Phosphate Acquisition and Virulence in Human Fungal Pathogens
Abstract
:1. Introduction
2. The PHO System
2.1. S. cerevisiae
2.2. C. glabrata
2.3. C. albicans
2.4. C. neoformans
2.5. A. fumigatus
3. Phosphate Acquisition and Stress Resistance
3.1. Alkaline pH Resistance
3.2. Cation Resistance
3.3. Resistance to Oxidative and Nitrosative Stresses
4. Polyphosphate
5. Phosphate Acquisition and Virulence
6. Concluding Remarks
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Vale-Silva, L.A.; Sanglard, D. Tipping the balance both ways: Drug resistance and virulence in Candida glabrata. FEMS Yeast Res. 2015, 15, fov025. [Google Scholar] [CrossRef] [PubMed]
- Fleck, C.B.; Schobel, F.; Brock, M. Nutrient acquisition by pathogenic fungi: Nutrient availability, pathway regulation, and differences in substrate utilization. Int. J. Med. Microbiol. 2011, 301, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Malavia, D.; Crawford, A.; Wilson, D. Nutritional immunity and fungal pathogenesis: The struggle for micronutrients at the host-pathogen interface. Adv. Microb. Physiol. 2017, 70, 85–103. [Google Scholar] [PubMed]
- Lamarche, M.G.; Wanner, B.L.; Crepin, S.; Harel, J. The phosphate regulon and bacterial virulence: A regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol. Rev. 2008, 32, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Lenburg, M.E.; O’Shea, E.K. Signaling phosphate starvation. Trends Biochem. Sci. 1996, 21, 383–387. [Google Scholar] [CrossRef]
- Secco, D.; Wang, C.; Shou, H.; Whelan, J. Phosphate homeostasis in the yeast Saccharomyces cerevisiae, the key role of the SPX domain-containing proteins. FEBS Lett. 2012, 586, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Mouillon, J.M.; Persson, B.L. New aspects on phosphate sensing and signaling in Saccharomyces cerevisiae. FEMS Yeast Res. 2006, 6, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, N.; DeRisi, J.; Brown, P.O. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol. Biol. Cell 2000, 11, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; O’Shea, E.K. Integrated approaches reveal determinants of genome-wide binding and function of the transcription factor Pho4. Mol. Cell 2011, 42, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.J.; Pillus, L.; Ghosh, G. Pho5p and newly identified nucleotide pyrophosphatases/phosphodiesterases regulate extracellular nucleotide phosphate metabolism in Saccharomyces cerevisiae. Eukaryot. Cell 2005, 4, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.L.; Lagerstedt, J.O.; Pratt, J.R.; Pattison-Granberg, J.; Lundh, K.; Shokrollahzadeh, S.; Lundh, F. Regulation of phosphate acquisition in Saccharomyces cerevisiae. Curr. Genet. 2003, 43, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, D.D.; Rizvi, A.H.; Raser, J.M.; Margolin, B.; O’Shea, E.K. Positive feedback regulates switching of phosphate transporters in S. cerevisiae. Mol. Cell 2007, 27, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Almaguer, C.; Mantella, D.; Perez, E.; Patton-Vogt, J. Inositol and phosphate regulate GIT1 transcription and glycerophosphoinositol incorporation in Saccharomyces cerevisiae. Eukaryot. Cell 2003, 2, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.; Almaguer, C.; Holic, R.; Griac, P.; Patton-Vogt, J. Glycerophosphocholine-dependent growth requires Gde1p (YPL110c) and Git1p in Saccharomyces cerevisiae. J. Biol. Chem. 2005, 280, 36110–36117. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, M.; Neumann, H.; Lenherr, E.D.; Wehner, M.; Rybin, V.; Hassa, P.O.; Uttenweiler, A.; Reinhardt, M.; Schmidt, A.; Seiler, J.; et al. Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase. Science 2009, 324, 513–516. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.M.; Kaffman, A.; Jolly, E.R.; O’Shea, E.K. Regulation of PHO4 nuclear localization by the PHO80-PHO85 cyclin-CDK complex. Science 1996, 271, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.R.; Smith, R.L.; O’Shea, E.K. Phosphate-regulated inactivation of the kinase PHO80-PHO85 by the CDK inhibitor PHO81. Science 1994, 266, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Kaffman, A.; Rank, N.M.; O’Neill, E.M.; Huang, L.S.; O’Shea, E.K. The receptor Msn5 exports the phosphorylated transcription factor Pho4 out of the nucleus. Nature 1998, 396, 482–486. [Google Scholar] [PubMed]
- Komeili, A.; O’Shea, E.K. Roles of phosphorylation sites in regulating activity of the transcription factor Pho4. Science 1999, 284, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Isasa, M.; Rose, C.M.; Elsasser, S.; Navarrete-Perea, J.; Paulo, J.A.; Finley, D.J.; Gygi, S.P. Multiplexed, proteome-wide protein expression profiling: Yeast deubiquitylating enzyme knockout strains. J. Proteome Res. 2015, 14, 5306–5317. [Google Scholar] [CrossRef] [PubMed]
- Wild, R.; Gerasimaite, R.; Jung, J.Y.; Truffault, V.; Pavlovic, I.; Schmidt, A.; Saiardi, A.; Jessen, H.J.; Poirier, Y.; Hothorn, M.; et al. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 2016, 352, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Mulugu, S.; York, J.D.; O’Shea, E.K. Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science 2007, 316, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Lonetti, A.; Szijgyarto, Z.; Bosch, D.; Loss, O.; Azevedo, C.; Saiardi, A. Identification of an evolutionarily conserved family of inorganic polyphosphate endopolyphosphatases. J. Biol. Chem. 2011, 286, 31966–31974. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Huang, K.; Quiocho, F.A.; O’Shea, E.K. Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate. Nat. Chem. Biol. 2008, 4, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hurlimann, H.C.; Pinson, B.; Stadler-Waibel, M.; Zeeman, S.C.; Freimoser, F.M. The SPX domain of the yeast low-affinity phosphate transporter Pho90 regulates transport activity. EMBO Rep. 2009, 10, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Janbon, G.; Ormerod, K.L.; Paulet, D.; Byrnes, E.J., 3rd; Yadav, V.; Chatterjee, G.; Mullapudi, N.; Hon, C.C.; Billmyre, R.B.; Brunel, F.; et al. Analysis of the genome and transcriptome of Cryptococcus neoformans var. Grubii reveals complex RNA expression and microevolution leading to virulence attenuation. PLoS Genet. 2014, 10, e1004261. [Google Scholar] [CrossRef]
- Li, W.; Cowley, A.; Uludag, M.; Gur, T.; McWilliam, H.; Squizzato, S.; Park, Y.M.; Buso, N.; Lopez, R. The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Res. 2015, 43, W580–W584. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, C.L.; Wykoff, D.D. Candida glabrata Pho4 is necessary and sufficient for Pho2-independent transcription of phosphate starvation genes. Genetics 2009, 182, 471–479. [Google Scholar] [CrossRef] [PubMed]
- He, B.Z.; Zhou, X.; O’Shea, E.K. Evolution of reduced co-activator dependence led to target expansion of a starvation response pathway. eLife 2017, 6, 25157. [Google Scholar] [CrossRef] [PubMed]
- Homann, O.R.; Dea, J.; Noble, S.M.; Johnson, A.D. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet. 2009, 5, e1000783. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, K.; Zaborin, A.; Valuckaite, V.; Rolfes, R.J.; Babrowski, T.; Bethel, C.; Olivas, A.; Zaborina, O.; Alverdy, J.C. Candida albicans isolates from the gut of critically ill patients respond to phosphate limitation by expressing filaments and a lethal phenotype. PLoS ONE 2012, 7, e30119. [Google Scholar] [CrossRef] [PubMed]
- Ikeh, M.A.; Kastora, S.L.; Day, A.M.; Herrero-de-Dios, C.M.; Tarrant, E.; Waldron, K.J.; Banks, A.P.; Bain, J.M.; Lydall, D.; Veal, E.A.; et al. Pho4 mediates phosphate acquisition in Candida albicans and is vital for stress resistance and metal homeostasis. Mol. Biol. Cell 2016, 27, 2784–2801. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.C.; Ganguly, S.; Solis, N.V.; Cooley, B.M.; Jensen-Seaman, M.I.; Filler, S.G.; Mitchell, A.P.; Patton-Vogt, J. Glycerophosphocholine utilization by Candida albicans: Role of the Git3 transporter in virulence. J. Biol. Chem. 2013, 288, 33939–33952. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.C.; Sun, T.; Johnson, M.E.; Bruno, V.M.; Patton-Vogt, J. Robust utilization of phospholipase-generated metabolites, glycerophosphodiesters, by Candida albicans: Role of the CaGit1 permease. Eukaryot. Cell 2011, 10, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.N.; Flanagan, P.R.; Zeng, J.; Jani, N.M.; Cardenas, M.E.; Moran, G.P.; Kohler, J.R. Phosphate is the third nutrient monitored by TOR in Candida albicans and provides a target for fungal-specific indirect TOR inhibition. Proc. Natl. Acad. Sci. USA 2017, 114, 6346–6351. [Google Scholar] [CrossRef] [PubMed]
- Urrialde, V.; Prieto, D.; Pla, J.; Alonso-Monge, R. The Pho4 transcription factor mediates the response to arsenate and arsenite in Candida albicans. Front. Microbiol. 2015, 6, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urrialde, V.; Prieto, D.; Pla, J.; Alonso-Monge, R. The Candida albicans Pho4 transcription factor mediates susceptibility to stress and influences fitness in a mouse commensalism model. Front. Microbiol. 2016, 7, 1062. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, Y. Identification of a Candida albicans homologue of the PHO85 gene, a negative regulator of the PHO system in Saccharomyces cerevisiae. Yeast 2000, 16, 1045–1051. [Google Scholar] [CrossRef]
- Serrano, R.; Ruiz, A.; Bernal, D.; Chambers, J.R.; Arino, J. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: Evidence for calcium-mediated signaling. Mol. Microbiol. 2002, 46, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, M.; Reiner, E.; Hu, G.; Tam, N.; Oliveira, D.L.; Caza, M.; Yeon, J.H.; Kim, J.; Kastrup, C.J.; Jung, W.H.; et al. Defects in phosphate acquisition and storage influence virulence of Cryptococcus neoformans. Infect. Immun. 2014, 82, 2697–2712. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Yang, D.H.; Maeng, S.; Lee, K.T.; So, Y.S.; Hong, J.; Choi, J.; Byun, H.J.; Kim, H.; Bang, S.; et al. Systematic functional profiling of transcription factor networks in Cryptococcus neoformans. Nat. Commun. 2015, 6, 6757. [Google Scholar] [CrossRef] [PubMed]
- Toh-E, A.; Ohkusu, M.; Li, H.M.; Shimizu, K.; Takahashi-Nakaguchi, A.; Gonoi, T.; Kawamoto, S.; Kanesaki, Y.; Yoshikawa, H.; Nishizawa, M. Identification of genes involved in the phosphate metabolism in Cryptococcus neoformans. Fungal Genet. Biol. 2015, 80, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Lev, S.; Kaufman-Francis, K.; Desmarini, D.; Juillard, P.G.; Li, C.; Stifter, S.A.; Feng, C.G.; Sorrell, T.C.; Grau, G.E.; Bahn, Y.S.; et al. Pho4 is essential for dissemination of Cryptococcus neoformans to the host brain by promoting phosphate uptake and growth at alkaline pH. mSphere 2017, 2, e00381-16. [Google Scholar] [CrossRef] [PubMed]
- De Gouvea, P.F.; Soriani, F.M.; Malavazi, I.; Savoldi, M.; Goldman, M.H.; Loss, O.; Bignell, E.; da Silva Ferreira, M.E.; Goldman, G.H. Functional characterization of the Aspergillus fumigatus PHO80 homologue. Fungal Genet. Biol. 2008, 45, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Serra-Cardona, A.; Canadell, D.; Arino, J. Coordinate responses to alkaline pH stress in budding yeast. Microb. Cell 2015, 2, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Sambade, M.; Alba, M.; Smardon, A.M.; West, R.W.; Kane, P.M. A genomic screen for yeast vacuolar membrane ATPase mutants. Genetics 2005, 170, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Ghillebert, R.; Swinnen, E.; De Snijder, P.; Smets, B.; Winderickx, J. Differential roles for the low-affinity phosphate transporters Pho87 and Pho90 in Saccharomyces cerevisiae. Biochem. J. 2011, 434, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Samyn, D.R.; Ruiz-Pavon, L.; Andersson, M.R.; Popova, Y.; Thevelein, J.M.; Persson, B.L. Mutational analysis of putative phosphate- and proton-binding sites in the Saccharomyces cerevisiae Pho84 phosphate:H(+) transceptor and its effect on signaling to the PKA and PHO pathways. Biochem. J. 2012, 445, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, L.; Reddi, A.R.; Leung, E.; Aranda, K.; Jensen, L.T.; Culotta, V.C. The effect of phosphate accumulation on metal ion homeostasis in Saccharomyces cerevisiae. J. Biol. Inorg. Chem. 2010, 15, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Festa, R.A.; Sun, T.S.; Wang, Z.Y. Iron and copper as virulence modulators in human fungal pathogens. Mol. Microbiol. 2014, 93, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Gerasimaite, R.; Mayer, A. Enzymes of yeast polyphosphate metabolism: Structure, enzymology and biological roles. Biochem. Soc. Trans. 2016, 44, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.N.; Gomez-Garcia, M.R.; Kornberg, A. Inorganic polyphosphate: Essential for growth and survival. Annu. Rev. Biochem. 2009, 78, 605–647. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J.; Jakob, U. Oxidative stress protection by polyphosphate—New roles for an old player. Curr. Opin. Microbiol. 2015, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J.; Wholey, W.Y.; Wagner, N.O.; Cremers, C.M.; Mueller-Schickert, A.; Hock, N.T.; Krieger, A.G.; Smith, E.M.; Bender, R.A.; Bardwell, J.C.; et al. Polyphosphate is a primordial chaperone. Mol. Cell 2014, 53, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Bru, S.; Ribeiro, M.P.; Clotet, J. Polyphosphate: Popping up from oblivion. Curr. Genet. 2017, 63, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R.; Ulrich, P.; Moreno, S.N. Evolution of acidocalcisomes and their role in polyphosphate storage and osmoregulation in eukaryotic microbes. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010, 365, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.A.; Rodrigues, C.O.; Docampo, R. Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation, and environmental stress in Trypanosoma cruzi. J. Biol. Chem. 2001, 276, 26114–26121. [Google Scholar] [CrossRef] [PubMed]
- Albi, T.; Serrano, A. Inorganic polyphosphate in the microbial world. Emerging roles for a multifaceted biopolymer. World J. Microbiol. Biotechnol. 2016, 32, 27. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.L.; Gomes, F.M.; Girard-Dias, W.; Almeida, F.P.; Albuquerque, P.C.; Kretschmer, M.; Kronstad, J.W.; Frases, S.; de Souza, W.; Rodrigues, M.L.; et al. Phosphorus-rich structures and capsular architecture in Cryptococcus neoformans. Future Microbiol. 2017, 12, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.H.; Choi, S.H.; Smith, S.A. Polyphosphate: An ancient molecule that links platelets, coagulation, and inflammation. Blood 2012, 119, 5972–5979. [Google Scholar] [CrossRef] [PubMed]
- Bru, S.; Martinez-Lainez, J.M.; Hernandez-Ortega, S.; Quandt, E.; Torres-Torronteras, J.; Marti, R.; Canadell, D.; Arino, J.; Sharma, S.; Jimenez, J.; et al. Polyphosphate is involved in cell cycle progression and genomic stability in Saccharomyces cerevisiae. Mol. Microbiol. 2016, 101, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.; Livermore, T.; Saiardi, A. Protein polyphosphorylation of lysine residues by inorganic polyphosphate. Mol. Cell 2015, 58, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Fradin, C.; De Groot, P.; MacCallum, D.; Schaller, M.; Klis, F.; Odds, F.C.; Hube, B. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol. Microbiol. 2005, 56, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Thewes, S.; Kretschmar, M.; Park, H.; Schaller, M.; Filler, S.G.; Hube, B. In vivo and ex vivo comparative transcriptional profiling of invasive and non-invasive Candida albicans isolates identifies genes associated with tissue invasion. Mol. Microbiol. 2007, 63, 1606–1628. [Google Scholar] [CrossRef] [PubMed]
- Zakikhany, K.; Naglik, J.R.; Schmidt-Westhausen, A.; Holland, G.; Schaller, M.; Hube, B. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell Microbiol. 2007, 9, 2938–2954. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, D.M.; Castillo, L.; Nather, K.; Munro, C.A.; Brown, A.J.; Gow, N.A.; Odds, F.C. Property differences among the four major Candida albicans strain clades. Eukaryot. Cell 2009, 8, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.S.; Rhie, G.E.; Oh, J.H.; Huh, W.K.; Yim, H.S.; Kang, S.O. Copper- and zinc-containing superoxide dismutase (Cu/ZnSod) is required for the protection of Candida albicans against oxidative stresses and the expression of its full virulence. Microbiology 2002, 148, 3705–3713. [Google Scholar] [CrossRef] [PubMed]
- Arana, D.M.; Alonso-Monge, R.; Du, C.; Calderone, R.; Pla, J. Differential susceptibility of mitogen-activated protein kinase pathway mutants to oxidative-mediated killing by phagocytes in the fungal pathogen Candida albicans. Cell Microbiol. 2007, 9, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Pukkila-Worley, R.; Peleg, A.Y.; Tampakakis, E.; Mylonakis, E. Candida albicans hyphal formation and virulence assessed using a Caenorhabditis elegans infection model. Eukaryot. Cell 2009, 8, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Kraus, P.R.; Boily, M.J.; Heitman, J. Cryptococcus neoformans gene expression during murine macrophage infection. Eukaryot. Cell 2005, 4, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Lev, S.; Crossett, B.; Cha, S.Y.; Desmarini, D.; Li, C.; Chayakulkeeree, M.; Wilson, C.F.; Williamson, P.R.; Sorrell, T.C.; Djordjevic, J.T. Identification of Aph1, a phosphate-regulated, secreted, and vacuolar acid phosphatase in Cryptococcus neoformans. MBio 2014, 5, e01649-14. [Google Scholar] [CrossRef] [PubMed]
- Lev, S.; Li, C.; Desmarini, D.; Saiardi, A.; Fewings, N.L.; Schibeci, S.D.; Sharma, R.; Sorrell, T.C.; Djordjevic, J.T. Fungal inositol pyrophosphate IP7 is crucial for metabolic adaptation to the host environment and pathogenicity. MBio 2015, 6, e00531-15. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lev, S.; Saiardi, A.; Desmarini, D.; Sorrell, T.C.; Djordjevic, J.T. Identification of a major IP5 kinase in Cryptococcus neoformans confirms that pp-IP5/IP7, not IP6, is essential for virulence. Sci. Rep. 2016, 6, 23927. [Google Scholar] [CrossRef] [PubMed]
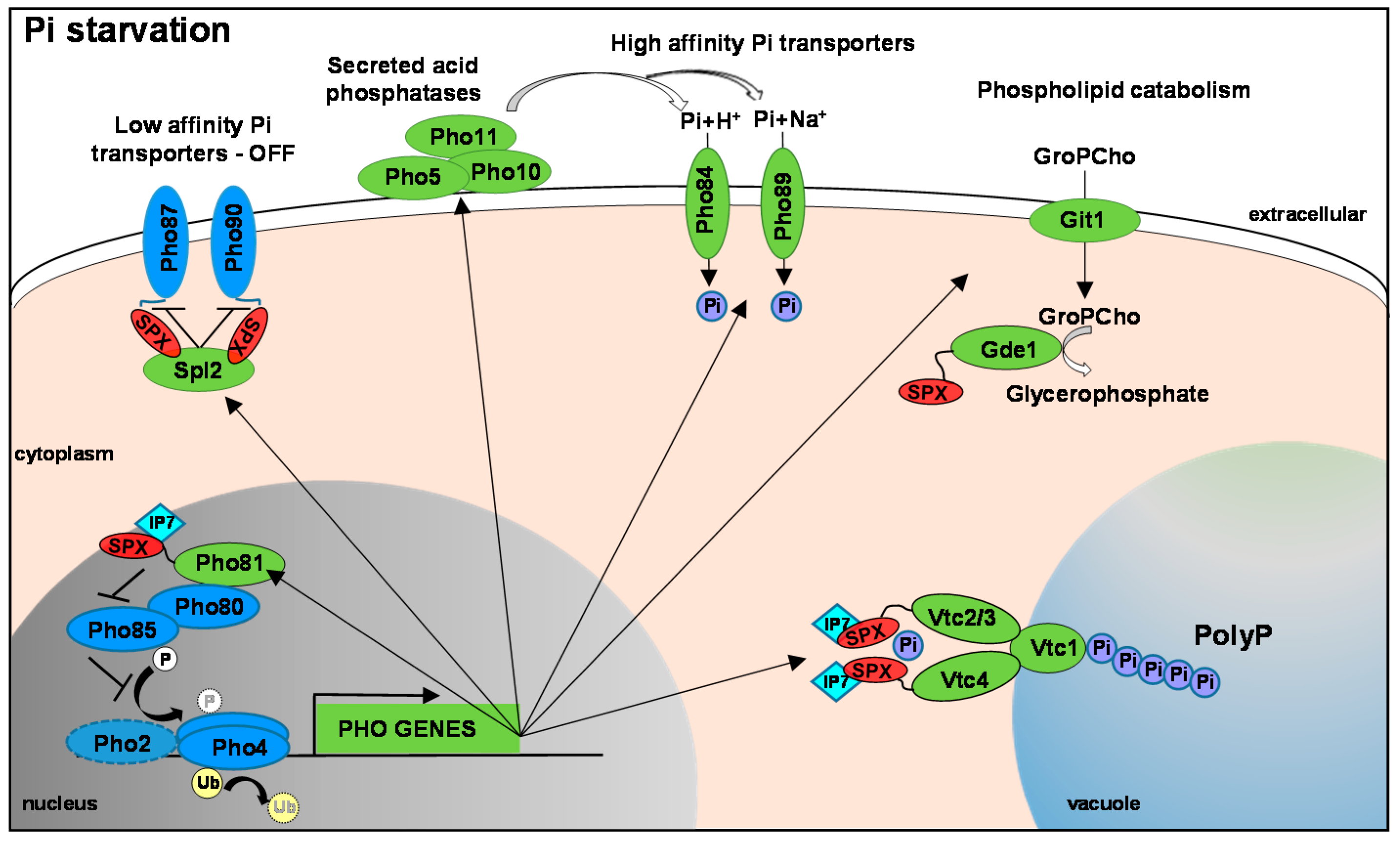
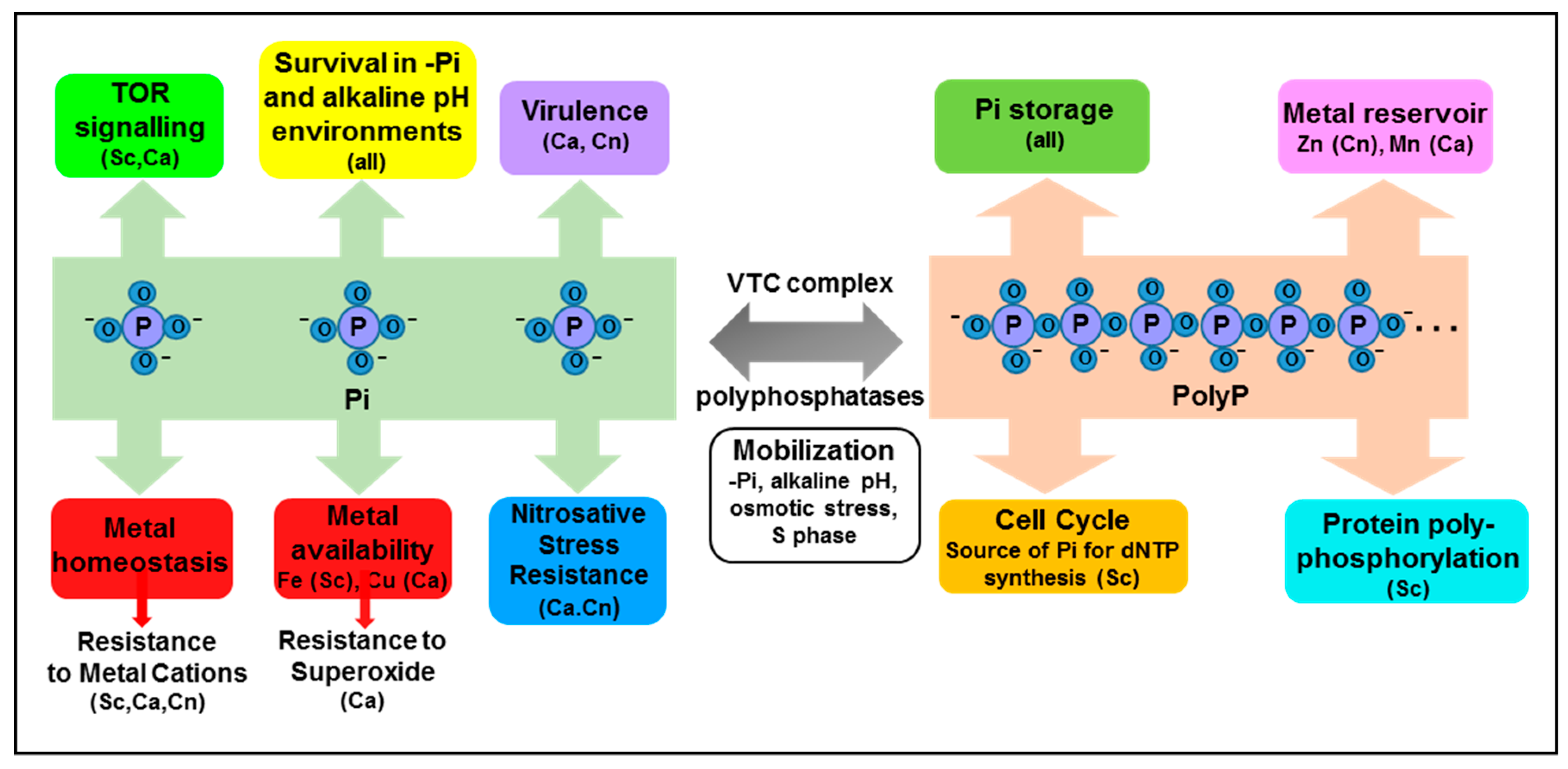
| Gene | Function in S. cerevisiae | C. glabrata 1 Orthologue and % Identity | C. albicans 1 Orthologue and % Identity | C. neoformans 2 Orthologue and % Identity | A. fumigatus 3 Orthologue and % Identity | Key Differences Compared to S. cerevisiae |
|---|---|---|---|---|---|---|
| PHO4 | Basic helix-loop-helix transcription factor; activates transcription cooperatively with Pho2p in response to phosphate limitation. Required for polyP production | PHO4 (CAGL0D05170g) 35% | PHO4 (C4_05680W_A) 29% | PHO4/HLH3 (CNAG_06751) 29% | Afu5g04190 33% | Significantly larger than Sc Pho4; homology mainly restricted to DNA binding domain. CnPHO4 displays auto-regulation. No impact on polyP levels in Cn pho4Δ cells. |
| PHO2 | Homeodomain transcription factor; activates transcription cooperatively with Pho4p in response to phosphate limitation. | PHO2 (CAGL0L07436g) 39% | GRF10 (C5_05080W_A) 34% | Afu4g10220 25% | Role of Pho2 largely restricted to Sc. No clear orthologue in Cn. | |
| PHO80 | Cyclin component of the Pho80-Pho85 CDK complex. See Pho85 description. | PHO80 (CAGL0E02541g) 53% | PHO80 (C6_03810W_A) 49% | PHO80 (CNAG_01922) 34% | phoB (Afu1g07070) 48% | PHO80 is induced in Cn following Pi starvation. |
| PHO85 | Cyclin dependent kinase of the Pho80-Pho85 CDK complex. Phosphorylates Pho4 under phosphate replete conditions preventing nuclear accumulation. | PHO85 (CAGL0L12474g) 89% | PHO85 (C1_04520C_A) 67% | CMGC/CDK/CDK5 (CNAG_08022) 60% | phoA Afu5g04130 68% | PHO85 is induced in Cn following Pi starvation. |
| PHO81 | CDK inhibitor that counteracts Pho85-Pho80 activity in low phosphate conditions. Induced following Pi starvation. | PHO81 (CAGL0L06622g) 42% | PHO81 (CR_00590W_A) 37% | PHO81 (CNAG_02541) 29% | phoC (Afu4g06020) 30% | |
| PHO84 | High affinity phosphate transporter. Loss of PHO84 results in constitutive activation of the PHO pathway but negligible phosphate uptake. | PHO84 (CAGL0B02475g) 77% | PHO84 (C1_11480W_A) 61% | PHO84 (CNAG_02777) 47% | phoD Afu2g10690 55% | Cn PHO84 displays functional redundancy with PHO840 and PHO89. Possible functional redundancy between Af phoD and other Pi transporters. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeh, M.; Ahmed, Y.; Quinn, J. Phosphate Acquisition and Virulence in Human Fungal Pathogens. Microorganisms 2017, 5, 48. https://doi.org/10.3390/microorganisms5030048
Ikeh M, Ahmed Y, Quinn J. Phosphate Acquisition and Virulence in Human Fungal Pathogens. Microorganisms. 2017; 5(3):48. https://doi.org/10.3390/microorganisms5030048
Chicago/Turabian StyleIkeh, Mélanie, Yasmin Ahmed, and Janet Quinn. 2017. "Phosphate Acquisition and Virulence in Human Fungal Pathogens" Microorganisms 5, no. 3: 48. https://doi.org/10.3390/microorganisms5030048




