Genome Sequence of Rhodoferax antarcticus ANT.BRT; A Psychrophilic Purple Nonsulfur Bacterium from an Antarctic Microbial Mat
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Genome Properties
3.2. Major Photosynthesis Genes
3.3. Light-Harvesting Complexes
3.4. Carbon Metabolism
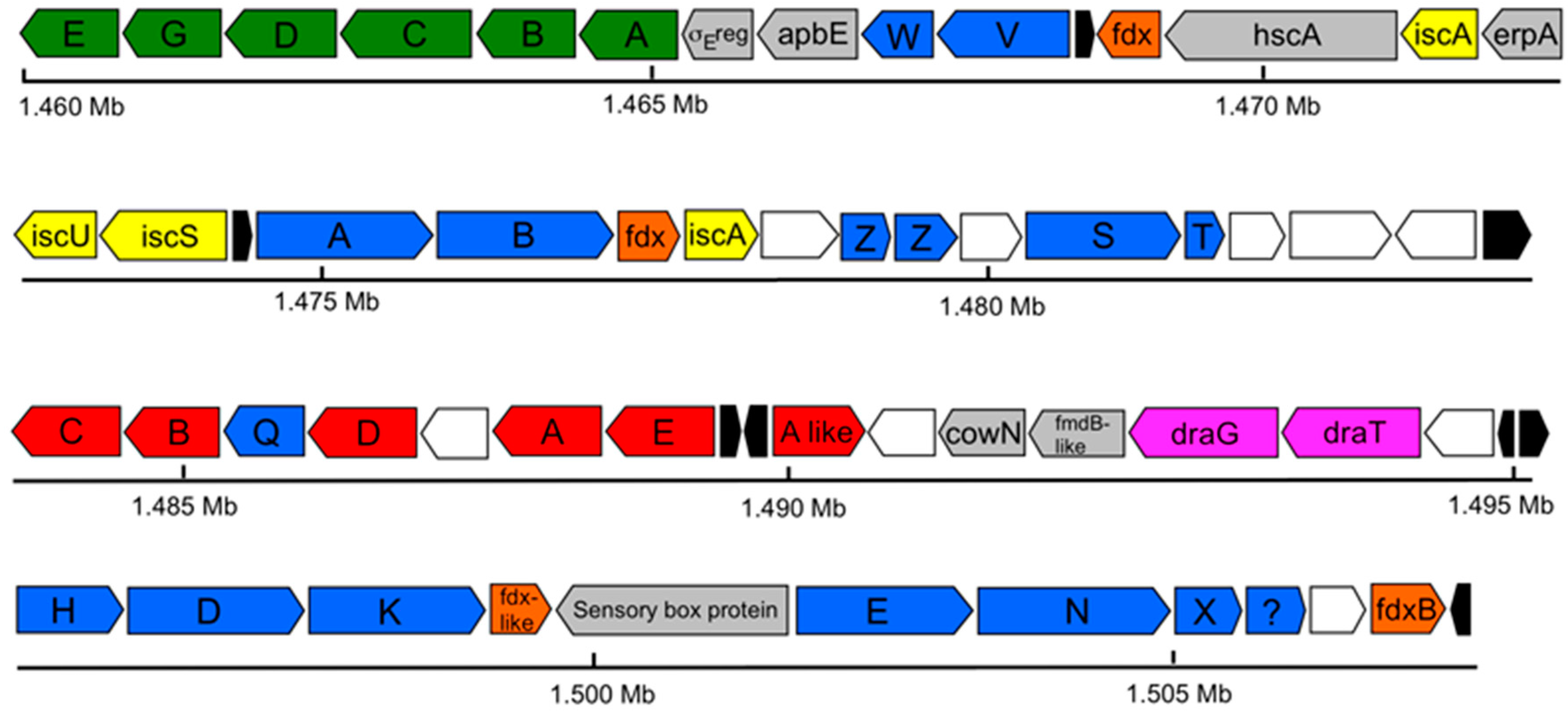
3.5. Nitrogen Fixation
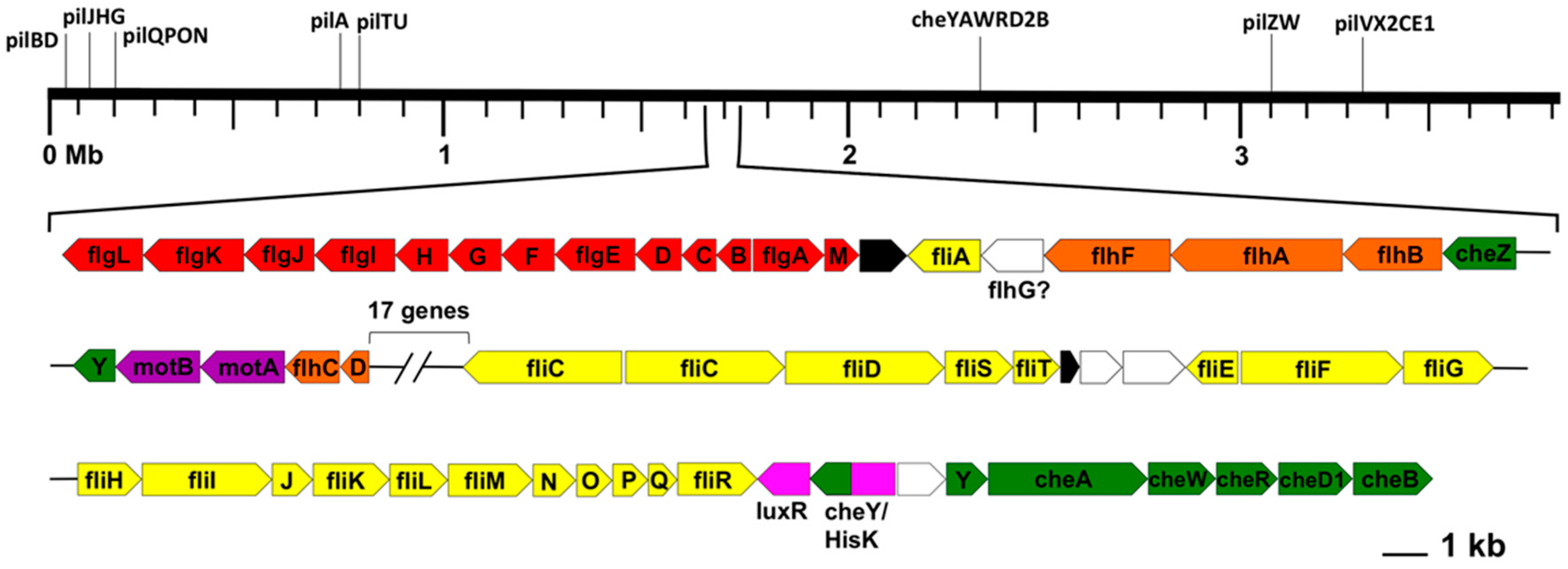
3.6. Motility
3.7. Plasmid Features
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Madigan, M.T.; Jung, D.O. An overview of purple bacteria: Systematics, physiology, and habitats. In The Purple Phototrophic Bacteria; Hunter, C.N., Daldal, F., Thurnauer, C., Beatty, J.T., Eds.; Springer: Dordrecht, the Netherlands, 2009; Volume 28, pp. 1–15. [Google Scholar]
- Madigan, M.T. Anoxygenic phototrophic bacteria from extreme environments. Photosynth. Res. 2003, 76, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.T.; Jung, D.O.; Woese, C.R.; Achenbach, L.A. Rhodoferax antarcticus sp. nov., a moderately psychrophilic purple nonsulfur bacterium isolated from an Antarctic microbial mat. Arch. Microbiol. 2000, 173, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Morita, R.Y. Psychrophilic bacteria. Bacteriol. Rev. 1975, 39, 144–167. [Google Scholar] [PubMed]
- Jung, D.O.; Achenbach, L.A.; Karr, E.A.; Takaichi, S.; Madigan, M.T. A gas vesiculate strain of the purple non-sulfur bacterium Rhodoferax antarcticus isolated from Lake Fryxell, Dry Valleys, Antarctica. Arch. Microbiol. 2004, 182, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2016, 44, D67–D72. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stetcher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 30, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Jukes, T.H.; Cantor, C.R. Evolution of protein molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; pp. 21–132. [Google Scholar]
- Choudhary, M.; Mackenzie, C.; Donohue, T.J.; Kaplan, S. Purple bacterial genomics. In The Purple Phototrophic Bacteria; Hunter, C.N., Daldal, F., Thurnauer, C., Beatty, J.T., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 28, pp. 691–706. [Google Scholar]
- Zerbino, D.R. Using the Velvet de novo assembler for short-read sequencing technologies. Curr. Protoc. Bioinform. 2010, 31, 11.5.1–11.5.12. [Google Scholar] [CrossRef]
- Galens, K.; Orvis, J.; Daugherty, S.; Creasy, H.H.; Angiuoli, S.; White, O.; Wortman, J.; Mahurkar, A.; Giglio, M.G. The IGS Standard Operating Procedure for Automated Prokaryotic Annotation. Stand. Genom. Sci. 2011, 4, 244–251. [Google Scholar] [CrossRef] [PubMed]
- University of Maryland School of Medicine Institute for Genome Sciences. Available online: http://www.igs.umaryland.edu/research/bioinformatics/analysis/ (accessed on 21 February 2017).
- BLAST-Extend-Repraze. Available online: http://ber.sourceforge.net/ (accessed on 21 February 2017).
- Manatee. Available online: http://manatee.sourceforge.net (accessed on 21 February 2017).
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A.; Larrson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol., 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Grissa, I.; Vergnaud, G.; Pourcel, C. CRISPRFinder: A web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res. 2007, 35, W52–W57. [Google Scholar] [CrossRef] [PubMed]
- Addlesee, H.A.; Fiedor, L.; Hunter, C.N. Physical mapping of bchG, orf427, and orf177 in the photosynthesis gene cluster of Rhodobacter. sphaeroides: Functional assignment of the bacteriochlorophyll synthetase gene. J. Bacteriol. 2000, 182, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T. Genome sequencing and analysis of the psychrophilic anoxygenic phototrophic bacterium Rhodoferax antarcticus sp. ANT.BR. Master’s Thesis, Arizona State University, Tempe, AZ, USA, July 2011. [Google Scholar]
- Weckesser, J.; Drews, G.; Tauschel, H.-D. Zur Feinstruktur und Taxonomie von Rhodopseudomonas. gelatinosa. Arch. Mikrobiol. 1969, 65, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Drews, G.; Golecki, J. Structure, molecular organization, and biosynthesis of membranes of purple bacteria. In Anoxygenic Photosynthetic Bacteria; Blankenship, R.E., Madigan, M.T., Bauer, C.E., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 1995; Volume 2, pp. 231–257. [Google Scholar]
- Ochane, S.; Steunou, A.-S.; Picaud, M.; Astier, C. Aerobic and anaerobic Mg-protoporphyrin monomethyl ester cyclases in purple bacteria: A strategy adopted to bypass the repressive oxygen control system. J. Biol. Chem. 2004, 279, 6385–6394. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.-H.; Wen, J.; Li, X.; Blankenship, R.E. Role of the AcsF protein in Chloroflexus. aurantiacus. J. Bacteriol. 2009, 191, 3580–3587. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis, 2nd ed.; Wiley Blackwell: Oxford, UK, 2014. [Google Scholar]
- Tuveson, R.W.; Larson, R.A.; Kagan, J. Role of cloned carotenoid genes expressed in Escherichia coli in protecting against inactivation by near-UV light and specific phototoxic molecules. J. Bacteriol. 1988, 170, 4675–4680. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhao, C.; Yue, H.; Yang, S. A unique low light adaptation mechanism in Rhodobacter. azotoformans. J. Basic Microbiol. 2014, 54, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Mascle-Allemand, C.; Duquesne, K.; Lebrun, R.; Scheuring, S.; Sturgis, J.N. Antenna mixing in photosynthetic membranes from Phaeospirillum. molischianum. Proc. Natl. Acad. Sci. USA 2010, 107, 5357–5362. [Google Scholar] [CrossRef] [PubMed]
- McLuskey, K.; Prince, S.M.; Cogdell, R.J.; Isaacs, N.W. The crystallographic structure of the B800-820 LH3 light-harvesting complex from the purple bacteria Rhodopseudomonas. acidophila strain 7050. Biochemistry 2001, 40, 8783–8789. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, A.T.; Cogdell, R.J.; Takaichi, S. The effect of growth conditions on the light-harvesting apparatus in Rhodopseudomonas acidophila. Photosynth. Res. 1993, 38, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Cogdell, R.J.; University of Glasgow, Glasgow, Scotland, UK. Personal communication, 2016.
- Prince, S.M.; Papiz, M.Z.; Freer, A.A.; McDermott, G.; Hawthornthwaite-Lawless, A.M.; Cogdell, R.J.; Isaacs, N.W. Apoprotein structure in the LH2 complex from Rhodopseudomonas. acidophila strain 10050: Modular assembly and protein pigment interactions. J. Mol. Biol. 1997, 268, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Horibe, T.; Qian, P.; Hunter, C.N.; Hashimoto, H. Stark absorption spectroscopy on the carotenoids bound to B800-820 and B800-850 type LH2 complexes from a purple photosynthetic bacterium, Phaeospirillum. molischianum strain DSM120. Arch. Biochem. Biophys. 2014, 572, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Vincent, W.F.; Downes, M.T.; Castenholz, R.W.; Howard-Williams, C. Community structure and pigment organization of cyanobacteria-dominated microbial mats in Antarctica. Eur. J. Phycol. 1993, l28, 213–221. [Google Scholar] [CrossRef]
- Fowler, G.J.S.; Visschers, R.W.; Grief, G.G.; van Grondelle, R.; Hunter, C.N. Genetically modified photosynthetic antenna complexes with blueshifted absorbance bands. Nature 1992, 355, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Badger, M.R.; Bek, E.J. Multiple Rubisco forms in proteobacteria: Their functional significance in relation to CO2 acquisition by the CBB cycle. J. Exp. Bot. 2008, 59, 1525–1541. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-K.; Marden, J.; Han, M.; Swingley, W.D.; Mastrian, S.D.; Chowdhury, S.R.; Hao, J.; Helmy, T.; Kim, S.; Kurdoglu, A.A.; et al. Metabolic flexibility revealed in the genome of the cyst-forming α-1 proteobacterium Rhodospirillum. centenum. BMC Genom. 2010, 11, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Sauer, U.; Eikmanns, B.J. The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol. Rev. 2005, 29, 765–794. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ribbe, M.W. Nitrogenase and homologs. J. Biol. Inorg. Chem. 2015, 20, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Bellenger, J.P.; Xu, Y.; Zhang, X.; Morel, F.M.M.; Kraepiel, A.M.L. Possible contribution of alternative nitrogenases to nitrogen fixation by asymbiotic N2-fixing bacteria in soils. Soil Biol. Biochem. 2013, 69, 413–420. [Google Scholar] [CrossRef]
- Miller, R.W.; Eady, R.R. Molybdenum and vanadium nitrogenases of Azotobacter. chroococcum: Low temperature favors N2 reduction by vanadium nitrogenase. Biochem. J. 1988, 256, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.; Siefert, J.L.; Staples, C.R.; Blankenship, R.E. The natural history of nitrogen fixation. Mol. Biol. Evol. 2004, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, J.; Kennedy, C. Temperature-dependent regulation by molybdenum and vanadium of expression of the structural genes encoding three nitrogenases in Azotobacter. vinelandii. Appl. Environ. Microbiol. 1991, 57, 622–624. [Google Scholar] [PubMed]
- Dos Santos, P.C.; Fang, Z.; Mason, S.W.; Setubal, J.; Dixon, R. Distribution of nitrogen fixation and nitrogenase-like sequences amongst microbial genomes. BMC Genom. 2012, 13, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Q. Perspectives in biological nitrogen fixation research. J. Integr. Plant. Biol. 2008, 50, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.C.; Smith, A.D.; Frazzon, J.; Cash, V.L.; Johnson, M.K.; Dean, D.R. Iron-sulfur cluster assembly: NifU-directed activation of the nitrogenase Fe protein. J. Biol. Chem. 2004, 279, 19705–19711. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.S.; Costas, A.M.G.; Hamilton, T.L.; Mus, F.; Peters, J.W. Evolution of molybdenum nitrogenase during the transition from anaerobic to aerobic metabolism. J. Bacteriol. 2015, 197, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Liu, Z.; Zhao, D.; Liu, X.; Zhang, B.; Xie, J.; Hong, Y.; Li, P.; Chen, S.; Dixon, R.; Li, J. A minimal nitrogen fixation cluster from Paenibacillus. sp. WLY78 enables expression of active nitrogenase in Escherichia coli. PLoS Genet. 2013, 9, e1003865. [Google Scholar] [CrossRef]
- Krebs, C.; Agar, J.N.; Smith, A.D.; Frazzon, J.; Dean, D.R.; Huynh, B.H.; Johnson, M.K. IscA, an alternate scaffold for Fe-S cluster biosynthesis. Biochemistry 2001, 40, 14069–14080. [Google Scholar] [CrossRef] [PubMed]
- Blanc, B.; Gerez, C.; de Choudens, S.O. Assembly of Fe/S proteins in bacterial systems: Biochemistry of the bacterial ISC system. Biochim. Biophys. Acta. 2014, 1853, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ochman, H. Origins of flagellar gene operons and secondary flagellar systems. J. Bacteriol. 2007, 189, 7098–7104. [Google Scholar] [CrossRef] [PubMed]
- Iida, Y.; Hobley, L.; Lambert, C.; Fenton, A.K.; Sockett, R.E.; Aizawa, S.-I. Roles of multiple flagellins in flagellar formation and flagellar growth post bdelloplast lysis in Bdellovibrio. bacteriovorus. J. Mol. Biol. 2009, 394, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Dziewit, L.; Bartosik, D. Plasmids of psychrophilic and psychrotolerant bacteria and their role in adaptation to cold environments. Front. Microbiol. 2014, 5, 596. [Google Scholar] [CrossRef] [PubMed]
- Cooper, V.S.; Vohr, S.H.; Wrocklage, S.C.; Hatcher, P.J. Why Genes Evolve Faster on Secondary Chromosomes in Bacteria. PLoS Comput. Biol. 2010, 6, e1000732. [Google Scholar] [CrossRef] [PubMed]
- Arutyunov, D.; Frost, L.S. F conjugation: Back to the beginning. Plasmid 2013, 70, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Vollmers, J.; Voget, S.; Dietrich, S.; Gollnow, K.; Smits, M.; Meyer, K.; Brinkhoff, T.; Simon, M.; Daniel, R. Poles apart: Arctic and Antarctic Octadecabacter. strains share high genome plasticity and a new type of xanthorhodopsin. PLoS ONE 2013, 8, e63422. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R.; Durbin, R. RNA sequence analysis using covariance models. Nucleic Acids Res. 1994, 22, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Math, R.K.; Jin, H.M.; Kim, J.M.; Hahn, Y.; Park, W.; Madsen, E.L.; Jeon, C.O. Comparative genomics reveals adaptation by Alteromonas. sp. SN2 to marine tidal-flat conditions: Cold tolerance and aromatic hydrocarbon metabolism. PLoS ONE 2012, 7, e35784. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.; Staley, J.T.; Danchin, A.; Wang, T.Z.; Brettin, T.S.; Hauser, L.J.; Land, M.L.; Thompson, L.S. Genomics of an extreme psychrophile, Psychromonas. ingrahamii. BMC Genom. 2008, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Dalluge, J.J.; Hamamoto, T.; Horikoshi, K.; Morita, R.Y.; Stetter, K.O.; McCloskey, J.A. Posttranscriptional modification of tRNA in psychrophilic bacteria. J. Bacteriol. 1997, 79, 1918–1923. [Google Scholar] [CrossRef]
- Qin, Q.-L.; Xie, B.-B.; Yu, Y.; Shu, Y.-L.; Rong, J.-C.; Zhang, Y.-J.; Zhao, D.-L.; Chen, X.-L.; Zhang, X.-Y.; Chen, B.; et al. Comparative genomics of the marine bacterial genus Glaciecola. reveals the high degree of genomic diversity and genomic characteristic for cold adaptation. Environ. Microbiol. 2014, 16, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
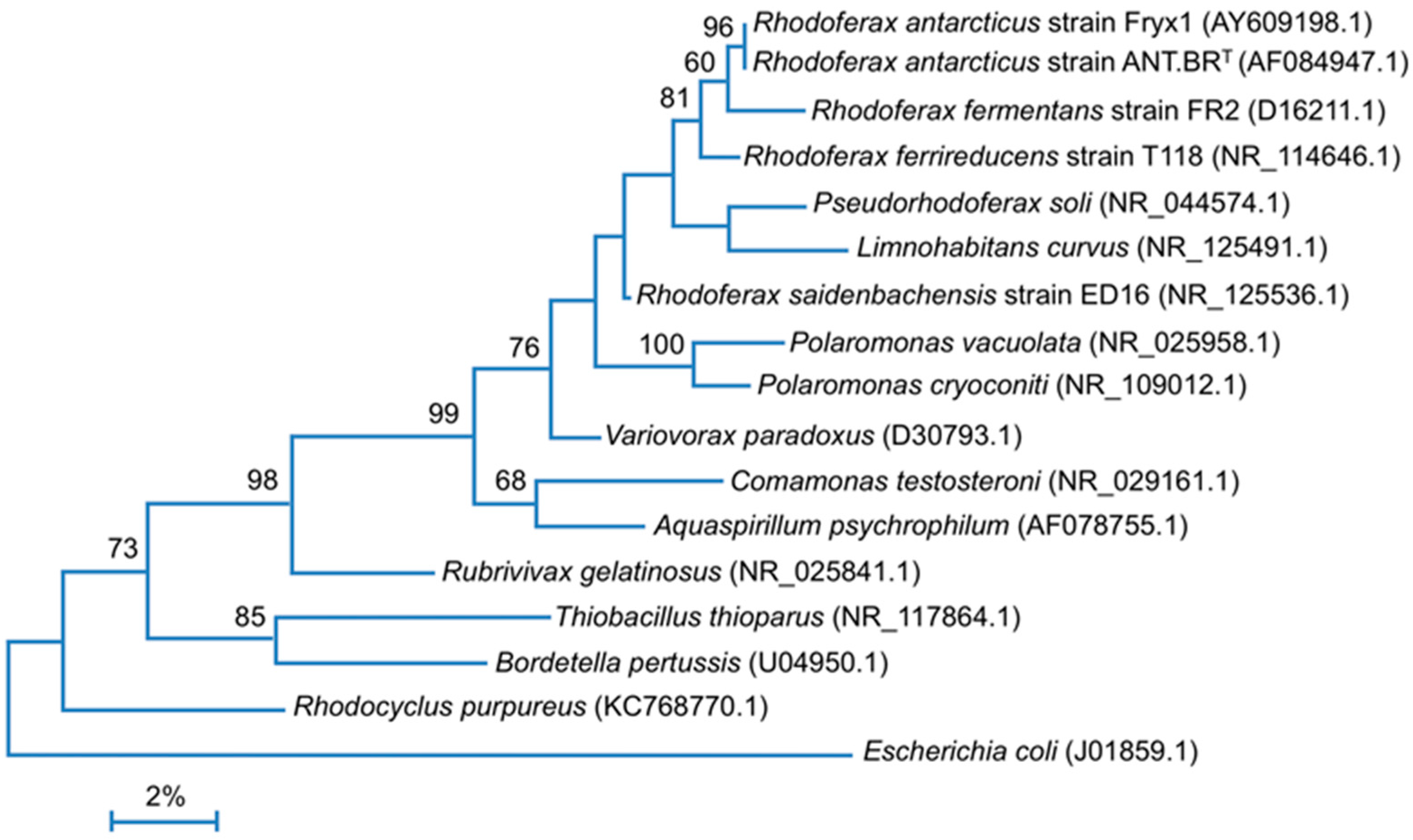
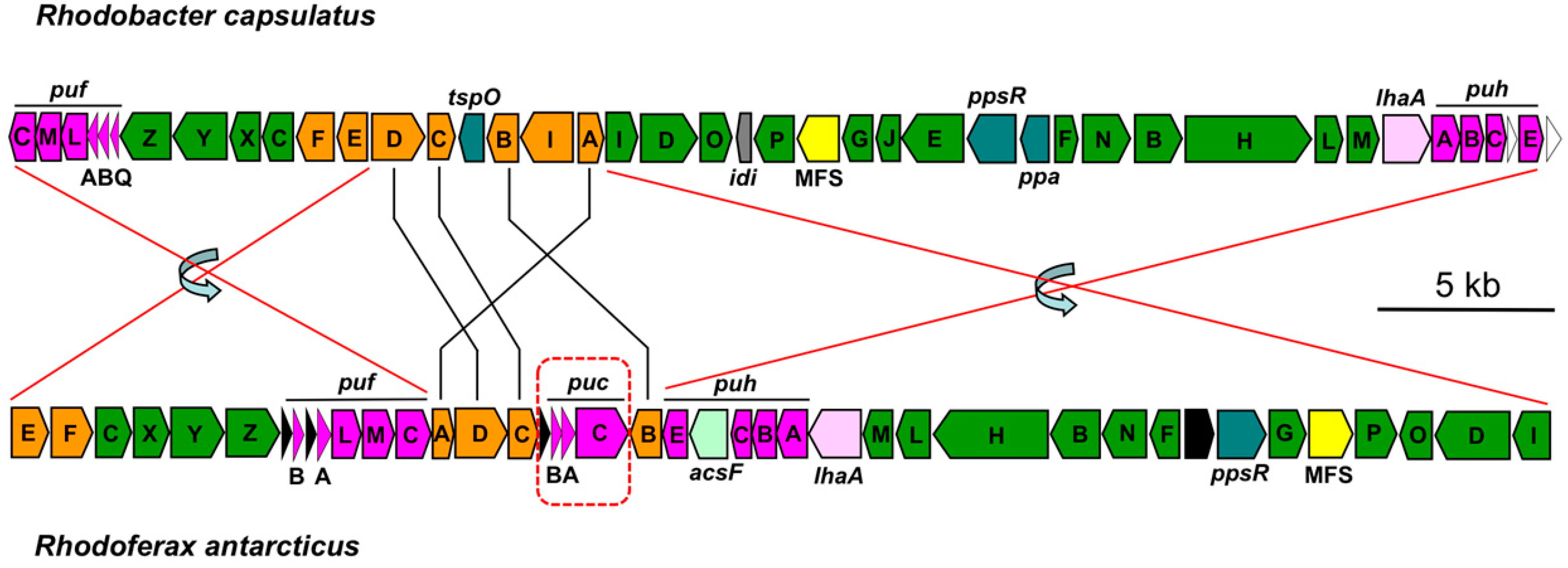
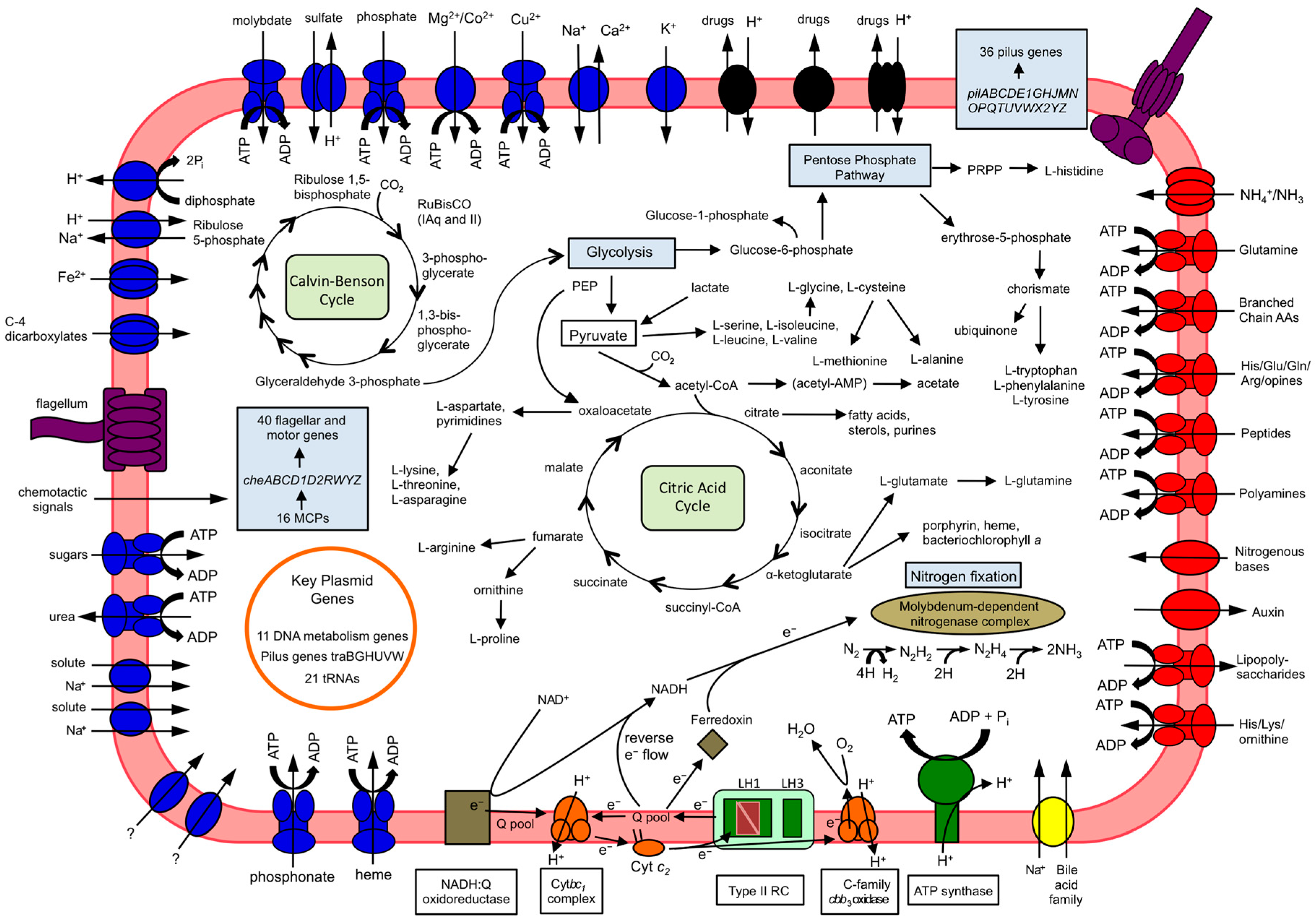
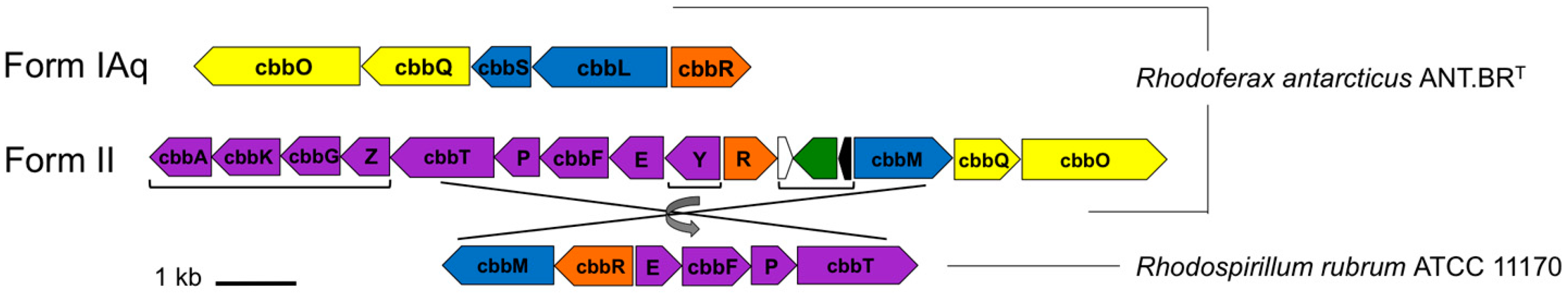
| Property | Term |
|---|---|
| Classification | Domain: Bacteria |
| Phylum: Proteobacteria | |
| Class: Betaproteobacteria | |
| Order: Burkholderiales | |
| Family: Comamonadaceae | |
| Genus: Rhodoferax | |
| Species: Rhodoferax antarcticus | |
| Type strain: ANT.BRT (ATCC 700587; DSMZ 24876) | |
| Gram stain | Negative |
| Cell shape | Vibrio to spirillum |
| Motility | Highly motile |
| Endosporulation | Non-endospore forming |
| Temperature range | 0–25 °C |
| Optimum temperature | 15–18 °C |
| pH range; Optimum | 6–8; 7 |
| Carbon sources | Acetate, pyruvate, lactate, succinate, malate, fumarate, glucose, fructose, sucrose, citrate, aspartate |
| Habitat | Algal–bacterial microbial mat |
| Salinity | 0%–2% NaCl (w/v) |
| Oxygen requirement | Facultative aerobe |
| Biotic relationship | Free-living |
| Pathogenicity | Non-pathogenic |
| Geographic location | Cape Royds, Ross Island, Antarctica |
| Sample collection | December, 1993 |
| Latitude | 77.55° S |
| Longitude | 166.16° E |
| Altitude | 20 m |
| Attribute | Value | % of Total |
|---|---|---|
| Genome size (bp) | 4,007,881 | 100.0 |
| Chromosome size (bp) | 3,809,266 | 95.0 |
| Plasmid size (bp) | 198,615 | 5.0 |
| DNA coding (bp) | 3,564,951 | 88.9 |
| Chromosome G + C content | 59.1 | – |
| Plasmid G + C content | 48.4 | – |
| Total genes | 4324 | 100.0 |
| Protein-encoding genes | 4257 | 98.5 |
| RNA genes | 67 | 1.5 |
| Pseudogenes (putative) | 228 | 5.3 |
| Genes with function prediction | 2606 | 60.2 |
| Genes with Pfam domains | 3130 | 72.3 |
| Genes with signal peptides | 211 | 4.9 |
| Genes with transmembrane helices | 800 | 18.5 |
| CRISPR repeats | 5 | 0.1 |
| Characteristic | Genes | % of Genome Content * |
|---|---|---|
| Energy and central intermediary metabolism | 438 | 10.3 |
| Amino acid biosynthesis | 107 | 2.5 |
| Transport and binding proteins | 342 | 8.0 |
| Cofactor and prosthetic group biosynthesis | 206 | 4.8 |
| DNA metabolism and nucleotide synthesis | 252 | 5.9 |
| Transcription | 76 | 1.8 |
| Protein synthesis, modification, and degradation | 332 | 7.8 |
| Regulatory functions and signal transduction | 299 | 7.0 |
| Cellular processes (division, chemotaxis, motility, toxin production and resistance, detoxification) | 262 | 6.2 |
| Fatty acid and phospholipid metabolism | 71 | 1.7 |
| Mobile and extrachromosomal element functions | 159 | 3.7 |
| Cell envelope | 251 | 5.9 |
| Proteins with family/domain assignments | 407 | 9.6 |
| Hypothetical proteins | 754 | 17.7 |
| Conserved hypothetical proteins | 558 | 13.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, J.M.; Riester, C.J.; Skinner, B.M.; Newell, A.W.; Swingley, W.D.; Madigan, M.T.; Jung, D.O.; Asao, M.; Chen, M.; Loughlin, P.C.; et al. Genome Sequence of Rhodoferax antarcticus ANT.BRT; A Psychrophilic Purple Nonsulfur Bacterium from an Antarctic Microbial Mat. Microorganisms 2017, 5, 8. https://doi.org/10.3390/microorganisms5010008
Baker JM, Riester CJ, Skinner BM, Newell AW, Swingley WD, Madigan MT, Jung DO, Asao M, Chen M, Loughlin PC, et al. Genome Sequence of Rhodoferax antarcticus ANT.BRT; A Psychrophilic Purple Nonsulfur Bacterium from an Antarctic Microbial Mat. Microorganisms. 2017; 5(1):8. https://doi.org/10.3390/microorganisms5010008
Chicago/Turabian StyleBaker, Jennifer M., Carli J. Riester, Blair M. Skinner, Austin W. Newell, Wesley D. Swingley, Michael T. Madigan, Deborah O. Jung, Marie Asao, Min Chen, Patrick C. Loughlin, and et al. 2017. "Genome Sequence of Rhodoferax antarcticus ANT.BRT; A Psychrophilic Purple Nonsulfur Bacterium from an Antarctic Microbial Mat" Microorganisms 5, no. 1: 8. https://doi.org/10.3390/microorganisms5010008






