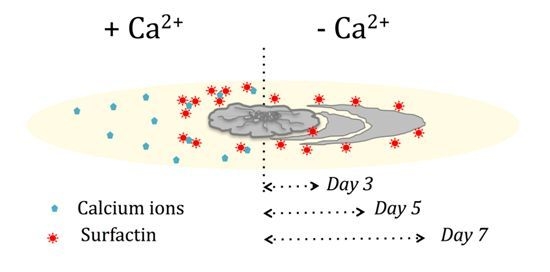
Presence of Calcium Lowers the Expansion of Bacillus subtilis Colony Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Media
2.2. Colony Biofilm Formation
2.3. Swarming and Sliding
2.4. Imaging and Colony Size Measurements
2.5. Growth and Fluorescent Reporter Assays
2.6. Surface Tension Measurements
3. Results
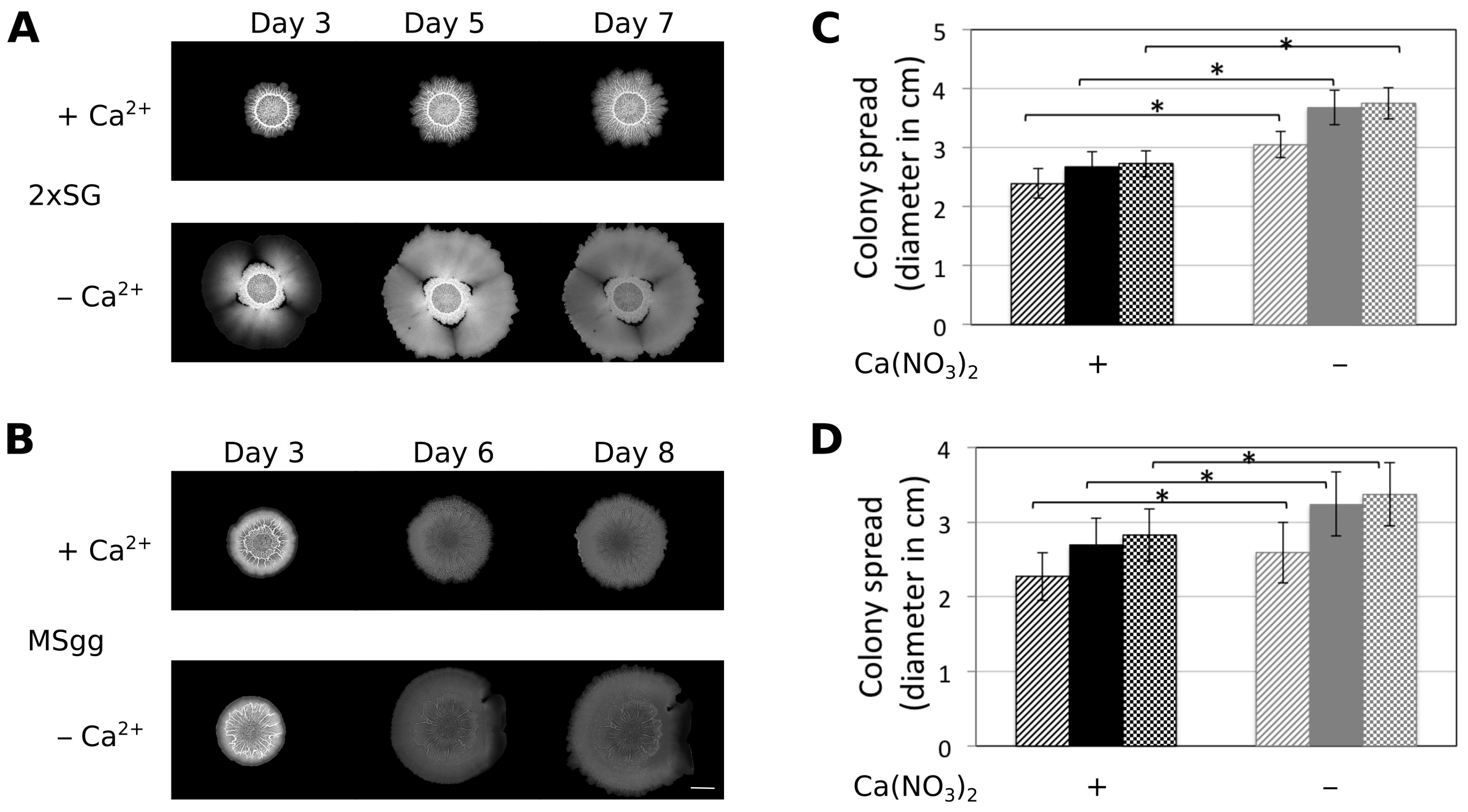
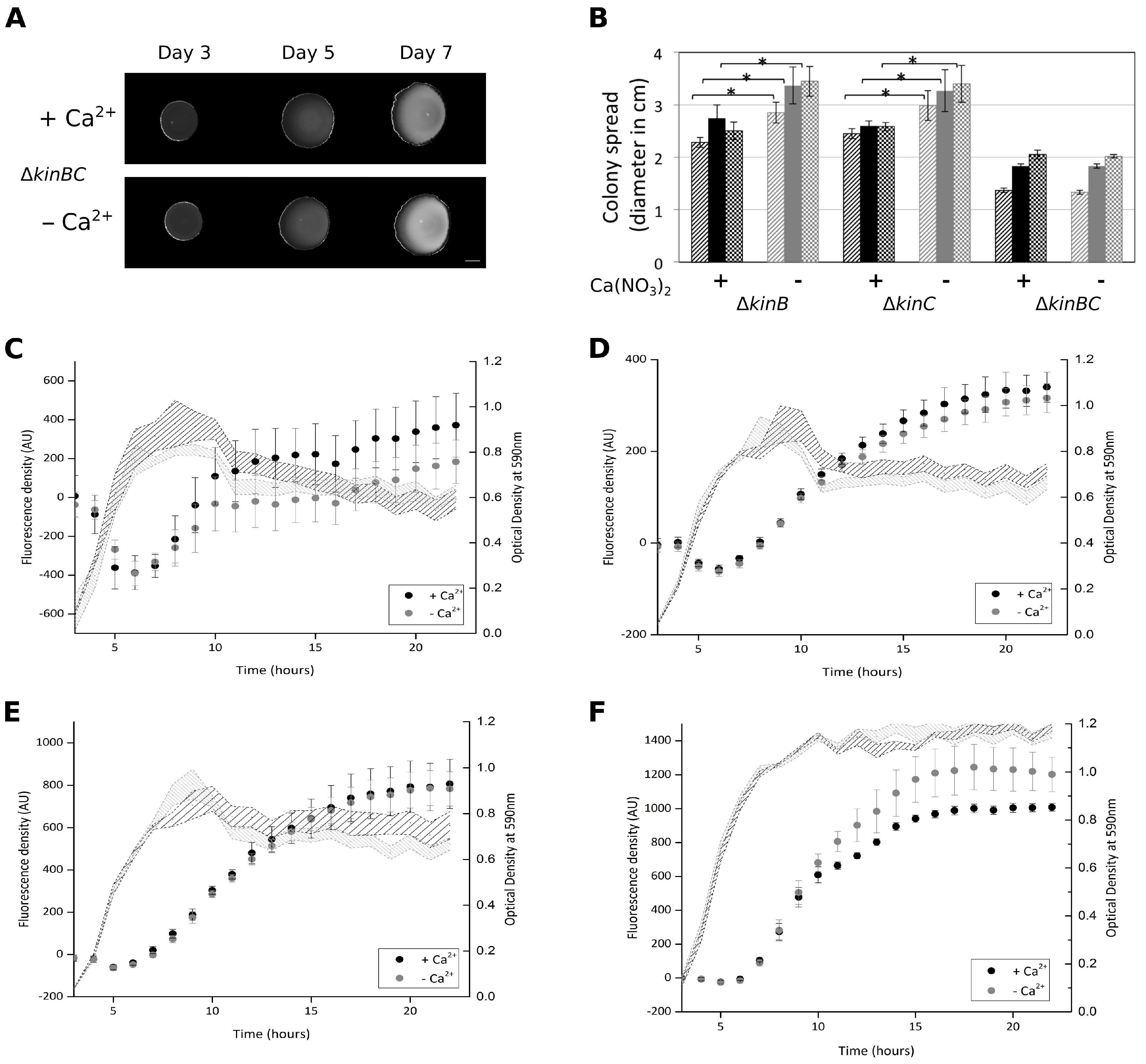
3.1. Presence of Ca2+ Prevents Cells to Spread Out from Matured Biofilm Colonies
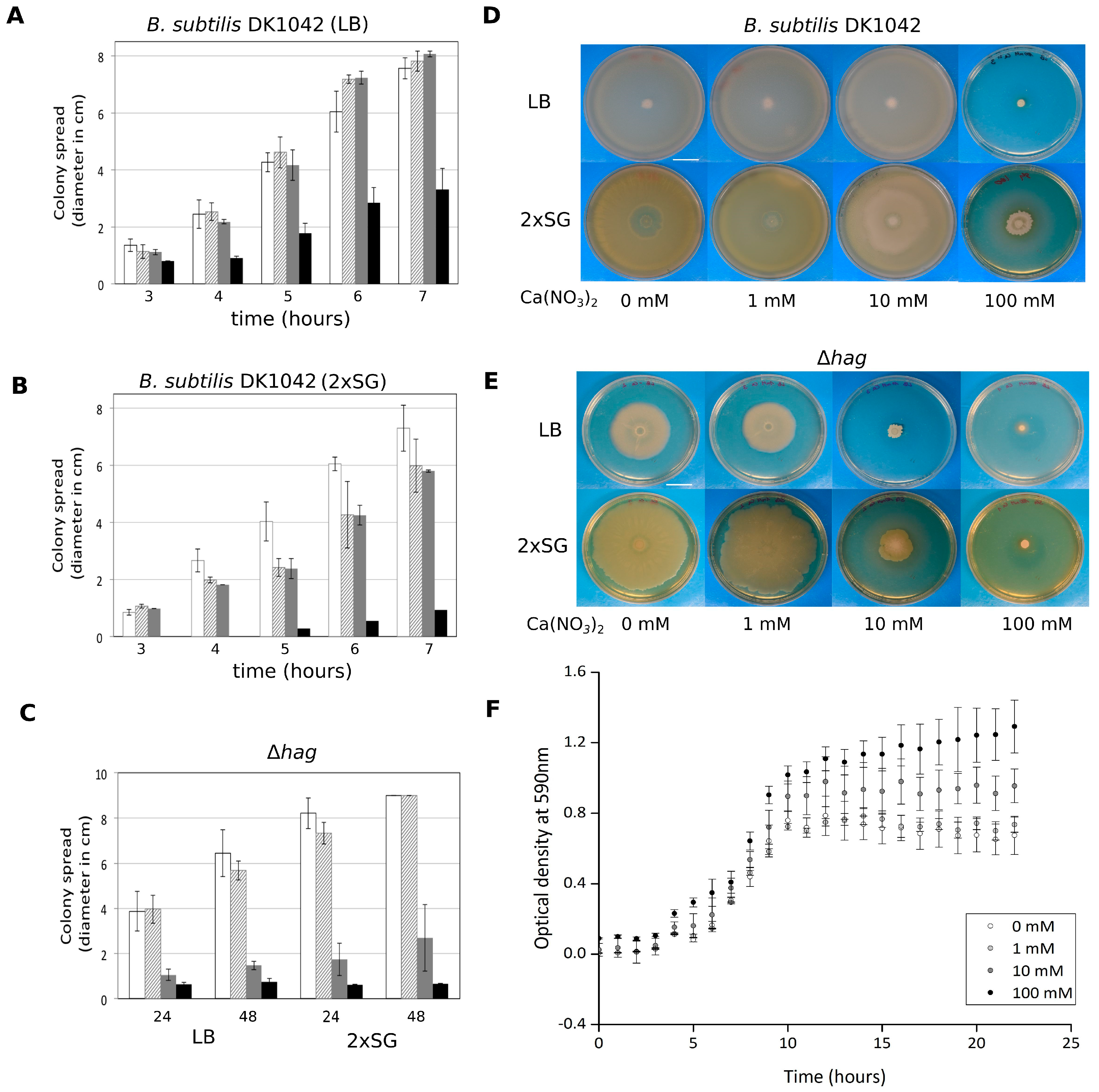
3.2. Ca2+ Restricts Flagellum-Independent Expansion of Biofilm Colonies
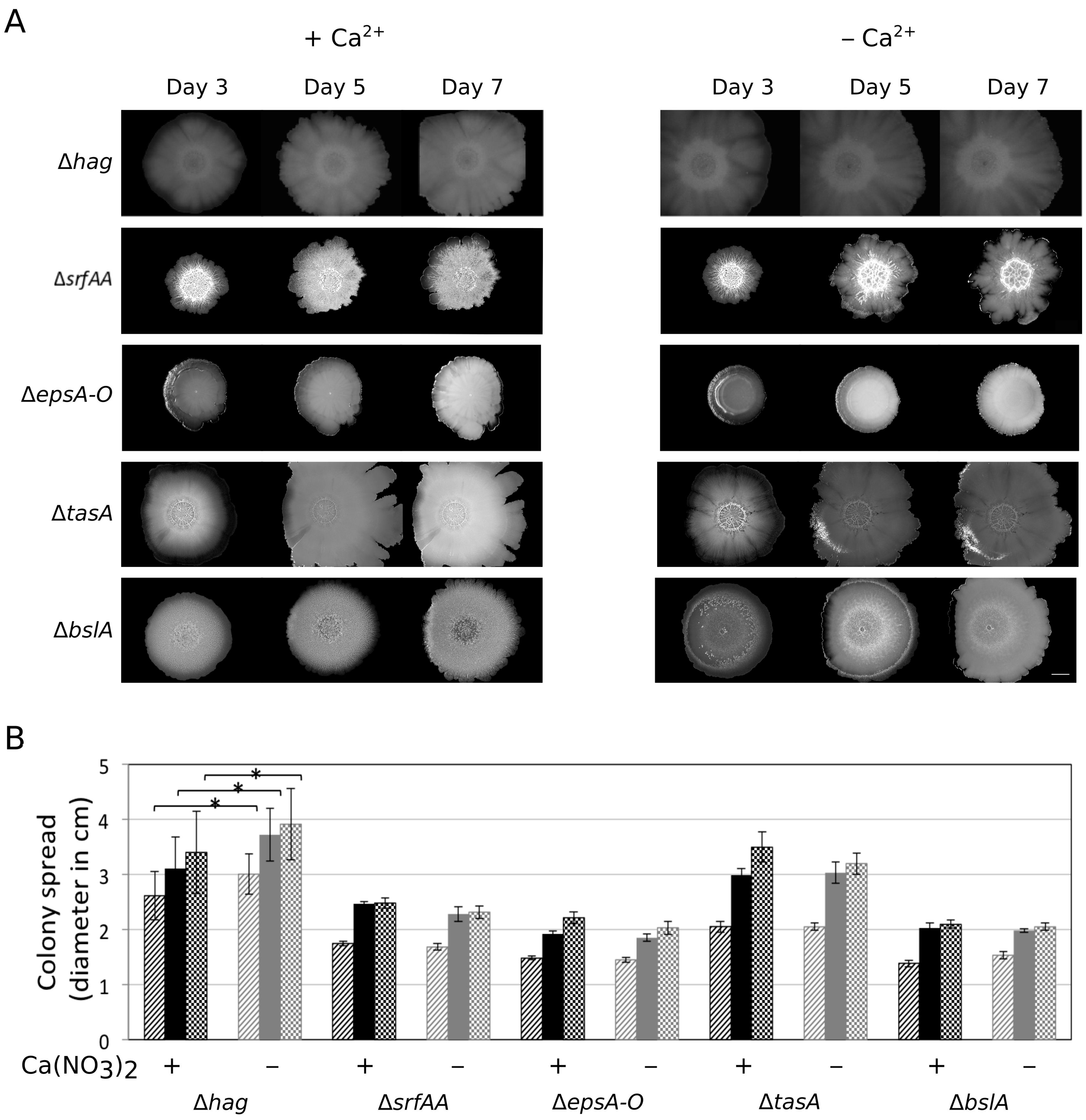
3.3. Importance of the Components Required for Sliding on Colony Expansion
3.4. Colony Expansion on Ca2+ Limited Medium Depends on KinB and KinC, the Major Sliding-Inducing Sensor Kinases
3.5. Ca2+ Does Not Impact the Expression of the EPS, tasA, and srfA Genes in Planktonic Cultures
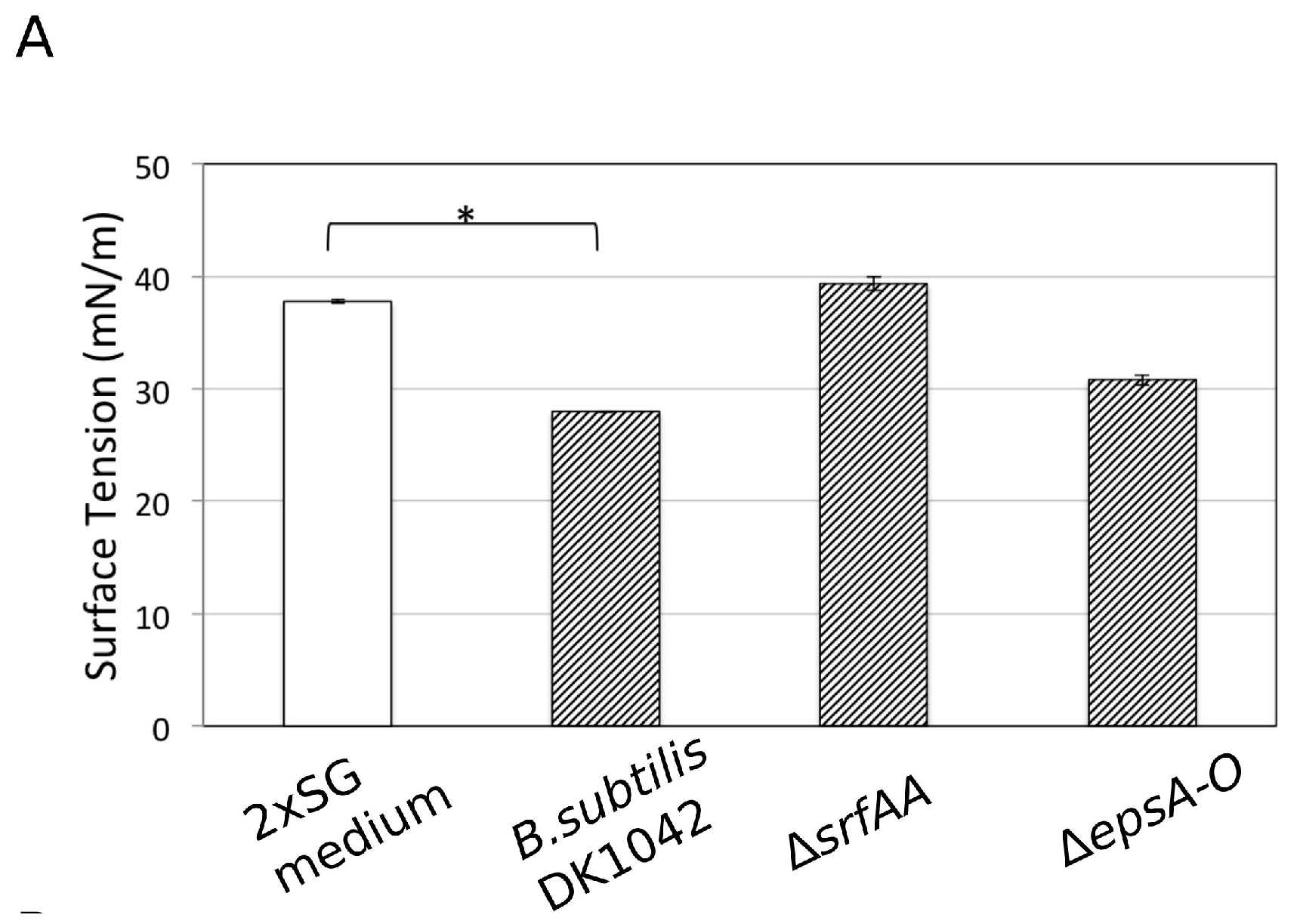
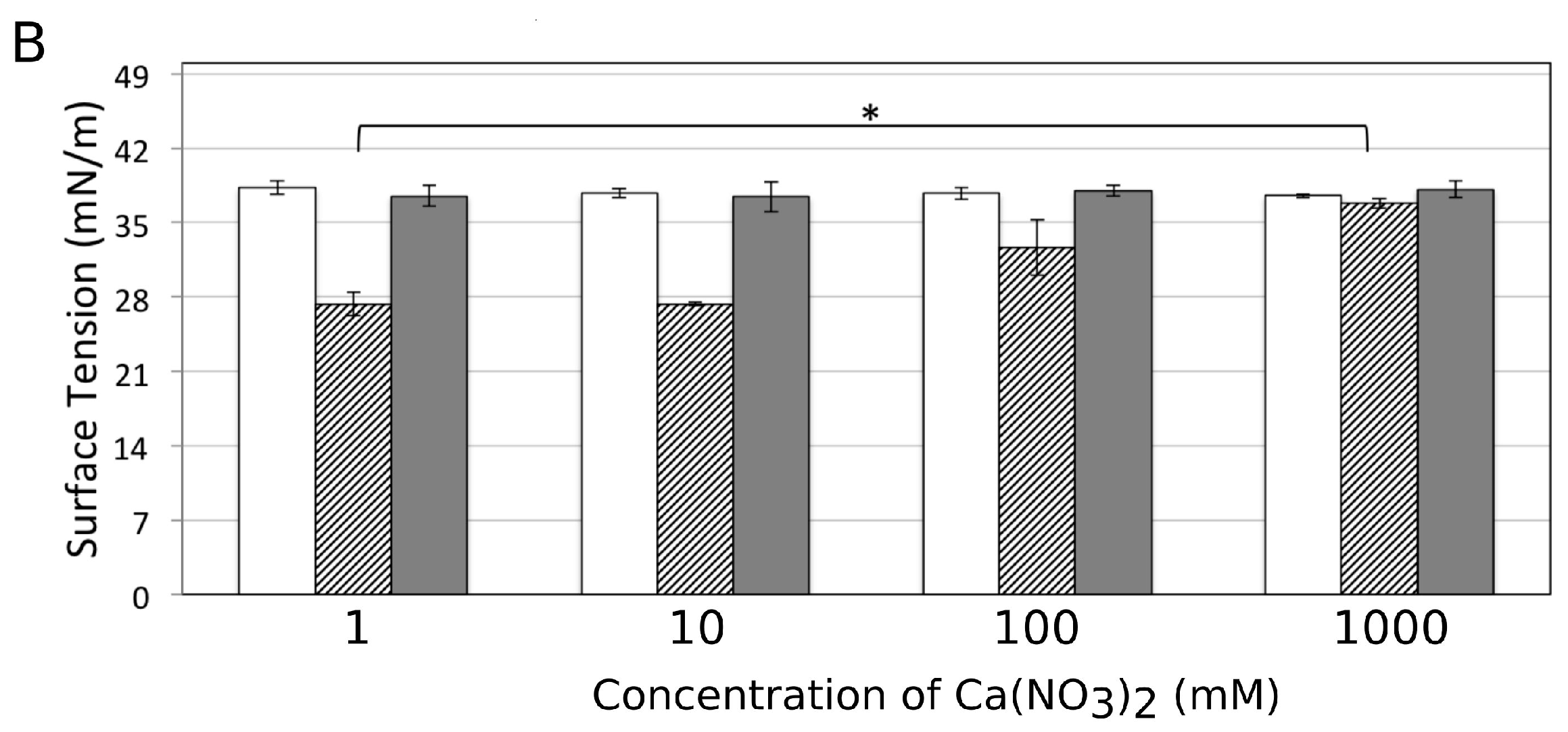
3.6. Influence of Ca2+ on the Amphiphilic Properties of Surfactin Molecules
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of interest
References
- Branda, S.S.; González-Pastor, J.E.; Ben-Yehuda, S.; Losick, R.; Kolter, R. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2001, 98, 11621–11626. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.A. Thinking about bacterial populations as multicellular organisms. Annu. Rev. Microbiol. 1998, 52, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Branda, S.S.; Chu, F.; Kearns, D.B.; Losick, R.; Kolter, R. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 2006, 59, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Hobley, L.; Ostrowski, A.; Rao, F.V.; Bromley, K.M.; Porter, M.; Prescott, A.R.; MacPhee, C.E.; Van Aalten, D.M.; Stanley-Wall, N.R. BslA is a self-assembling bacterial hydrophobin that coats the Bacillus subtilis biofilm. Proc. Natl. Acad. Sci. USA 2013, 110, 13600–13605. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Iwano, M. BslA (YuaB) forms a hydrophobic layer on the surface of Bacillus subtilis biofilms. Mol. Microbiol. 2012, 85, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Vlamakis, H.; Chai, Y.; Beauregard, P.; Losick, R.; Kolter, R. Sticking together: Building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 2013, 11, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Dragoš, A.; Kovács, Á.T. The peculiar functions of bacterial extracellular matrix. Trends Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Á.T.; van Gestel, J.; Kuipers, O.P. The protective layer of biofilm: A repellent function for a new class of amphiphilic proteins. Mol. Microbiol. 2012, 85, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Seminara, A.; Angelini, T.E.; Wilking, J.N.; Vlamakis, H.; Ebrahim, S.; Kolter, R.; Weitz, D.A.; Brenner, M.P. Osmotic spreading of Bacillus subtilis biofilms driven by an extracellular matrix. Proc. Natl. Acad. Sci. USA 2012, 109, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Grau, R.R.; de Oña, P.; Kunert, M.; Leñini, C.; Gallegos-Monterrosa, R.; Mhatre, E.; Vileta, D.; Donato, V.; Hölscher, T.; Boland, W.; et al. A duo of potassium-responsive histidine kinases govern the multicellular destiny of Bacillus subtilis. mBio 2015, 6, e00581-15. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, J.; Vlamakis, H.; Kolter, R. From cell differentiation to cell collectives: Bacillus subtilis uses division of labor to migrate. PLoS Biol. 2015, 13, e1002141. [Google Scholar] [CrossRef]
- Kinsinger, R.F.; Shirk, M.C.; Fall, R. Rapid surface motility in Bacillus subtilis is dependent on extracellular surfactin and potassium ion. J. Bacteriol. 2003, 185, 5627–5631. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Á.T. Bacterial differentiation via gradual activation of global regulators. Curr. Genet. 2016, 62, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, E.; Troszok, A.; Gallegos-Monterrosa, R.; Lindstädt, S.; Hölscher, T.; Kuipers, O.P.; Kovács, Á.T. The impact of manganese on biofilm development of Bacillus subtilis. Microbiology 2016, 162, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, C.E.; Huang, S.; Hanstein, C.G.; Strauch, M.A.; Burbulys, D.; Wang, L.; Hoch, J.A.; Whiteley, J.M. Synergistic kinetic interactions between components of the phosphorelay controlling sporulation in Bacillus subtilis. Biochemstry 1998, 37, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Shao, W.; Perego, M.; Hoch, J.A. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol. Microbiol. 2000, 38, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Prindle, A.; Humphries, J.; Gabalda-Sagarra, M.; Asally, M.; Lee, D.Y.; Ly, S.; Garcia-Ojalvo, J.; Suel, G.M. Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature 2015, 523, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K. Bacillus subtilis pellicle formation proceeds through genetically defined morphological changes. J. Bacteriol. 2007, 189, 4920–4931. [Google Scholar] [CrossRef] [PubMed]
- Konkol, M.A.; Blair, K.M.; Kearns, D.B. Plasmid-encoded ComI inhibits competence in the ancestral 3610 strain of Bacillus subtilis. J. Bacteriol. 2013, 195, 4085–4093. [Google Scholar] [CrossRef] [PubMed]
- Veening, J.-W.; Murray, H.; Errington, J. A mechanism for cell cycle regulation of sporulation initiation in Bacillus subtilis. Genes Dev. 2009, 23, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, J.; Weissing, F.J.; Kuipers, O.P.; Kovács, A.T. Density of founder cells affects spatial pattern formation and cooperation in Bacillus subtilis biofilms. ISME J. 2014, 8, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Susanna, K.A.; Mironczuk, A.M.; Smits, W.K.; Hamoen, L.W.; Kuipers, O.P. A single, specific thymine mutation in the ComK-binding site severely decreases binding and transcription activation by the competence transcription factor ComK of Bacillus subtilis. J. Bacteriol. 2007, 189, 4718–4728. [Google Scholar] [CrossRef] [PubMed]
- López, D.; Vlamakis, H.; Losick, R.; Kolter, R. Paracrine signaling in a bacterium. Genes Dev. 2009, 23, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Verhamme, D.T.; Murray, E.J.; Stanley-Wall, N.R. DegU and Spo0A jointly control transcription of two loci required for complex colony development by Bacillus subtilis. J. Bacteriol. 2009, 191, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Á.T.; Kuipers, O.P. Rok regulates yuaB expression during architecturally complex colony development of Bacillus subtilis 168. J. Bacteriol. 2011, 193, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Kraas, F.I.; Helmetag, V.; Wittmann, M.; Strieker, M.; Marahiel, M.A. Functional dissection of surfactin synthetase initiation module reveals insights into the mechanism of lipoinitiation. Chem. Biol. 2010, 17, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Gallegos-Monterrosa, R.; Mhatre, E.; Kovács, Á.T. Specific Bacillus subtilis 168 variants do form biofilms on nutrient rich medium. Microbiology 2016, 162, 1922–1932. [Google Scholar] [PubMed]
- Oslizlo, A.; Stefanic, P.; Dogsa, I.; Mandic-Mulec, I. Private link between signal and response in Bacillus subtilis quorum sensing. Proc. Natl. Acad. Sci. USA 2014, 111, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, T.; Dragoš, A.; Gallegos-Monterrosa, R.; Martin, M.; Mhatre, E.; Richter, A.; Kovács, Á.T. Monitoring spatial segregation in surface colonizing microbial populations. J. Vis. Exp. 2016, 116, e54752. [Google Scholar] [CrossRef] [PubMed]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012, 10, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kearns, D.B.; Losick, R. Swarming motility in undomesticated Bacillus subtilis. Mol. Microbiol. 2003, 49, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer-Shaanan, Y.; Sibony-Nevo, O.; Bloom-Ackermann, Z.; Suissa, R.; Steinberg, N.; Kartvelishvily, E.; Brumfeld, V.; Kolodkin-Gal, I. Spatio-temporal assembly of functional mineral scaffolds within microbial biofilms. NPJ Biofilms Microbiomes 2016, 2, 15031. [Google Scholar] [CrossRef]
- Barabesi, C.; Galizzi, A.; Mastromei, G.; Rossi, M.; Tamburini, E.; Perito, B. Bacillus subtilis gene cluster involved in calcium carbonate biomineralization. J. Bacteriol. 2007, 189, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K. Gradual activation of the response regulator DegU controls serial expression of genes for flagellum formation and biofilm formation in Bacillus subtilis. Mol. Microbiol. 2007, 66, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Marlow, V.L.; Porter, M.; Hobley, L.; Kiley, T.B.; Swedlow, J.R.; Davidson, F.A.; Stanley-Wall, N.R. Phosphorylated DegU manipulates cell fate differentiation in the Bacillus subtilis biofilm. J. Bacteriol. 2014, 196, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Greenwich, J.; Li, Y.; Wang, Q.; Chai, Y. The bacterial tyrosine kinase activator TkmA contributes to biofilm formation largely independently of the cognate kinase PtkA in Bacillus subtilis. J. Bacteriol. 2015, 197, 3421–3432. [Google Scholar] [CrossRef] [PubMed]
- Arutchelvi, J.; Sangeetha, J.; Philip, J.; Doble, M. Self-assembly of surfactin in aqueous solution: Role of divalent counterions. Colloids Surf. B Biointerfaces 2014, 116, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmy, L. Über die ahhängigkeit der capillaritäts—Constanten des alkohols von substanz und gestalt des benetzten festen körpers. Ann. Phys. Chem. 1863, 119, 177–217. [Google Scholar] [CrossRef]
- López, D.; Gontang, E.A.; Kolter, R. Potassium sensing histidine kinase in Bacillus subtilis. Methods Enzymol. 2010, 471, 229–251. [Google Scholar] [PubMed]
- Shemesh, M.; Chai, Y. A combination of glycerol and manganese promotes biofilm formation in Bacillus subtilis via histidine kinase kind signaling. J. Bacteriol. 2013, 195, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Oknin, H.; Steinberg, D.; Shemesh, M. Magnesium ions mitigate biofilm formation of Bacillus species via downregulation of matrix genes expression. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Herbaud, M.-L.; Guiseppi, A.; Denizot, F.; Haiech, J.; Kilhoffer, M.-C. Calcium signalling in Bacillus subtilis. Biochim. Biophys. Acta 1998, 1448, 212–226. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, W.; Tsai, S.-M.; Zehnder, S.; Sarntinoranont, M.; Angelini, T. Surface indentation and fluid intake generated by the polymer matrix of Bacillus subtilis biofilms. Soft Matter 2015, 11, 3612–3617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Nie, K.; Li, M.; Sun, Q. Simulation of Bacillus subtilis biofilm growth on agar plate by diffusion–reaction based continuum model. Phys. Biol. 2016, 13, 046002. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M. Attachment of Pseudomonas fluorescens to glass and influence of electrolytes on bacterium-substratum separation distance. J. Bacteriol. 1988, 170, 2027–2030. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kakii, K. Intergeneric coaggregations among Oligotropha carboxidovorans and Acinetobacter species present in activated sludge. FEMS Microbiol. Lett. 2003, 224, 23–28. [Google Scholar] [CrossRef]
- Meng, Y.; Dong, G.; Zhang, C.; Ren, Y.; Qu, Y.; Chen, W. Calcium regulates glutamate dehydrogenase and poly-γ-glutamic acid synthesis in Bacillus natto. Biotechnol. Lett. 2016, 38, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Grau, A.; Fernandez, J.C.G.; Peypoux, F.; Ortiz, A. A study on the interactions of surfactin with phospholipid vesicles. Biochim. Biophys. Acta 1999, 1418, 307–319. [Google Scholar] [CrossRef]
| Strain | Genotype | Reference, Source, or Construction |
|---|---|---|
| DK1042 | 3610 comIQ12I | [20] |
| TB500 | 3610 comIQ12I amyE::Physperpank-gfp(specR) | pTB497 → DK1042 |
| TB602 | 3610 comIQ12I ΔtasA::specR | TB163 [11] → DK1042 |
| TB277 | 3610 comIQ12I ΔsrfAA::Cm | RG551 [11] → DK1042 |
| TB530 | 3610 comIQ12I Δhag::neo | TB24 [11] → TB500 |
| TB524 | 3610 comIQ12I ΔepsA-O::tetR | DL1032 [24] → TB500 |
| TB526 | 3610 comIQ12I ΔbslA::cmR | NRS 2097 [25] → TB500 |
| TB398 | 3610 comIQ12I ΔkinA::mlsR | JH12638 [11] → DK1042 |
| TB399 | 3610 comIQ12I ΔkinB::tetR | JH19980 [11] → DK1042 |
| TB400 | 3610 comIQ12I ΔkinC::specR | BAL393 [11] → DK1042 |
| TB401 | 3610 comIQ12I ΔkinD::cmR | BAL691 [11] → DK1042 |
| TB402 | 3610 comIQ12I ΔkinE::cmR | BAL692 [11] → DK1042 |
| TB672 | 3610 comIQ12I ΔkinB::tetR ΔkinC::specR | TB400 → TB399 |
| TB656 | 3610 comIQ12I ΔkinC::specR ΔkinD::cmR | TB400 → TB401 |
| TB671 | 3610 comIQ12I ΔdegU::neoR | ΔdegU [26]→ DK1042 |
| TB51 | 3610 comIQ12I ΔlcfA::mlsR | MW2 [27] → DK1042 |
| TB363 | 3610 comIQ12I sacA::PepsA-gfp(neoR) | [28] |
| TB373 | 3610 comIQ12I sacA::PtapA-gfp(neoR) | [28] |
| TB685 | 3610 comIQ12I amyE::PbslA-gfp(cmR) | pTB670 → DK1042 |
| TB740 | 3610 comIQ12I PsrfAA-gfp(specR) | BD4720 [29] → DK1042 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhatre, E.; Sundaram, A.; Hölscher, T.; Mühlstädt, M.; Bossert, J.; Kovács, Á.T. Presence of Calcium Lowers the Expansion of Bacillus subtilis Colony Biofilms. Microorganisms 2017, 5, 7. https://doi.org/10.3390/microorganisms5010007
Mhatre E, Sundaram A, Hölscher T, Mühlstädt M, Bossert J, Kovács ÁT. Presence of Calcium Lowers the Expansion of Bacillus subtilis Colony Biofilms. Microorganisms. 2017; 5(1):7. https://doi.org/10.3390/microorganisms5010007
Chicago/Turabian StyleMhatre, Eisha, Anandaroopan Sundaram, Theresa Hölscher, Mike Mühlstädt, Jörg Bossert, and Ákos T. Kovács. 2017. "Presence of Calcium Lowers the Expansion of Bacillus subtilis Colony Biofilms" Microorganisms 5, no. 1: 7. https://doi.org/10.3390/microorganisms5010007