormone. N. Engl. J. Med. 1985, 313, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Britton, T.C.; Al-Sarraj, S.; Shaw, C.; Campbell, T.; Collinge, J. Sporadic creutzfeldt-jakob disease in a 16-year-old in the UK. Lancet 1995, 346, 1155. [Google Scholar] [CrossRef]
- Bateman, D.; Hilton, D.; Love, S.; Zeidler, M.; Beck, J.; Collinge, J. Sporadic creutzfeldt-jakob disease in a 18-year-old in the UK. Lancet 1995, 346, 1155–1156. [Google Scholar] [CrossRef]
- Will, R.G.; Ironside, J.W.; Zeidler, M.; Cousens, S.N.; Estibeiro, K.; Alperovitch, A.; Poser, S.; Pocchiari, M.; Hofman, A.; Smith, P.G. A new variant of creutzfeldt-jakob disease in the uk. Lancet 1996, 347, 921–925. [Google Scholar] [CrossRef]
- Collinge, J.; Sidle, K.C.; Meads, J.; Ironside, J.; Hill, A.F. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ cjd. Nature 1996, 383, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.F.; Desbruslais, M.; Joiner, S.; Sidle, K.C.; Gowland, I.; Collinge, J.; Doey, L.J.; Lantos, P. The same prion strain causes vcjd and bse. Nature 1997, 389, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, N.; Wilesmith, J.; Griot, C. Bovine spongiform encephalopathy (bse): Causes and consequences of a common source epidemic. Am. J. Epidemiol. 1997, 145, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W.; Williams, E.S.; McCarty, C.W.; Spraker, T.R.; Kreeger, T.J.; Larsen, C.T.; Thorne, E.T. Epizootiology of chronic wasting disease in free-ranging cervids in colorado and wyoming. J. Wildl. Dis. 2000, 36, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Haley, N.J.; Hoover, E.A. Chronic wasting disease of cervids: Current knowledge and future perspectives. Annu. Rev. Anim. Biosci. 2015, 3, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Benestad, S.L.; Mitchell, G.; Simmons, M.; Ytrehus, B.; Vikoren, T. First case of chronic wasting disease in europe in a norwegian free-ranging reindeer. Vet. Res. 2016, 47, 88. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.S. Selective vulnerability to neurodegenerative disease: The curious case of prion protein. Dis. Model. Mech. 2014, 7, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; McLean, C.A.; Masters, C.L. Gerstmann-straussler-scheinker syndrome, fatal familial insomnia, and kuru: A review of these less common human transmissible spongiform encephalopathies. J. Clin. Neurosci. 2001, 8, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Montagna, P.; Gambetti, P.; Cortelli, P.; Lugaresi, E. Familial and sporadic fatal insomnia. Lancet Neurol. 2003, 2, 167–176. [Google Scholar] [CrossRef]
- Bueler, H.; Fischer, M.; Lang, Y.; Bluethmann, H.; Lipp, H.P.; DeArmond, S.J.; Prusiner, S.B.; Aguet, M.; Weissmann, C. Normal development and behaviour of mice lacking the neuronal cell-surface prp protein. Nature 1992, 356, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.C.; Clarke, A.R.; Hooper, M.L.; Aitchison, L.; McConnell, I.; Hope, J. 129/ola mice carrying a null mutation in prp that abolishes mrna production are developmentally normal. Mol. Neurobiol. 1994, 8, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Chen, J.; Yu, H.; Liu, S.; Chen, J.; Xu, X.; Sha, H.; Zhang, X.; Wu, G.; Xu, S.; et al. Functional disruption of the prion protein gene in cloned goats. J. Gen. Virol. 2006, 87, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Richt, J.A.; Kasinathan, P.; Hamir, A.N.; Castilla, J.; Sathiyaseelan, T.; Vargas, F.; Sathiyaseelan, J.; Wu, H.; Matsushita, H.; Koster, J.; et al. Production of cattle lacking prion protein. Nat. Biotechnol. 2007, 25, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.D.; Lindquist, S.; Aguzzi, A. The prion protein knockout mouse: A phenotype under challenge. Prion 2007, 1, 83–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLennan, N.F.; Brennan, P.M.; McNeill, A.; Davies, I.; Fotheringham, A.; Rennison, K.A.; Ritchie, D.; Brannan, F.; Head, M.W.; Ironside, J.W.; et al. Prion protein accumulation and neuroprotection in hypoxic brain damage. Am. J. Pathol. 2004, 165, 227–235. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Katamine, S.; Nishida, N.; Moriuchi, R.; Shigematsu, K.; Sugimoto, T.; Nakatani, A.; Kataoka, Y.; Houtani, T.; Shirabe, S.; et al. Loss of cerebellar purkinje cells in aged mice homozygous for a disrupted prp gene. Nature 1996, 380, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.C.; Lee, I.Y.; Silverman, G.L.; Harrison, P.M.; Strome, R.; Heinrich, C.; Karunaratne, A.; Pasternak, S.H.; Chishti, M.A.; Liang, Y.; et al. Ataxia in prion protein (prp)-deficient mice is associated with upregulation of the novel prp-like protein doppel. J. Mol. Biol. 1999, 292, 797–817. [Google Scholar] [CrossRef] [PubMed]
- Katamine, S.; Nishida, N.; Sugimoto, T.; Noda, T.; Sakaguchi, S.; Shigematsu, K.; Kataoka, Y.; Nakatani, A.; Hasegawa, S.; Moriuchi, R.; et al. Impaired motor coordination in mice lacking prion protein. Cell. Mol. Neurobiol. 1998, 18, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Sakaguchi, S.; Shigematsu, K.; Okimura, N.; Katamine, S. Doppel-induced purkinje cell death is stoichiometrically abrogated by prion protein. Biochem. Biophys. Res. Commun. 2004, 319, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Peoc’h, K.; Volland, H.; De Gassart, A.; Beaudry, P.; Sazdovitch, V.; Sorgato, M.C.; Creminon, C.; Laplanche, J.L.; Lehmann, S. Prion-like protein doppel expression is not modified in scrapie-infected cells and in the brains of patients with creutzfeldt-jakob disease. FEBS Lett. 2003, 536, 61–65. [Google Scholar] [CrossRef]
- Bueler, H.; Aguzzi, A.; Sailer, A.; Greiner, R.A.; Autenried, P.; Aguet, M.; Weissmann, C. Mice devoid of prp are resistant to scrapie. Cell 1993, 73, 1339–1347. [Google Scholar] [CrossRef]
- Brandner, S.; Isenmann, S.; Raeber, A.; Fischer, M.; Sailer, A.; Kobayashi, Y.; Marino, S.; Weissmann, C.; Aguzzi, A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature 1996, 379, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Mallucci, G.; Dickinson, A.; Linehan, J.; Klohn, P.C.; Brandner, S.; Collinge, J. Depleting neuronal prp in prion infection prevents disease and reverses spongiosis. Science 2003, 302, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Chesebro, B.; Trifilo, M.; Race, R.; Meade-White, K.; Teng, C.; LaCasse, R.; Raymond, L.; Favara, C.; Baron, G.; Priola, S.; et al. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 2005, 308, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.F.; Collinge, J. Subclinical prion infection in humans and animals. Br. Med. Bull. 2003, 66, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Lasmezas, C.I.; Deslys, J.P.; Robain, O.; Jaegly, A.; Beringue, V.; Peyrin, J.M.; Fournier, J.G.; Hauw, J.J.; Rossier, J.; Dormont, D. Transmission of the bse agent to mice in the absence of detectable abnormal prion protein. Science 1997, 275, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Manuelidis, L.; Fritch, W.; Xi, Y.G. Evolution of a strain of cjd that induces bse-like plaques. Science 1997, 277, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Nitrini, R.; Rosemberg, S.; Passos-Bueno, M.R.; da Silva, L.S.; Iughetti, P.; Papadopoulos, M.; Carrilho, P.M.; Caramelli, P.; Albrecht, S.; Zatz, M.; et al. Familial spongiform encephalopathy associated with a novel prion protein gene mutation. Ann. Neurol. 1997, 42, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Boyd, A.; Fletcher, A.; Byron, K.; Harper, C.; McLean, C.A.; Masters, C.L. Novel prion protein gene mutation in an octogenarian with creutzfeldt-jakob disease. Arch. Neurol. 2000, 57, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Grasbon-Frodl, E.; Lorenz, H.; Mann, U.; Nitsch, R.M.; Windl, O.; Kretzschmar, H.A. Loss of glycosylation associated with the t183a mutation in human prion disease. Acta Neuropathol. 2004, 108, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Hegde, R.S.; Mastrianni, J.A.; Scott, M.R.; DeFea, K.A.; Tremblay, P.; Torchia, M.; DeArmond, S.J.; Prusiner, S.B.; Lingappa, V.R. A transmembrane form of the prion protein in neurodegenerative disease. Science 1998, 279, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.F.; Joiner, S.; Linehan, J.; Desbruslais, M.; Lantos, P.L.; Collinge, J. Species-barrier-independent prion replication in apparently resistant species. Proc. Natl. Acad. Sci. USA 2000, 97, 10248–10253. [Google Scholar] [CrossRef] [PubMed]
- Race, R.; Raines, A.; Raymond, G.J.; Caughey, B.; Chesebro, B. Long-term subclinical carrier state precedes scrapie replication and adaptation in a resistant species: Analogies to bovine spongiform encephalopathy and variant creutzfeldt-jakob disease in humans. J. Virol. 2001, 75, 10106–10112. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.F.; Collinge, J. Subclinical prion infection. Trends Microbiol. 2003, 11, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Bolton, D.C.; Bendheim, P.E. Purification of scrapie agents: How far have we come? Curr. Top. Microbiol. Immunol. 1991, 172, 39–55. [Google Scholar] [PubMed]
- Silveira, J.R.; Raymond, G.J.; Hughson, A.G.; Race, R.E.; Sim, V.L.; Hayes, S.F.; Caughey, B. The most infectious prion protein particles. Nature 2005, 437, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Tzaban, S.; Friedlander, G.; Schonberger, O.; Horonchik, L.; Yedidia, Y.; Shaked, G.; Gabizon, R.; Taraboulos, A. Protease-sensitive scrapie prion protein in aggregates of heterogeneous sizes. Biochemistry 2002, 41, 12868–12875. [Google Scholar] [PubMed]
- Pastrana, M.A.; Sajnani, G.; Onisko, B.; Castilla, J.; Morales, R.; Soto, C.; Requena, J.R. Isolation and characterization of a proteinase k-sensitive PrPSc fraction. Biochemistry 2006, 45, 15710–15717. [Google Scholar] [CrossRef] [PubMed]
- Sajnani, G.; Silva, C.J.; Ramos, A.; Pastrana, M.A.; Onisko, B.C.; Erickson, M.L.; Antaki, E.M.; Dynin, I.; Vazquez-Fernandez, E.; Sigurdson, C.J.; et al. Pk-sensitive prp is infectious and shares basic structural features with pk-resistant prp. PLoS Pathog. 2012, 8, e1002547. [Google Scholar] [CrossRef] [PubMed]
- Bueler, H.; Raeber, A.; Sailer, A.; Fischer, M.; Aguzzi, A.; Weissmann, C. High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted prp gene. Mol. Med. 1994, 1, 19–30. [Google Scholar] [PubMed]
- Biasini, E.; Seegulam, M.E.; Patti, B.N.; Solforosi, L.; Medrano, A.Z.; Christensen, H.M.; Senatore, A.; Chiesa, R.; Williamson, R.A.; Harris, D.A. Non-infectious aggregates of the prion protein react with several PrPSc-directed antibodies. J. Neurochem. 2008, 105, 2190–2204. [Google Scholar] [CrossRef] [PubMed]
- Riesner, D.; Kellings, K.; Post, K.; Wille, H.; Serban, H.; Groth, D.; Baldwin, M.A.; Prusiner, S.B. Disruption of prion rods generates 10-nm spherical particles having high alpha-helical content and lacking scrapie infectivity. J. Virol. 1996, 70, 1714–1722. [Google Scholar] [PubMed]
- Morales, R.; Abid, K.; Soto, C. The prion strain phenomenon: Molecular basis and unprecedented features. Biochim. Biophys. Acta 2007, 1772, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Safar, J.; Wille, H.; Itri, V.; Groth, D.; Serban, H.; Torchia, M.; Cohen, F.E.; Prusiner, S.B. Eight prion strains have prp(sc) molecules with different conformations. Nat. Med. 1998, 4, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.K.; McKinley, M.P.; Bowman, K.A.; Braunfeld, M.B.; Barry, R.A.; Prusiner, S.B. Separation and properties of cellular and scrapie prion proteins. Proc. Natl. Acad. Sci. USA 1986, 83, 2310–2314. [Google Scholar] [CrossRef] [PubMed]
- Korth, C.; Stierli, B.; Streit, P.; Moser, M.; Schaller, O.; Fischer, R.; Schulz-Schaeffer, W.; Kretzschmar, H.; Raeber, A.; Braun, U.; et al. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 1997, 390, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Jucker, M. The amyloid state of proteins in human diseases. Cell 2012, 148, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.K.; Al-Doujaily, H.; Sharps, B.; Clarke, A.R.; Collinge, J. Prion propagation and toxicity in vivo occur in two distinct mechanistic phases. Nature 2011, 470, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Udgaonkar, J.B. Molecular mechanism of the misfolding and oligomerization of the prion protein: Current understanding and its implications. Biochemistry 2015, 54, 4431–4442. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskaite, J.; Young, A.; Gardner, C.E.; Macpherson, J.V.; Venien-Bryan, C.; Pinheiro, T.J. An unusual soluble beta-turn-rich conformation of prion is involved in fibril formation and toxic to neuronal cells. Biochem. Biophys. Res. Commun. 2005, 328, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Novitskaya, V.; Bocharova, O.V.; Bronstein, I.; Baskakov, I.V. Amyloid fibrils of mammalian prion protein are highly toxic to cultured cells and primary neurons. J. Biol. Chem. 2006, 281, 13828–13836. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, S.; Rezaei, H.; Sales, N.; Kaiser-Schulz, G.; Lefebvre-Roque, M.; Vidal, C.; Fournier, J.G.; Comte, J.; Wopfner, F.; Grosclaude, J.; et al. In vitro and in vivo neurotoxicity of prion protein oligomers. PLoS Pathog. 2007, 3, e125. [Google Scholar] [CrossRef] [PubMed]
- Ugalde, C.L.; Finkelstein, D.I.; Lawson, V.A.; Hill, A.F. Pathogenic mechanisms of prion protein, amyloid-beta and alpha-synuclein misfolding: The prion concept and neurotoxicity of protein oligomers. J. Neurochem. 2016, 139, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Resenberger, U.K.; Harmeier, A.; Woerner, A.C.; Goodman, J.L.; Muller, V.; Krishnan, R.; Vabulas, R.M.; Kretzschmar, H.A.; Lindquist, S.; Hartl, F.U.; et al. The cellular prion protein mediates neurotoxic signalling of beta-sheet-rich conformers independent of prion replication. EMBO J. 2011, 30, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, J.W.; Schlote, W.; Tateishi, J. Neuronal autophagy in experimental creutzfeldt-jakob’s disease. Acta Neuropathol. 1989, 78, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, J.W.; Kao, M.; Schlote, W.; Diringer, H. Neuronal autophagy in experimental scrapie. Acta Neuropathol. 1991, 82, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Liberski, P.P.; Yanagihara, R.; Gibbs, C.J., Jr.; Gajdusek, D.C. Neuronal autophagic vacuoles in experimental scrapie and creutzfeldt-jakob disease. Acta Neuropathol. 1992, 83, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, B.; Liberski, P.P.; Giraud, P.; Kopp, N.; Brown, P. Autophagy is a part of ultrastructural synaptic pathology in creutzfeldt-jakob disease: A brain biopsy study. Int. J. Biochem. Cell Biol. 2004, 36, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tian, C.; Wang, S.B.; Xie, W.L.; Guo, Y.; Zhang, J.; Shi, Q.; Chen, C.; Dong, X.P. Activation of the macroautophagic system in scrapie-infected experimental animals and human genetic prion diseases. Autophagy 2012, 8, 1604–1620. [Google Scholar] [CrossRef] [PubMed]
- Heiseke, A.; Aguib, Y.; Riemer, C.; Baier, M.; Schatzl, H.M. Lithium induces clearance of protease resistant prion protein in prion-infected cells by induction of autophagy. J. Neurochem. 2009, 109, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Karapetyan, Y.E.; Sferrazza, G.F.; Zhou, M.; Ottenberg, G.; Spicer, T.; Chase, P.; Fallahi, M.; Hodder, P.; Weissmann, C.; Lasmezas, C.I. Unique drug screening approach for prion diseases identifies tacrolimus and astemizole as antiprion agents. Proc. Natl. Acad. Sci. USA 2013, 110, 7044–7049. [Google Scholar] [CrossRef] [PubMed]
- Nakagaki, T.; Satoh, K.; Ishibashi, D.; Fuse, T.; Sano, K.; Kamatari, Y.O.; Kuwata, K.; Shigematsu, K.; Iwamaru, Y.; Takenouchi, T.; et al. Fk506 reduces abnormal prion protein through the activation of autolysosomal degradation and prolongs survival in prion-infected mice. Autophagy 2013, 9, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Cortes, C.J.; Qin, K.; Cook, J.; Solanki, A.; Mastrianni, J.A. Rapamycin delays disease onset and prevents prp plaque deposition in a mouse model of gerstmann-straussler-scheinker disease. J. Neurosci. 2012, 32, 12396–12405. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Giese, A.; Groschup, M.H.; Hess, B.; Kretzschmar, H.A. Neuronal cell death in scrapie-infected mice is due to apoptosis. Brain Pathol. 1995, 5, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Jesionek-Kupnicka, D.; Buczynski, J.; Kordek, R.; Liberski, P.P. Neuronal loss and apoptosis in experimental creutzfeldt-jakob disease in mice. Folia Neuropathol. 1999, 37, 283–286. [Google Scholar] [PubMed]
- Gray, F.; Chretien, F.; Adle-Biassette, H.; Dorandeu, A.; Ereau, T.; Delisle, M.B.; Kopp, N.; Ironside, J.W.; Vital, C. Neuronal apoptosis in creutzfeldt-jakob disease. J. Neuropathol. Exp. Neurol. 1999, 58, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Budka, H. Distribution of apoptosis-related proteins in sporadic creutzfeldt-jakob disease. Brain Res. 2010, 1323, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Drew, S.C.; Haigh, C.L.; Klemm, H.M.; Masters, C.L.; Collins, S.J.; Barnham, K.J.; Lawson, V.A. Optical imaging detects apoptosis in the brain and peripheral organs of prion-infected mice. J. Neuropathol. Exp. Neurol. 2011, 70, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Coulpier, M.; Messiaen, S.; Hamel, R.; de Marco, M.F.; Lilin, T.; Eloit, M. Bax deletion does not protect neurons from bse-induced death. Neurobiol. Dis. 2006, 23, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.D.; King, O.D.; Jackson, W.S.; Hetz, C.A.; Borkowski, A.W.; Thielen, P.; Wollmann, R.; Lindquist, S. Diminishing apoptosis by deletion of bax or overexpression of bcl-2 does not protect against infectious prion toxicity in vivo. J. Neurosci. 2007, 27, 13022–13027. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.D.; Hetz, C.; Yi, C.H.; Jackson, W.S.; Borkowski, A.W.; Yuan, J.; Wollmann, R.H.; Lindquist, S. Prion pathogenesis is independent of caspase-12. Prion 2007, 1, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Glickman, M.H.; Ciechanover, A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, C.; Goold, R.; Andre, R.; Devoy, A.; Ortega, Z.; Moonga, J.; Linehan, J.M.; Brandner, S.; Lucas, J.J.; Collinge, J.; et al. Prion-mediated neurodegeneration is associated with early impairment of the ubiquitin-proteasome system. Acta Neuropathol. 2016, 131, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Andre, R.; Tabrizi, S.J. Misfolded prp and a novel mechanism of proteasome inhibition. Prion 2012, 6, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Deriziotis, P.; Andre, R.; Smith, D.M.; Goold, R.; Kinghorn, K.J.; Kristiansen, M.; Nathan, J.A.; Rosenzweig, R.; Krutauz, D.; Glickman, M.H.; et al. Misfolded prp impairs the ups by interaction with the 20s proteasome and inhibition of substrate entry. EMBO J. 2011, 30, 3065–3077. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, M.; Deriziotis, P.; Dimcheff, D.E.; Jackson, G.S.; Ovaa, H.; Naumann, H.; Clarke, A.R.; van Leeuwen, F.W.; Menendez-Benito, V.; Dantuma, N.P.; et al. Disease-associated prion protein oligomers inhibit the 26s proteasome. Mol. Cell 2007, 26, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhao, D.; Yang, L. Interaction between misfolded prp and the ubiquitin-proteasome system in prion-mediated neurodegeneration. Acta Biochim. Biophys. Sin. 2013, 45, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Dron, M.; Dandoy-Dron, F.; Farooq Salamat, M.K.; Laude, H. Proteasome inhibitors promote the sequestration of PrPSc into aggresomes within the cytosol of prion-infected cad neuronal cells. J. Gen. Virol. 2009, 90, 2050–2060. [Google Scholar] [CrossRef] [PubMed]
- Homma, T.; Ishibashi, D.; Nakagaki, T.; Satoh, K.; Sano, K.; Atarashi, R.; Nishida, N. Increased expression of p62/sqstm1 in prion diseases and its association with pathogenic prion protein. Sci. Rep. 2014, 4, 4504. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Russelakis-Carneiro, M.; Maundrell, K.; Castilla, J.; Soto, C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003, 22, 5435–5445. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Matamala, J.M.; Duran-Aniotz, C.; Cornejo, V.H.; Foley, A.; Hetz, C. Er stress signaling and neurodegeneration: At the intersection between alzheimer's disease and prion-related disorders. Virus Res. 2015, 207, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Halliday, M.; Radford, H.; Mallucci, G.R. Prions: Generation and spread versus neurotoxicity. J. Biol. Chem. 2014, 289, 19862–19868. [Google Scholar] [CrossRef] [PubMed]
- Halliday, M.; Radford, H.; Sekine, Y.; Moreno, J.; Verity, N.; le Quesne, J.; Ortori, C.A.; Barrett, D.A.; Fromont, C.; Fischer, P.M.; et al. Partial restoration of protein synthesis rates by the small molecule isrib prevents neurodegeneration without pancreatic toxicity. Cell Death Dis. 2015, 6, e1672. [Google Scholar] [CrossRef] [PubMed]
- Halliday, M.; Radford, H.; Zents, K.A.M.; Molloy, C.; Moreno, J.A.; Verity, N.C.; Smith, E.; Ortori, C.A.; Barrett, D.A.; Bushell, M.; et al. Repurposed drugs targeting eif2alpha-p-mediated translational repression prevent neurodegeneration in mice. Brain 2017, 140, 1768–1783. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Halliday, M.; Molloy, C.; Radford, H.; Verity, N.; Axten, J.M.; Ortori, C.A.; Willis, A.E.; Fischer, P.M.; Barrett, D.A.; et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 2013, 5, 206ra138. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Radford, H.; Peretti, D.; Steinert, J.R.; Verity, N.; Martin, M.G.; Halliday, M.; Morgan, J.; Dinsdale, D.; Ortori, C.A.; et al. Sustained translational repression by eif2alpha-p mediates prion neurodegeneration. Nature 2012, 485, 507–511. [Google Scholar] [PubMed]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under er stress and beyond. Nat. Rev. Mol. Cell. Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Schroder, M. The unfolded protein response. Mol. Biotechnol. 2006, 34, 279–290. [Google Scholar] [CrossRef]
- Schroder, M.; Kaufman, R.J. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005, 74, 739–789. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Novoa, I.; Zeng, H.; Harding, H.P.; Ron, D. Feedback inhibition of the unfolded protein response by gadd34-mediated dephosphorylation of eif2alpha. J. Cell Biol. 2001, 153, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Axten, J.M.; Medina, J.R.; Feng, Y.; Shu, A.; Romeril, S.P.; Grant, S.W.; Li, W.H.; Heerding, D.A.; Minthorn, E.; Mencken, T.; et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1h-indol-5-yl)-7h-p yrrolo[2,3-d]pyrimidin-4-amine (gsk2606414), a potent and selective first-in-class inhibitor of protein kinase r (pkr)-like endoplasmic reticulum kinase (perk). J. Med. Chem. 2012, 55, 7193–7207. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Zyryanova, A.; Crespillo-Casado, A.; Fischer, P.M.; Harding, H.P.; Ron, D. Stress responses. Mutations in a translation initiation factor identify the target of a memory-enhancing compound. Science 2015, 348, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Doronina, V.A.; Staniforth, G.L.; Speldewinde, S.H.; Tuite, M.F.; Grant, C.M. Oxidative stress conditions increase the frequency of de novo formation of the yeast [psi+] prion. Mol. Microbiol. 2015, 96, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Redecke, L.; Binder, S.; Elmallah, M.I.; Broadbent, R.; Tilkorn, C.; Schulz, B.; May, P.; Goos, A.; Eich, A.; Rubhausen, M.; et al. Uv-light-induced conversion and aggregation of prion proteins. Free Radic. Biol. Med. 2009, 46, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yang, L.; Zhang, Z.; Wu, W.; Zhou, X.; Yin, X.; Zhao, D. Cellular prion protein (PrPC) of the neuron cell transformed to a pk-resistant protein under oxidative stress, comprising main mitochondrial damage in prion diseases. J. Mol. Neurosci. 2013, 51, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Besinger, A. Prion protein expression and superoxide dismutase activity. Biochem. J. 1998, 334 Pt 2, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Sakudo, A.; Lee, D.C.; Li, S.; Nakamura, T.; Matsumoto, Y.; Saeki, K.; Itohara, S.; Ikuta, K.; Onodera, T. Prp cooperates with sti1 to regulate sod activity in prp-deficient neuronal cell line. Biochem. Biophys. Res. Commun. 2005, 328, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.G.; Kim, J.I.; Lee, H.P.; Jin, J.K.; Choi, E.K.; Carp, R.I.; Kim, Y.S. Induction of heme oxygenase-1 in the brains of scrapie-infected mice. Neurosci. Lett. 2000, 289, 173–176. [Google Scholar] [CrossRef]
- Choi, S.I.; Ju, W.K.; Choi, E.K.; Kim, J.; Lea, H.Z.; Carp, R.I.; Wisniewski, H.M.; Kim, Y.S. Mitochondrial dysfunction induced by oxidative stress in the brains of hamsters infected with the 263 k scrapie agent. Acta Neuropathol. 1998, 96, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Sohn, H.O.; Lim, H.B.; Lee, Y.G.; Kim, Y.S.; Carp, R.I.; Wisniewski, H.M. Alteration of free radical metabolism in the brain of mice infected with scrapie agent. Free Radic. Res. 1999, 30, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Belichenko, P.V.; Brown, D.; Jeffrey, M.; Fraser, J.R. Dendritic and synaptic alterations of hippocampal pyramidal neurones in scrapie-infected mice. Neuropathol. Appl. Neurobiol. 2000, 26, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, M.; Halliday, W.G.; Bell, J.; Johnston, A.R.; MacLeod, N.K.; Ingham, C.; Sayers, A.R.; Brown, D.A.; Fraser, J.R. Synapse loss associated with abnormal prp precedes neuronal degeneration in the scrapie-infected murine hippocampus. Neuropathol. Appl. Neurobiol. 2000, 26, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Belichenko, P.; Sales, J.; Jeffrey, M.; Fraser, J.R. Early loss of dendritic spines in murine scrapie revealed by confocal analysis. Neuroreport 2001, 12, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.C.; Siskova, Z.; Perry, V.H.; O'Connor, V. Selective presynaptic degeneration in the synaptopathy associated with me7-induced hippocampal pathology. Neurobiol. Dis. 2009, 35, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Mallucci, G.R.; White, M.D.; Farmer, M.; Dickinson, A.; Khatun, H.; Powell, A.D.; Brandner, S.; Jefferys, J.G.; Collinge, J. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron 2007, 53, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; Deacon, R.; Wells, H.; Boche, D.; Waters, S.; Diniz, C.P.; Scott, H.; Rawlins, J.N.; Perry, V.H. Synaptic changes characterize early behavioural signs in the me7 model of murine prion disease. Eur. J. Neurosci. 2003, 17, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Chiti, Z.; Knutsen, O.M.; Betmouni, S.; Greene, J.R. An integrated, temporal study of the behavioural, electrophysiological and neuropathological consequences of murine prion disease. Neurobiol. Dis. 2006, 22, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Clinton, J.; Forsyth, C.; Royston, M.C.; Roberts, G.W. Synaptic degeneration is the primary neuropathological feature in prion disease: A preliminary study. Neuroreport 1993, 4, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Sassoon, J.; Daniels, M.; Brown, D.R. Astrocytic regulation of nmda receptor subunit composition modulates the toxicity of prion peptide prp106-126. Mol. Cell. Neurosci. 2004, 25, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Ratte, S.; Prescott, S.A.; Collinge, J.; Jefferys, J.G. Hippocampal bursts caused by changes in nmda receptor-dependent excitation in a mouse model of variant cjd. Neurobiol. Dis. 2008, 32, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Khosravani, H.; Zhang, Y.; Tsutsui, S.; Hameed, S.; Altier, C.; Hamid, J.; Chen, L.; Villemaire, M.; Ali, Z.; Jirik, F.R.; et al. Prion protein attenuates excitotoxicity by inhibiting nmda receptors. J. Cell Biol. 2008, 181, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Lashuel, H.A.; Hartley, D.; Petre, B.M.; Walz, T.; Lansbury, P.T., Jr. Neurodegenerative disease: Amyloid pores from pathogenic mutations. Nature 2002, 418, 291. [Google Scholar] [CrossRef] [PubMed]
- Quist, A.; Doudevski, I.; Lin, H.; Azimova, R.; Ng, D.; Frangione, B.; Kagan, B.; Ghiso, J.; Lal, R. Amyloid ion channels: A common structural link for protein-misfolding disease. Proc. Natl. Acad. Sci. USA 2005, 102, 10427–10432. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.A.; Martin, D.; Manuelidis, L. Microglia from creutzfeldt-jakob disease-infected brains are infectious and show specific mrna activation profiles. J. Virol. 2002, 76, 10905–10913. [Google Scholar] [CrossRef] [PubMed]
- Giese, A.; Brown, D.R.; Groschup, M.H.; Feldmann, C.; Haist, I.; Kretzschmar, H.A. Role of microglia in neuronal cell death in prion disease. Brain Pathol. 1998, 8, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Schmidt, B.; Kretzschmar, H.A. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 1996, 380, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Grizenkova, J.; Akhtar, S.; Brandner, S.; Collinge, J.; Lloyd, S.E. Microglial cx3cr1 knockout reduces prion disease incubation time in mice. BMC Neurosci. 2014, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Herrmann, U.S.; Falsig, J.; Abakumova, I.; Nuvolone, M.; Schwarz, P.; Frauenknecht, K.; Rushing, E.J.; Aguzzi, A. A neuroprotective role for microglia in prion diseases. J. Exp. Med. 2016, 213, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.M.; Field, R.H.; Perry, V.H.; Murray, C.L.; Cunningham, C. Microglia in the degenerating brain are capable of phagocytosis of beads and of apoptotic cells, but do not efficiently remove PrPSc, even upon lps stimulation. Glia 2010, 58, 2017–2030. [Google Scholar] [CrossRef] [PubMed]
- Mays, C.E.; Kim, C.; Haldiman, T.; van der Merwe, J.; Lau, A.; Yang, J.; Grams, J.; Di Bari, M.A.; Nonno, R.; Telling, G.C.; et al. Prion disease tempo determined by host-dependent substrate reduction. J. Clin. Investig. 2014, 124, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Lian, F.; Wen, Y.; Guo, C.; Lin, D. Prion protein oligomer and its neurotoxicity. Acta Biochim. Biophys. Sin. 2013, 45, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, L.; Hinrichs, W.; Groschup, M.H. Prion protein misfolding. Curr. Mol. Med. 2009, 9, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Vats, A.; Taneja, V. Toxic species in amyloid disorders: Oligomers or mature fibrils. Ann. Indian Acad. Neurol. 2015, 18, 138–145. [Google Scholar] [PubMed]
- Safar, J.G.; DeArmond, S.J.; Kociuba, K.; Deering, C.; Didorenko, S.; Bouzamondo-Bernstein, E.; Prusiner, S.B.; Tremblay, P. Prion clearance in bigenic mice. J. Gen. Virol. 2005, 86, 2913–2923. [Google Scholar] [CrossRef] [PubMed]
- White, M.D.; Farmer, M.; Mirabile, I.; Brandner, S.; Collinge, J.; Mallucci, G.R. Single treatment with rnai against prion protein rescues early neuronal dysfunction and prolongs survival in mice with prion disease. Proc. Natl. Acad. Sci. USA 2008, 105, 10238–10243. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Kawagoe, K.; Chen, C.J.; Teruya, K.; Sakasegawa, Y.; Doh-ura, K. Orally administered amyloidophilic compound is effective in prolonging the incubation periods of animals cerebrally infected with prion diseases in a prion strain-dependent manner. J. Virol. 2007, 81, 12889–12898. [Google Scholar] [CrossRef] [PubMed]
- Trevitt, C.R.; Collinge, J. A systematic review of prion therapeutics in experimental models. Brain 2006, 129, 2241–2265. [Google Scholar] [CrossRef] [PubMed]
- Sim, V.L.; Caughey, B. Recent advances in prion chemotherapeutics. Infect. Disord. Drug Targets 2009, 9, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Supattapone, S.; Nguyen, H.O.; Cohen, F.E.; Prusiner, S.B.; Scott, M.R. Elimination of prions by branched polyamines and implications for therapeutics. Proc. Natl. Acad. Sci. USA 1999, 96, 14529–14534. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hughes, D.; Halliday, M. What Is Our Current Understanding of PrPSc-Associated Neurotoxicity and Its Molecular Underpinnings? Pathogens 2017, 6, 63. https://doi.org/10.3390/pathogens6040063
Hughes D, Halliday M. What Is Our Current Understanding of PrPSc-Associated Neurotoxicity and Its Molecular Underpinnings? Pathogens. 2017; 6(4):63. https://doi.org/10.3390/pathogens6040063
Chicago/Turabian StyleHughes, Daniel, and Mark Halliday. 2017. "What Is Our Current Understanding of PrPSc-Associated Neurotoxicity and Its Molecular Underpinnings?" Pathogens 6, no. 4: 63. https://doi.org/10.3390/pathogens6040063




