A Comparative Overview of the Flagellar Apparatus of Dinoflagellate, Perkinsids and Colpodellids
Abstract
:1. Introduction
2. Diversity of the Flagellar Apparatus of the Dinoflagellates and Related Lineages
2.1. Flagellar Apparatus of Dinoflagellates
2.1.1. Dinokaryotes

2.1.2. Oxyrrhis marina: A Basal Lineage of Dinoflagellates

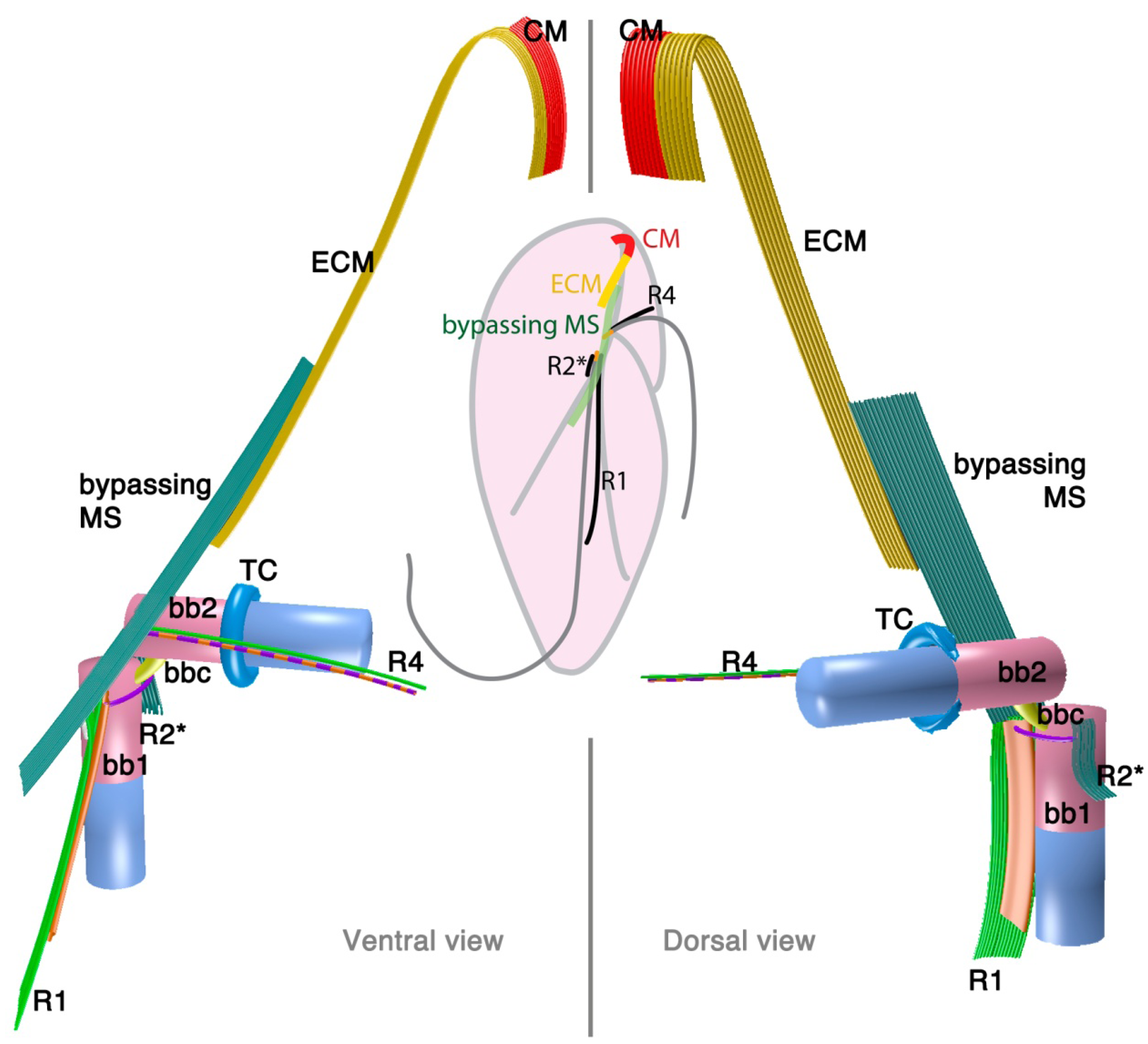
2.1.3. Psammosa pacifica
2.2. Perkinsids: The Sister Group of the Dinoflagellates
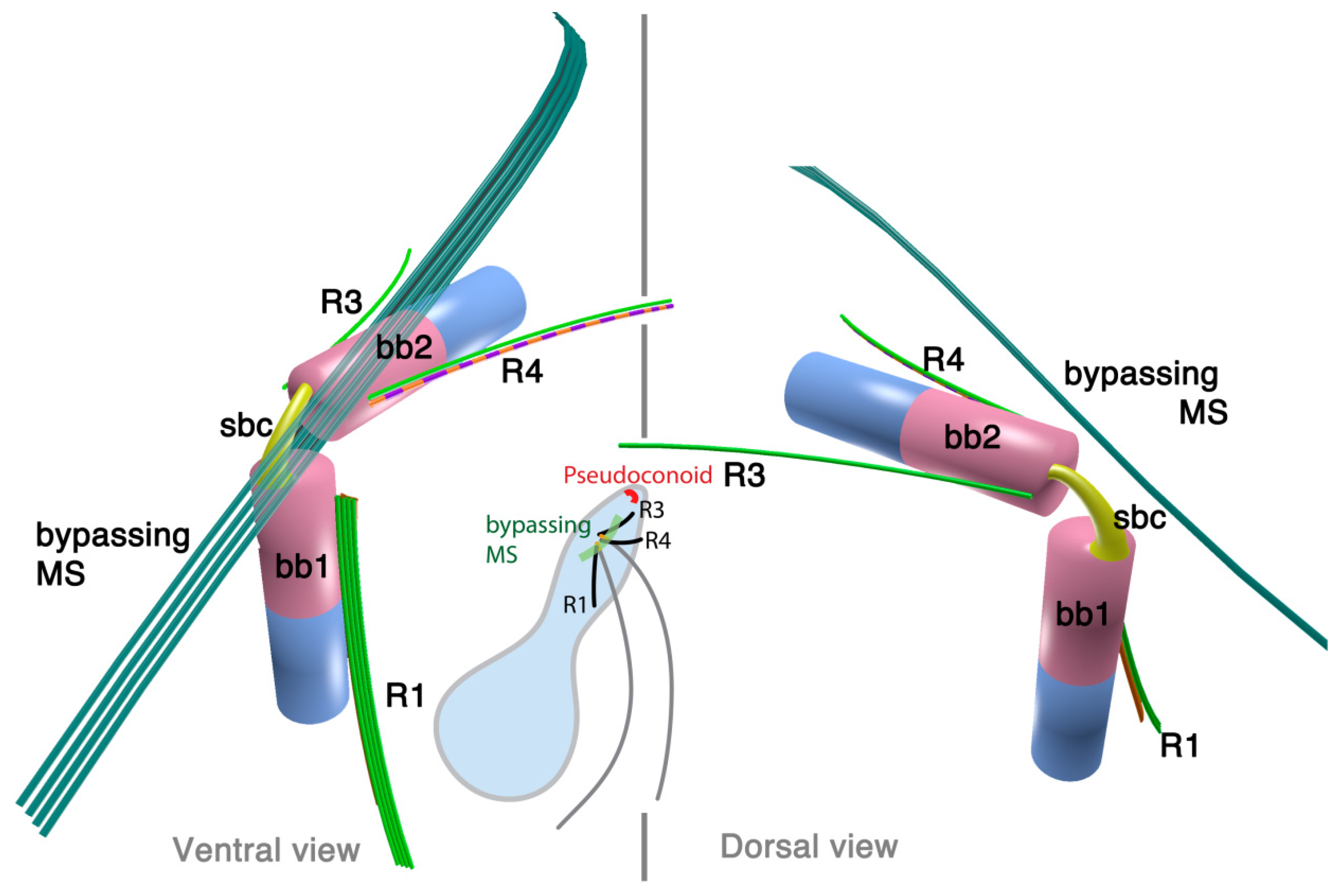
2.2.1. Parvilucifera infectans
2.2.2. Rastrimonas subtilis
2.3. Colpodellids: Sisters of the Apicomplexan Parasites
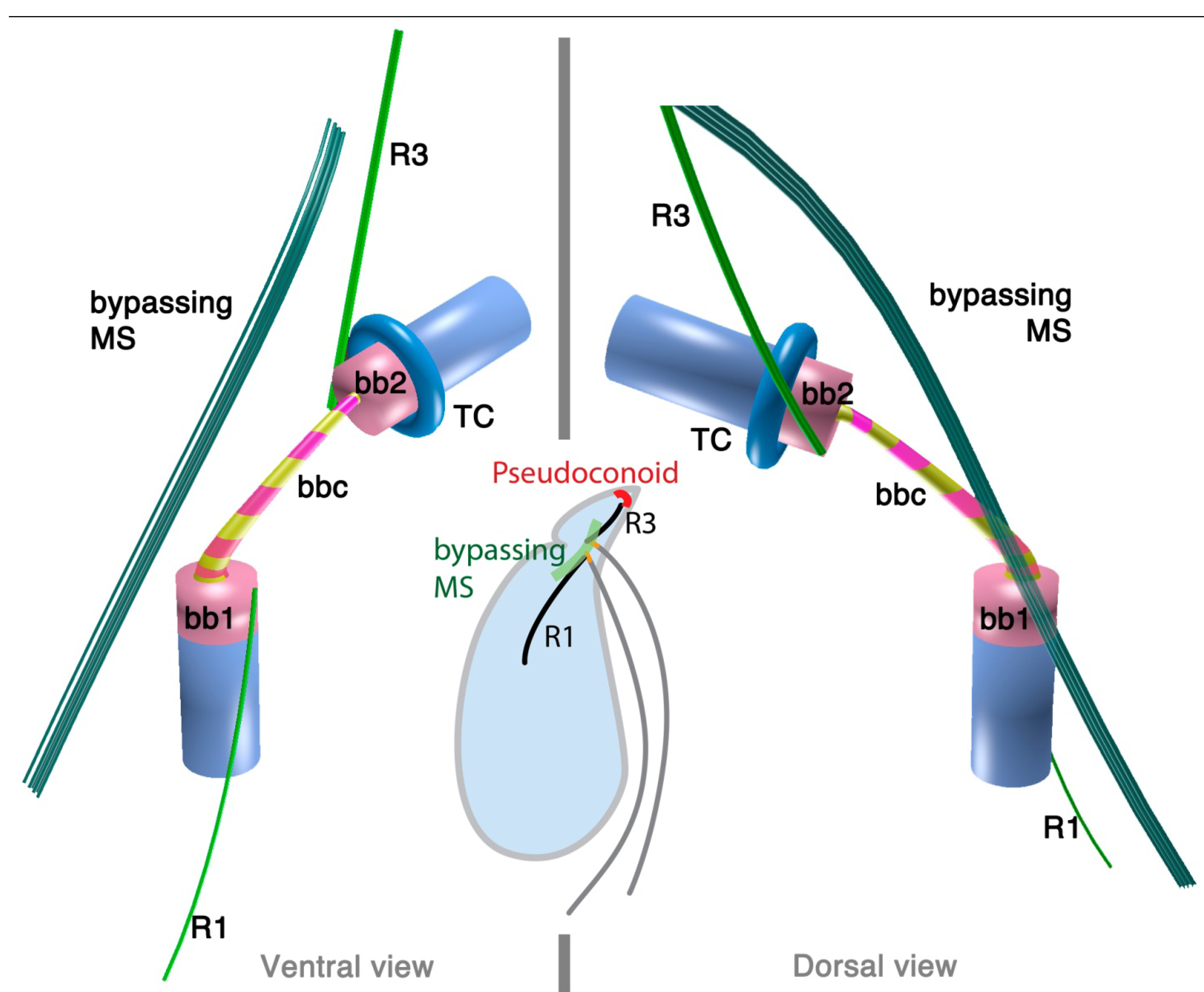
2.3.1. Colpodella vorax
2.3.2. Colpodella gonderi
2.3.3. Colpodella perforans
3. Character Evolution
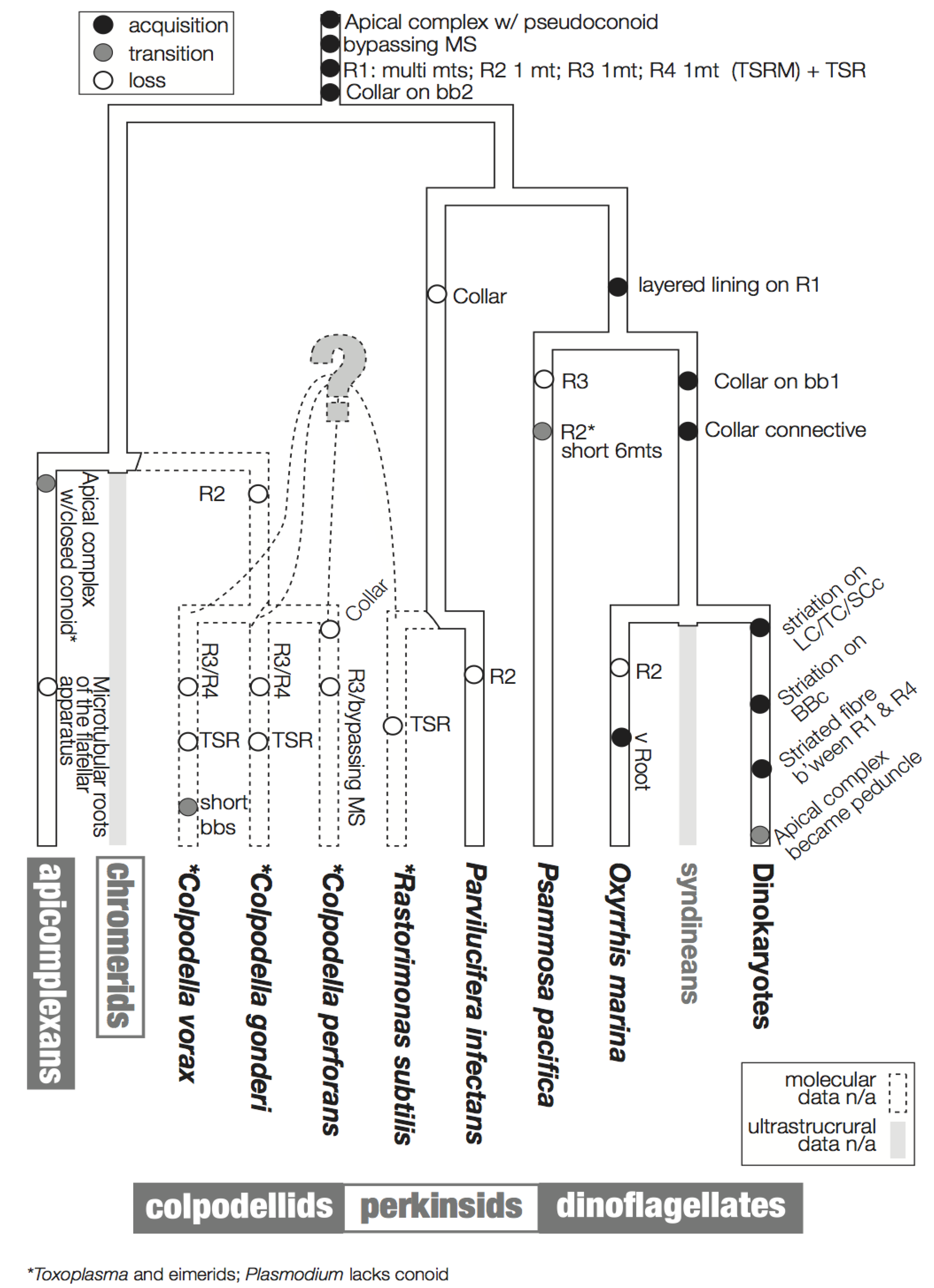
3.1. Microtubular Roots
3.2. Basal Body Collars and Fibrous Connective Structures
3.3. Is the Dinoflagellate Peduncle Homologous to the Apical Complex?
Acknowledgments
Conflicts of Interest
References
- Gómez, F. A quantitative review of the lifestyle, habitat and trophic diversity of dinoflagellates (Dinoflagellata, Alveolata). Syst. Biodivers. 2012, 10, 267–275. [Google Scholar] [CrossRef]
- Sherr, E.B.; Sherr, B.F. Heterotrophic dinoflagellates: A significant component of microzooplankton biomass and major grazers of diatoms in the sea. Mar. Ecol. Prog. Ser. 2007, 352, 187–197. [Google Scholar] [CrossRef]
- Stentiford, G.; Shields, J.D. A review of the parasitic dinoflagellates Hematodinium species and Hematodinium-like infections in marine crustaceans. Dis. Aquat. Organ 2005, 66, 47–70. [Google Scholar] [CrossRef]
- Berge, T.; Poulsen, L.K.; Moldrup, M.; Daugbjerg, N.; Hansen, P.J. Marine microalgae attack and feed on metazoans. ISME J. 2012, 6, 1926–1936. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Cavalier-Smith, T.; Chao, E.E. Protalveolate phylogeny and systematics and the origins of Sporozoa and dinoflagellates (phylum Myzozoa nom. nov.). Eur. J. Protistol. 2004, 40, 185–212. [Google Scholar] [CrossRef]
- Gornik, S.G.; Ford, K.L.; Mulhern, T.D.; Bacic, A.; Mcfadden, G.I.; Waller, R.F. Loss of nucleosomal DNA condensation coincides with appearance of a novel nuclear protein in dinoflagellates. Curr. Biol. 2012, 22, 2303–2312. [Google Scholar] [CrossRef]
- Okamoto, N.; Horak, A.; Keeling, P.J. Description of two species of early branching dinoflagellates, Psammosa pacifica n. g., n. sp. and P. atlantica n. sp. PLoS One 2012, 7, e34900. [Google Scholar] [CrossRef]
- Hearne, J.L.; Pitula, J.S. Identification of two spliced leader RNA transcripts from Perkinsus marinus. J. Eukaryot. Microbiol. 2011, 58, 266–268. [Google Scholar] [CrossRef]
- Zhang, H.; Dungan, C.F.; Lin, S. Introns, alternative splicing, spliced leader trans-splicing and differential expression of PCNA and cyclin in Perkinsus marinu. Protist 2011, 162, 154–167. [Google Scholar] [CrossRef]
- Zhang, H.; Campbell, D.A.; Sturm, N.R.; Dungan, C.F.; Lin, S. Spliced leader RNAs, mitochondrial gene frameshifts and multi-protein phylogeny expand support for the genus Perkinsus as a unique group of alveolates. PLoS One 2011, 6, e19933. [Google Scholar]
- Hoppenrath, M.; Leander, B.S. Molecular phylogeny of Parvilucifera prorocentri (Alveolata, Myzozoa): Insights into perkinsid character evolution. J. Eukaryot. Microbiol. 2009, 56, 251–256. [Google Scholar] [CrossRef]
- Fukuda, Y.; Endoh, H. Phylogenetic analyses of the dinoflagellate Noctiluca scintillans based on beta-tubulin and Hsp90 genes. Eur. J. Protistol. 2008, 44, 27–33. [Google Scholar] [CrossRef]
- Hoppenrath, M.; Leander, B.S. Dinoflagellate phylogeny as inferred from heat shock protein 90 and ribosomal gene sequences. PLoS One 2010, 5, e13220. [Google Scholar] [CrossRef]
- Mangot, J.-F.; Debroas, D.; Domaizon, I. Perkinsozoa, a well-known marine protozoan flagellate parasite group, newly identified in lacustrine systems: A review. Hydrobiologia 2011, 659, 37–48. [Google Scholar] [CrossRef]
- Leander, B.S.; Keeling, P.J. Morphostasis in alveolate evolution. Trends Ecol. Evol. 2003, 18, 395–402. [Google Scholar] [CrossRef]
- Siddall, M.E.; Reece, K.S.; Graves, J.E.; Burreson, E.M. “Total evidence” refutes the inclusion of Perkinsus species in the phylum Apicomplexa. Parasitology 1997, 115, 165–176. [Google Scholar] [CrossRef]
- Leander, B.S.; Kuvardina, O.; ALESHIN, V.V.; Myl’nikov, A.P.; Keeling, P.J. Molecular phylogeny and surface morphology of Colpodella edax (Alveolata): Insights into the phagotrophic ancestry of apicomplexans. J. Eukaryot. Microbiol. 2003, 50, 334–340. [Google Scholar] [CrossRef]
- Janouskovec, J.; Horak, A.; Barott, K.L.; Rohwer, F.L.; Keeling, P.J. Global analysis of plastid diversity reveals apicomplexan-related lineages in coral reefs. Curr. Biol. 2012, 22, R518–R519. [Google Scholar] [CrossRef]
- Moore, R.B.; Obornik, M.; Janouskovec, J.; Chrudimský, T.; Vancová, M.; Green, D.H.; Wright, S.W.; Davies, N.W.; Bolch, C.J.S.; Heimann, K.; et al. A photosynthetic alveolate closely related to apicomplexan parasites. Nature 2008, 451, 959–963. [Google Scholar] [CrossRef]
- Obornik, M.; Modrý, D.; Lukeš, M.; Černotíková-Stříbrná, E.; Cihlář, J.; Tesařová, M.; Kotabová, E.; Vancová, M.; Prášil, O.; Lukes, J. Morphology, ultrastructure and life cycle of Vitrella brassicaformis n. sp., n. gen., a novel chromerid from the Great Barrier Reef. Protist 2012, 163, 306–323. [Google Scholar] [CrossRef]
- Leander, B.S. Marine gregarines: Evolutionary prelude to the apicomplexan radiation? Trends Parasitol. 2008, 24, 60–67. [Google Scholar] [CrossRef]
- Kuvardina, O.N.; Leander, B.S.; Aleshin, V.V.; Myl’nikov, A.P.; Keeling, P.J.; Simdyanov, T.G. The phylogeny of colpodellids (Alveolata) using small subunit rRNA gene sequences suggests they are the free-living sister group to apicomplexans. J. Eukaryot. Microbiol. 2002, 49, 498–504. [Google Scholar] [CrossRef]
- Janouskovec, J.; Tikhonenkov, D.V.; Mikhailov, K.V.; Simdyanov, T.G.; Aleoshin, V.V.; Mylnikov, A.P.; Keeling, P.J. Colponemids represent multiple ancient alveolate lineages. Curr. Biol. 2012, 23, 1–7. [Google Scholar]
- Roberts, K.R.; Heimann, K.; Wetherbee, R. The flagellar apparatus and canal structure in Prorocentrum micans (Dinophyceae). Phycologia 1995, 34, 313–322. [Google Scholar] [CrossRef]
- Leadbeater, B.; Dodge, J.D. An electron microscope study of dinoflagellate flagella. J. Gen. Microbiol. 1967, 46, 305–314. [Google Scholar] [CrossRef]
- Leadbeater, B.S.C. The intracellular origin of flagellar hairs in the dinoflagellate Woloszynskia micra Leadbeater & Dodge. J. Cell Sci. 1971, 9, 443–451. [Google Scholar]
- Moestrup, Ø. Flagellar structure in algae: A review, with new observations particularly on the Chrysophyceae, Phaeophyceae (Fucophyceae), Euglenophyceae, and Reckertia. Phycologia 1982, 21, 427–528. [Google Scholar] [CrossRef]
- Roberts, K.R.; Roberts, J.E. The flagellar apparatus and cytoskeleton of the dinoflagellates. Protoplasma 1991, 164, 105–122. [Google Scholar] [CrossRef]
- Moestrup, Ø. The Flagellate Cytoskeleton: Introduction of a General Terminology for Microtubular Flagellar Roots in Protists. In The Flagellates. Unity, Diversity and Evolution; Leadbeater, B.S.C., Green, J.C., Eds.; Taylor & Francis: London, UK, 2000; pp. 69–94. [Google Scholar]
- Calado, S.C.F.C.M. Ultrastructure and phylogeny of peridinioid dinoflagellates. 2010. Available online: http://hdl.handle.net/10773/972 (accessed on 4 August 2012).
- Heimann, K.; Roberts, K.R.; Wetherbee, R. Flagellar apparatus transformation and development in Prorocentrum micans and P. minimum (Dinophyceae). Phycologia 1995, 34, 323–335. [Google Scholar] [CrossRef]
- Hansen, G.; Moestrup, Ø. Fine-structural characterization of Alexandrium catenella (Dinophyceae) with special emphasis on the flagellar apparatus. Eur. J. Phycol. 1998, 33, 281–291. [Google Scholar] [CrossRef]
- Hansen, G.; Daugbjerg, N. Symbiodinium natans sp. nov.: A “free-living” dinoflagellate from Tenerife (Northeast-Atlantic Ocean). J. Phycol. 2009, 45, 251–263. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Lindberg, K.; Daugbjerg, N. Studies on woloszynskioid dinoflagellates V. Ultrastructure of Biecheleriopsis gen. nov., with description of Biecheleriopsis adriatica sp. nov. Phycol. Res. 2009, 57, 221–237. [Google Scholar] [CrossRef]
- Roberts, K.R.; Rusche, M.L.; Taylor, M.F.J.R. The cortical microtubular cytoskeleton of Oxyrrhis marina (Dinophyceae) observed with immunofluorescence and electron microscopy. J. Phycol. 1993, 29, 642–649. [Google Scholar] [CrossRef]
- Craveiro, S.C.; Calado, A.J.; Daugbjerg, N.; Hansen, G.; Moestrup, Ø. Ultrastructure and LSU rDNA-based phylogeny of Peridinium lomnickii and description of Chimonodinium gen. nov. (Dinophyceae). Protist 2011, 162, 590–615. [Google Scholar] [CrossRef]
- Dodge, J.D.; Crawford, R.M. Fine structure of the dinoflagellate Oxyrrhis marina II. The flagellar system. Protistologica 1971, 7, 399–409. [Google Scholar]
- Roberts, K.R. The flagellar apparatus of Oxyrrhis marina (Pyrrophyta). J. Phycol. 1985, 21, 641–655. [Google Scholar] [CrossRef]
- Höhfeld, I.; Beech, P.L.; Melkonian, M.A. Immunolocalization of centrin in Oxyrrhis marina (Dinophyceae). J. Phycol. 1994, 30, 474–489. [Google Scholar]
- Okamoto, N.; Keeling, P.J. The 3D structure of the apical complex and association with the flagellar apparatus revealed by serial TEM tomography in Psammosa pacifica, a distant relative of the apicomplexa. PLoS One 2014, 9, e84653. [Google Scholar] [CrossRef]
- Norén, F.; Moestrup, Ø.; Rehnstam-Holm, A.S. Parvilucifera infectans Norén et Moestrup gen. et sp. nov.(perkinsozoa phylum nov.): A parasitic flagellate capable of killing toxic microalgae. Eur. J. Protistol. 1999, 35, 233–254. [Google Scholar] [CrossRef]
- Brugerolle, G. Cryptophagus subtilis: A new parasite of cryptophytes affiliated with the Perkinsozoa lineage. Eur. J. Protistol. 2002, 37, 379–390. [Google Scholar] [CrossRef]
- Leander, B.S.; Hoppenrath, M. Ultrastructure of a novel tube-forming, intracellular parasite of dinoflagellates: Parvilucifera prorocentri sp. nov. (Alveolata, Myzozoa). Eur. J. Protistol. 2008, 44, 55–70. [Google Scholar] [CrossRef]
- Brugerolle, G. Apicomplexan parasite Cryptophagus renamed Rastrimonas gen. nov. Eur. J. Protistol. 2003, 39, 101–101. [Google Scholar] [CrossRef]
- Simpson, A.G.B.; Patterson, D.J. Ultrastructure and identification of the predatory flagellate Colpodella pugnax Cienkowski (Apicomplexa) with a description of Colpodella turpis n. sp. and a review of the genus. Syst. Parasitol. 1996, 33, 187–198. [Google Scholar] [CrossRef]
- Yuan, C.L.; Keeling, P.J.; Krause, P.J.; Horak, A.; Bent, S.; Rollend, L.; Hua, X.G. Colpodella spp.-like parasite infection in woman, China. Emerg. Infect. Dis. 2012, 19, 125–127. [Google Scholar]
- Brugerolle, G. Colpodella vorax: Ultrastructure, predation, life-cycle mitosis, and phylogenetic relationships. Eur. J. Protistol. 2002, 38, 113–125. [Google Scholar] [CrossRef]
- Foissner, W.; Foissner, I. First record of an ectoplasitic flagellate on ciliates: An ultrastructural investigation of the morphology and the mode of attachment of Spiromonas gonderi nov. spec. (Zoomastigophora, Spiromonadidae) invading the pellicle of ciliates of the genus Colpoda (Ciliophora Colpodidae). Protistologica 1984, 20, 635–648. [Google Scholar]
- Myl’nikova, Z.M.; Myl’nikov, A.P. The morphology of predatory flagellate Colpodella pseudoedax Mylnikov et Mylnikov, 2007 (Colpodellida, Alveolata). Inland Water Biol. 2009, 2, 199–204. [Google Scholar] [CrossRef]
- Myl’nikov, A.P. Ultrastructure and phylogeny of colpodellids (Colpodellida, Alveolata). Biol. Bull. 2009, 36, 582–590. [Google Scholar] [CrossRef]
- Brugerolle, G.; Mignot, J.P. Observations sur le cycle l’ultrastructure et la position systématique de Spiromonas perforans (Bodo perforans Hollande 1938), flagellé parasite de Chilomonas paramecium: Ses relations a vec les dinoflagellés et sporozoaires. Protistologica 1979, 15, 183–196. [Google Scholar]
- Myl’nikov, A.P.; Myl’nikova, Z.M.; Tsvetkov, A.L. Fine structure of a predatory flagellate Colpodella sp. Inland Water Biol. 2000, 1, 1–8. [Google Scholar]
- Olmo, J.L.; Esteban, G.F.; Finlay, B.J. New records of the ectoparasitic flagellate Colpodella gonderi on non-Colpoda ciliates. Int. Microbiol. 2012, 14, 207–2011. [Google Scholar]
- Lynn, D.H. Cytoterminology of cortical components of ciliates: Somatic and oral kinetids. Biosystems 1988, 21, 299–307. [Google Scholar] [CrossRef]
- Hansen, G.; Daugbjerg, N. Ultrastructure of Gyrodinium spirale, the type species of Gyrodinium (Dinophyceae), including a phylogeny of G. dominans, G. rubrum and G. spirale deduced from partial LSU rDNA sequences. Protist 2004, 155, 271–294. [Google Scholar] [CrossRef]
- Melkonian, M.A.; Beech, P.L.; Katsaros, C.; Schulze, D. Centrin-Mediated Cell Motility in Algae. In Algal Cell Motility; Melkonian, M.A., Ed.; Springer: New York, NY, USA, 1991; pp. 179–221. [Google Scholar]
- Höhfeld, I.; Otten, J.; Melkonian, M.A. Contractile eukaryotic flagella: Centrin is involved. Protoplasma 1988, 147, 16–24. [Google Scholar] [CrossRef]
- Schnepf, E.; Deichgräber, G. “Myzocytosis,” a kind of endocytosis with implications to compartmentation in endosymbiosis. Naturwissenschaften 1984, 71, 218–219. [Google Scholar] [CrossRef]
- Gustafson, P.V.; Agar, H.D.; Cramer, D.I. An electron microscope study of Toxoplasma. Am. J. Trop. Med. Hyg. 1954, 3, 1008–1022. [Google Scholar]
- Scholtyseck, E.; Mehlhorn, H.; Friedhoff, K. The fine structure of the conoid of Sporozoa and related organisms. Parasitol. Res. 1970, 34, 68–94. [Google Scholar]
- Scholtyseck, E.; Melhorn, H. Ultrastructural study of characteristic organelles (paired organelles, micronemes, micropores) of Sporozoa and related organisms. Z. Parasitenk. 1970, 34, 97–127. [Google Scholar]
- Blackman, M.; Bannister, L. Apical organelles of Apicomplexa: Biology and isolation by subcellular fractionation. Mol. Biochem. Parasitol. 2001, 117, 11–25. [Google Scholar] [CrossRef]
- Morrissette, N.S.; Sibley, L.D. Cytoskeleton of apicomplexan parasites. Microbiol. Mol. Biol. Rev. 2002, 66, 21–38. [Google Scholar] [CrossRef]
- Dyson, J.; Grahame, J.; Evennett, P. The Apical complex of the gregarine Digyalum oweni (Protozoa, Apicomplexa). J. Nat. Hist. 1994, 28, 1–7. [Google Scholar]
- Garces, E.; Hoppenrath, M. Ultrastructure of the intracellular parasite Parvilucifera sinerae (Alveolata, Myzozoa) infecting the marine toxic planktonic dinoflagellate Alexandrium minutum (Dinophyceae). Harmful Algae 2010, 10, 64–70. [Google Scholar] [CrossRef]
- Perkins, F. The structure of Perkinsus marinus (Mackin, Owen and Collier, 1950) Levine, 1978 with comments on taxonomy and phylogeny of Perkinsus spp. J. Shellfish. Res. 1996, 15, 67–87. [Google Scholar]
- Perkins, F. Zoospores of the oyster pathogen, Dermocystidium marinum. I. Fine structure of the conoid and other sporozoan-like organelles. J. Parasitol. 1976, 62, 959–974. [Google Scholar] [CrossRef]
- Calado, A.J.; Moestrup, Ø. Ultrastructural study of the type species of Peridiniopsis, Peridiniopsis borgei (Dinophyceae), with special reference to the peduncle and flagellar apparatus. Phycologi 2002, 41, 567–584. [Google Scholar] [CrossRef]
- Calado, A.J.; Craveiro, S.C.; Daugbjerg, N.; Moestrup, Ø. Ultrastructure and LSU rDNA-based phylogeny of Esoptrodinium gemma (Dinophyceae), with notes on feeding behavior and the description of the flagellar base area of a planozygote. J. Phycol. 2006, 42, 434–452. [Google Scholar] [CrossRef]
- Jacobson, D.M.; Anderson, D.M. Widespread phagocytosis of ciliates and other protists by marine mixotrophic and heterotrophic thecate dinoflagellates. J. Phycol. 1996, 32, 279–285. [Google Scholar]
- Calado, A.J.; Hansen, G.; Moestrup, Ø. Architecture of the flagellar apparatus and related structures in the type species of Peridinium, P. cinctum (Dinophyceae). Eur. J. Phycol. 1999, 34, 179–191. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Hansen, G.; Daugbjerg, N.; Flaim, G.; D’Andrea, M. Studies on woloszynskioid dinoflagellates II: On Tovellia sanguinea sp. nov., the dinoflagellate responsible for the reddening of Lake Tovel, N. Italy. Eur. J. Phycol. 2006, 41, 47–65. [Google Scholar] [CrossRef]
- Hansen, G.; Daugbjerg, N.; Henriksen, P. Baldinia anauniensis gen. et sp. nov.: A “new” dinoflagellate from Lake Tovel, N. Italy. Phycologia 2007, 46, 86–108. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Hansen, G.; Daugbjerg, N. Studies on woloszynskioid dinoflageflates III: On the ultrastructure and phylogeny of Borghiella dodgei gen. et sp nov., a cold-water species from Lake Tovel, N. Italy, and on B. tenuissima comb. nov (syn. Woloszynskia tenuissima). Phycologia 2008, 47, 54–78. [Google Scholar] [CrossRef]
- Calado, A.J.; Craveiro, S.C.; Daugbjerg, N.; Moestrup, Ø. Description of Tyrannodinium gen. nov., a freshwater dinoflagellate closely related to the marine Pfiesteria-like species. J. Phycol. 2009, 45, 1195–1205. [Google Scholar] [CrossRef]
- Craveiro, S.C.; Calado, A.J.; Daugbjerg, N.; Moestrup, Ø. Ultrastructure and LSU rDNA-based revision of Peridinium group Palatinum (Dinophyceae) with the description of Palatinus gen. nov. J. Phycol. 2009, 45, 1175–1194. [Google Scholar] [CrossRef]
- Gubbels, M.-J.; Duraisingh, M.T. Evolution of apicomplexan secretory organelles. Int. J. Parasitol. 2012, 42, 1071–1081. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Okamoto, N.; Keeling, P.J. A Comparative Overview of the Flagellar Apparatus of Dinoflagellate, Perkinsids and Colpodellids. Microorganisms 2014, 2, 73-91. https://doi.org/10.3390/microorganisms2010073
Okamoto N, Keeling PJ. A Comparative Overview of the Flagellar Apparatus of Dinoflagellate, Perkinsids and Colpodellids. Microorganisms. 2014; 2(1):73-91. https://doi.org/10.3390/microorganisms2010073
Chicago/Turabian StyleOkamoto, Noriko, and Patrick J. Keeling. 2014. "A Comparative Overview of the Flagellar Apparatus of Dinoflagellate, Perkinsids and Colpodellids" Microorganisms 2, no. 1: 73-91. https://doi.org/10.3390/microorganisms2010073