Paralytic Shellfish Toxins and Cyanotoxins in the Mediterranean: New Data from Sardinia and Sicily (Italy)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dinoflagellates
2.1.1. Sampling and Cell Cultures
2.1.2. Morphological Analysis
2.1.3. Toxin Analysis
2.1.4. Biomolecular Analysis
2.2. Cyanobacteria
2.2.1. Sampling
2.2.2. Cyanobacterial Abundance and Species Composition in Natural Samples
2.2.3. Cell Cultures
2.2.4. Toxin Determination
3. Results
3.1. Dinoflagellates
3.1.1. Morphology of Alexandrium Strains
3.1.2. Toxins of Alexandrium Strains
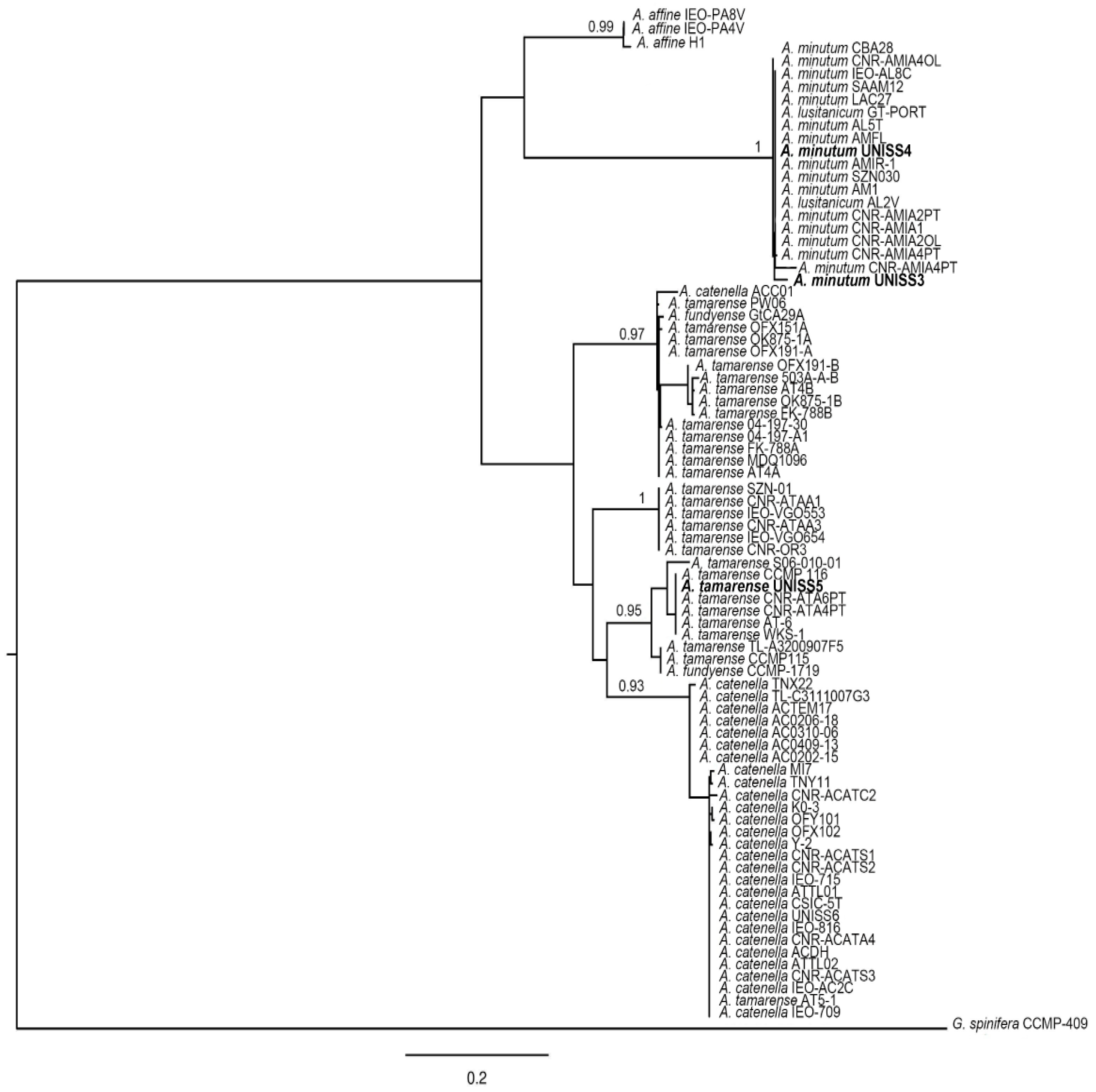
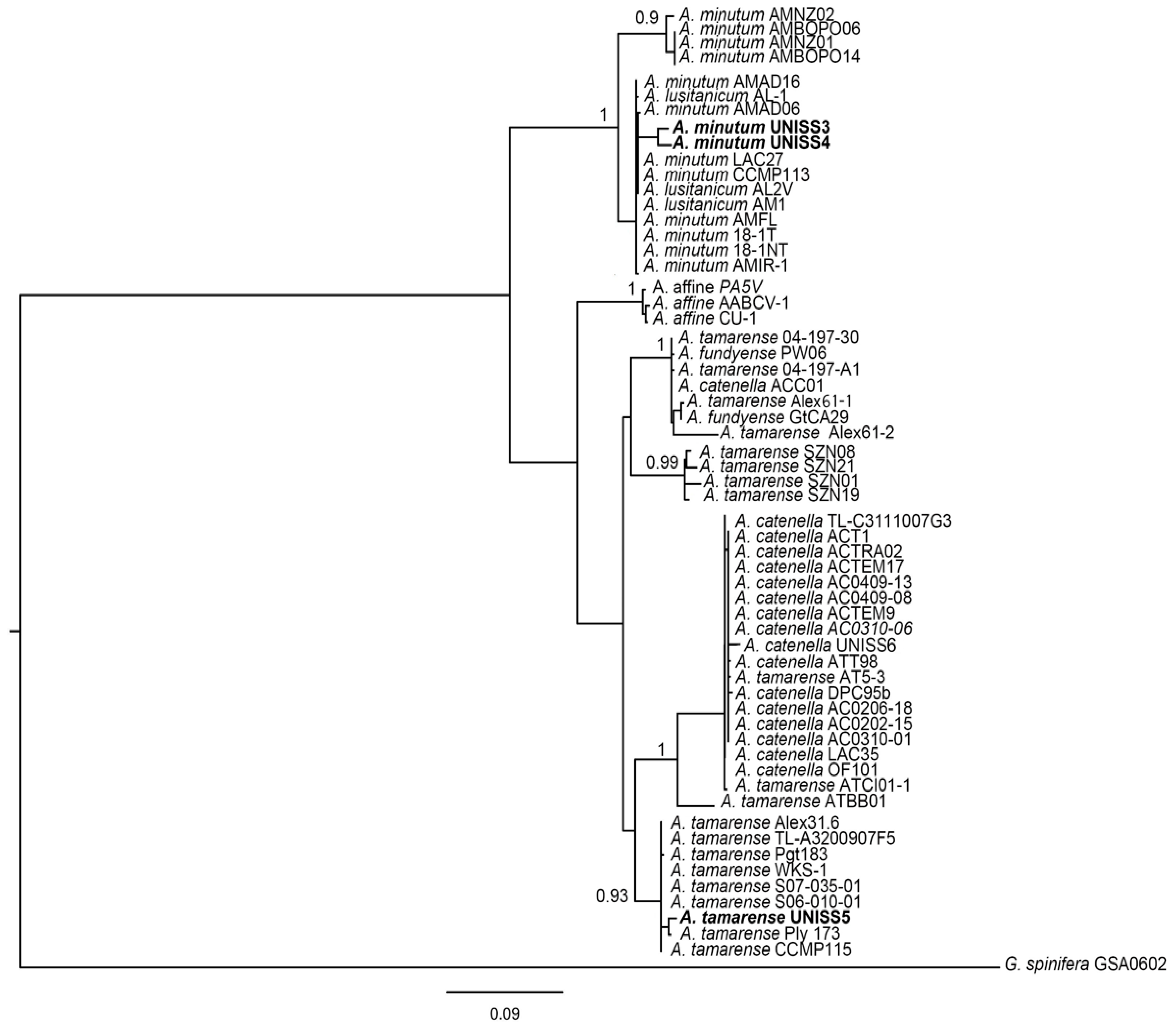
3.1.3. Genetics of Alexandrium Strains
3.2. Cyanobacteria
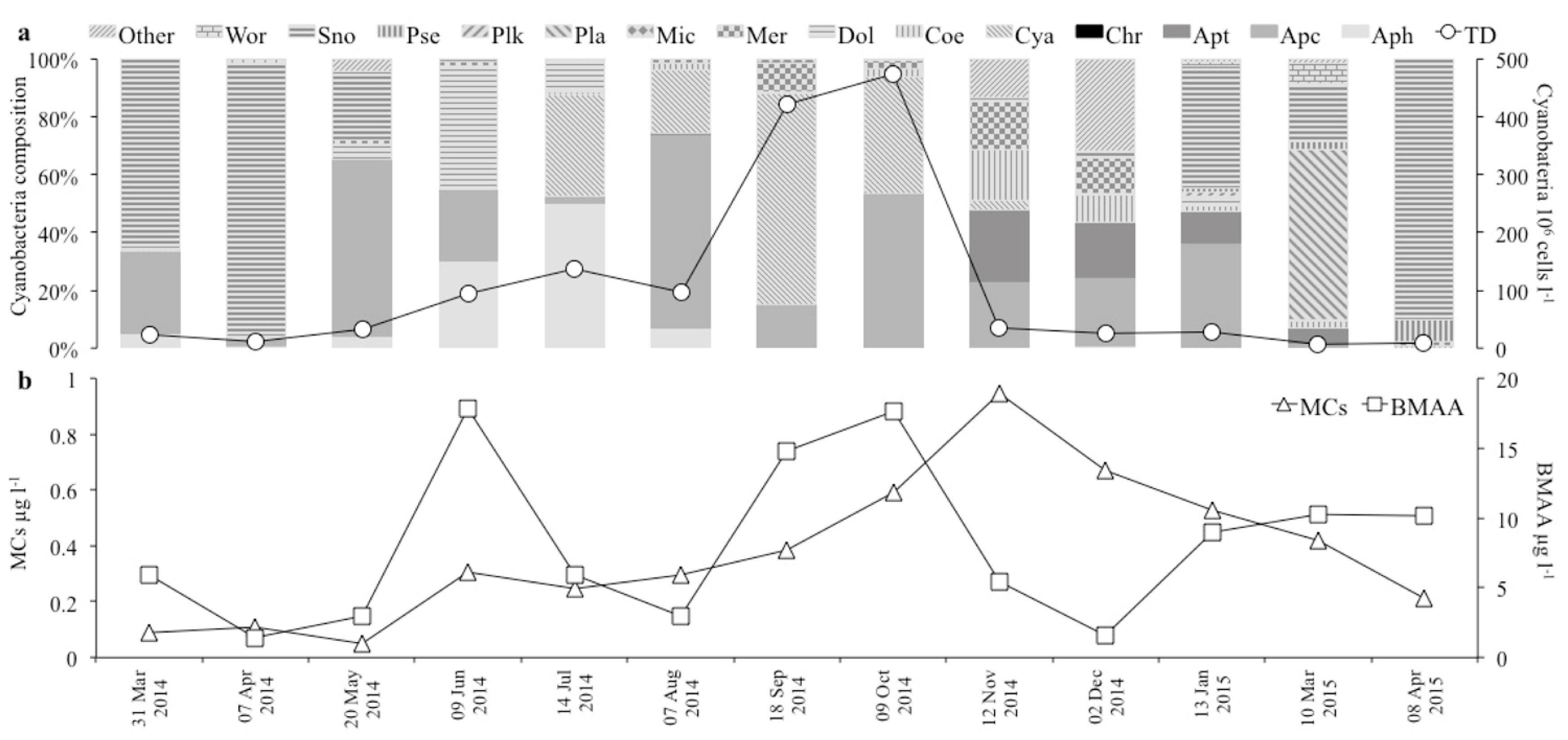
3.2.1. Species Composition and Cell Abundance
3.2.2. Toxins and Dominant Species
4. Discussion
4.1. Dinoflagellates
4.2. Cyanobacteria
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Glibert, P.M.; Anderson, D.M.; Gentien, P.; Granéli, E.; Sellner, K.G. The Global, Complex Phenomena of Harmful Algal Blooms. Oceanography 2005, 18, 130–141. [Google Scholar] [CrossRef]
- Masó, M.; Garcés, E. Harmful microalgae blooms (HAB): Problematic and conditions that induce them. Mar. Pollut. Bull. 2006, 53, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Smayda, T.J. Harmful phytoplankton blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42, 137–153. [Google Scholar] [CrossRef]
- Zingone, A.; Enevoldsen, H.O. The diversity of harmful algal blooms: A challenge for science and management. Ocean Coast. Manag. 2000, 43, 725–748. [Google Scholar] [CrossRef]
- Hoagland, P.; Anderson, D.M.; Kaoru, Y.; White, A.W. The Economic Effects of Harmful Algal Blooms in the United States: Estimates, Assessment Issues, and Information Needs. Estuaries 2002, 25, 819–837. [Google Scholar] [CrossRef]
- Morgan, K.L.; Larkin, S.L.; Adams, C.M. Firm-level economic effects of HABS: A tool for business loss assessment. Harmful Algae 2009, 8, 212–218. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.N.; Deplege, M.H.; Fleming, L.; Hess, P.; Lees, D.; Leonard, P.; Madsen, L.; Owen, R.; Pirlet, H.; Seys, J.; et al. Oceans and Human Health (OHH): A European Perspective from the Marine Board of the European Science Foundation (Marine Board-ESF). Microb. Ecol. 2013, 65, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Sciacca, S.; Fallico, R.; Fiore, M.; Oliveri Conti, G.; Ledda, C. Harmful Algal Blooms in the Mediterranean Sea: Effects on Human Health. Sci. Rep. 2013, 2, 587. [Google Scholar] [CrossRef]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, R.; Misner, I.; Tomas, C.; Wright, J. Occurrence of 12-methylgymnodimine in a spirolide-producing dinoflagellate Alexandrium peruvianum and the biogenetic implications. Tetrahedron Lett. 2011, 52, 4243–4246. [Google Scholar] [CrossRef]
- Martens, H.; Tillmann, U.; Harju, K.; Dell’Aversano, C.; Tartaglione, L.; Krock, B. Toxin Variability Estimations of 68 Alexandrium ostenfeldii (Dinophyceae) Strains from The Netherlands Reveal a Novel Abundant Gymnodimine. Microorganisms 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic Alkaloids: Saxitoxin and Its Analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Batoréu, M.C.C.; Dias, E.; Pereira, P.; Franca, S. Risk of human exposure to paralytic toxins of algal origin. Environ. Toxicol. Pharmacol. 2005, 19, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, A.M.; Caddeo, T.; Pulina, S.; Padedda, B.M.; Satta, C.T.; Sechi, N.; Lugliè, A. Spatial distribution and multiannual trends of potentially toxic microalgae in shellfish farms along the Sardinian coast (NW Mediterranean Sea). Environ. Monit. Assess. 2015, 187, 86. [Google Scholar] [CrossRef] [PubMed]
- Pistocchi, R.; Guerrini, F.; Pezzolesi, L.; Riccardi, M.; Vanucci, S.; Ciminiello, P.; Dell’Aversano, C.; Forino, M.; Fattorusso, E.; Tartaglione, L.; et al. Toxin levels and profiles in microalgae from the North-Western Adriatic Sea—15 Years of studies on cultured species. Mar. Drugs 2012, 10, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Zingone, A.; Siano, R.; D’Alelio, D.; Sarno, D. Potentially toxic and harmful microalgae from coastal waters of the Campania region (Tyrrhenian Sea Mediterranean Sea). Harmful Algae 2006, 5, 321–337. [Google Scholar] [CrossRef]
- Lugliè, A.; Satta, C.T.; Pulina, S.; Bazzoni, A.M.; Padedda, B.M.; Sechi, N. Harmful algal blooms in Sardinia. Biol. Mar. Med. 2011, 18, 2–9. [Google Scholar]
- Vila, M.; Giacobbe, M.G.; Masó, M.; Gangemi, E.; Penna, A.; Sampedro, N.; Azzaro, F.; Camp, J.; Galluzzi, L. A comparative study on recurrent blooms of Alexandrium minutum in two Mediterranean coastal areas. Harmful Algae 2005, 4, 673–695. [Google Scholar] [CrossRef]
- Welker, M.; von Döhren, H. Cyanobacterial peptides—Nature’s own combinatorial biosynthesis. FEMS Microbiol. Rev. 2006, 30, 530–563. [Google Scholar] [CrossRef] [PubMed]
- Ersmark, K.; Del Valle, J.R.; Hanessian, S. Chemistry and biology of the aeruginosin family of serine protease inhibitors. Angew. Chem. Int. Ed. Eng. 2008, 47, 1202–1223. [Google Scholar] [CrossRef] [PubMed]
- Ferranti, P.; Fabbrocino, S.; Cerulo, M.G.; Bruno, M.; Serpe, L.; Gallo, P. Characterization of biotoxins produced by a cyanobacteria bloom in Lake Averno using two LC–MS-based techniques. Food Addit. Contam. 2008, 25, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Gácsi, M.; Antal, O.; Vasas, G.; Máthé, C.; Borbély, G.; Saker, M.L.; Gyori, J.; Farkas, A.; Vehovszky, A.; Bánfalvi, G. Comparative study of cyanotoxins affecting cytoskeletal and chromatin structures in CHO-K1 cells. Toxicol. In Vitro 2009, 23, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Valério, E.; Chaves, S.; Tenreiro, R. Diversity and Impact of Prokaryotic Toxins on Aquatic Environments: A Review. Toxins 2010, 2, 2359–2410. [Google Scholar] [CrossRef] [PubMed]
- Faasen, E.J. Presence of the Neurotoxin BMAA in Aquatic Ecosystems: What Do We Really Know? Toxins 2014, 6, 1109–1138. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-N-methylamino-l-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Kostrzewa, R.M.; Guillemin, G.J. BMAA and Neurodegenerative Illness. Neurotox. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, O.; Berg, C.; Brittebo, E.B.; Lindquist, N.G. Retention of the cyanobacterial neurotoxin β-N-methylamino-l-alanine in melanin and neuromelanin-containing cells: A possible link between Parkinson-dementia complex and pigmentary retinopathy. Pigment Cell Melanoma Res. 2008, 22, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Maidana, M.; Carlis, V.; Galhardi, F.G.; Junes, J.S.; Geracitano, L.A.; Monserrat, J.M.; Barros, D.M. Effects of microcystins over short- and long-term memory and oxidative stress generation in hippocampus of rats. Chem. Biol. Interact. 2006, 159, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco, D.; Moreno, E.; Sanchis, D.; Wörmer, L.; Paniagua, T.; Del Cueto, A.; Quesada, A. Cyanobacterial abundance and microcystin occurrence in Mediterranean water reservoirs in Central Spain: Microcystins in the Madrid area. Eur. J. Phycol. 2006, 41, 281–291. [Google Scholar] [CrossRef]
- Messineo, V.; Bogialli, S.; Melchiorre, S.; Sechi, N.; Lugliè, A.; Casiddu, P.; Mariani, M.A.; Padedda, B.M.; Di Corcia, A.; Mazza, R.; et al. Cyanotoxins occurrence in Italian freshwaters. Limnologica 2009, 39, 95–106. [Google Scholar] [CrossRef]
- Falconer, I.R.; Humpage, A.R. Health Risk Assessment of Cyanobacterial (Blue-Green Algal) Toxins in Drinking Water. Int. J. Environ. Res. Public Health 2006, 2, 43–50. [Google Scholar] [CrossRef]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.A.; Padedda, B.M.; Kaštovský, J.; Buscarinu, P.; Sechi, N.; Virdis, T.; Lugliè, A. Effects of trophic status on microcystin production and the dominance of cyanobacteria in the phytoplankton assemblage of Mediterranean reservoirs. Sci. Rep. 2015, 5, 17964. [Google Scholar] [CrossRef] [PubMed]
- Prioli, G. La molluschicoltura in Italia. In Estado Actual del Cultivo y Manejo de Moluscos Bivalvos y su Proyección Futura: Factores Que Afectan su Sustentabilidad en América Latina; Lovatelli, A., Farías, A., Uriarte, I., Eds.; FAO Actas de Pesca y Acuicultura: Roma, Italy, 2008; No. 12; pp. 159–176. [Google Scholar]
- Balech, E. The Genus Alexandrium Halim (Dinoflagellata), 1st ed.; Sherkin Island Marine Station: Cork, Ireland, 1995; pp. 1–151. ISBN 1-870492-61-7. [Google Scholar]
- Fritz, L.; Triemer, R.E. A rapid simple technique utilizing Calcofluor White M2R for the visualization of dinoflagellate thecal plates. J. Phycol. 1985, 21, 662–664. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C. Quantitative Determination of Paralytic Shellfish Poisoning Toxins in Shellfish Using Pre-Chromatographic Oxidation and Liquid Chromatography with Fluorescence Detection. J. AOAC Int. 2005, 88, 1714–1732. [Google Scholar] [PubMed]
- Sullivan, J.J.; Wekell, M.M. Determination of paralytic shellfish poisoning toxins by high pressure liquid chromatography. In Seafood Toxins; Ragelis, E.P., Ed.; ACS Symposium Series 262; American Chemical Society: Washington DC, USA, 1984; pp. 197–205. [Google Scholar]
- Thielert, G.; Kaiser, I.; Luckas, B. HPLC determination of PSP toxins. In Proceedings of the Symposium on Marine Biotoxins, Paris, France, 30–31 January 1991; Frémy, J.M., Ed.; Editions CNEVA: Maison-Alfort, France, 1991; pp. 121–125. [Google Scholar]
- Oshima, Y. Postcolumn Derivatization Liquid Chromatographic Method for Paralytic Shellfish Toxins. J. AOAC Int. 1985, 78, 528–532. [Google Scholar]
- Quilliam, M.A.; Janecek, M.; Lawrence, J.F. Characterisation of the oxidation products of paralytic shellfish poisoning toxins by liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 1993, 7, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.F.; Ménard, C. Liquid Chromatographic Determination of Paralytic Shellfish Poisons in Shellfish after Prechromatographic Oxidation. J. Assoc. Off. Anal. Chem. 1991, 74, 1006–1012. [Google Scholar] [PubMed]
- Stacca, D. Molecular Characterization of Harmful Algal Species. Ph.D. Thesis, University of Sassari, Sardinia, Italy, November 2013. [Google Scholar]
- Penna, A.; Fraga, S.; Masó, M.; Giacobbe, M.G.; Bravo, I.; Garcés, E.; Vila, M.; Bertozzini, E.; Andreoni, F.; Lugliè, A.; et al. Phylogenetic relationships among the Mediterranean Alexandrium (Dinophyceae) species based on sequences of 5.8S gene and Internal Transcript Spacers of the rRNA operon. Eur. J. Phycol. 2008, 43, 163–178. [Google Scholar] [CrossRef]
- Penna, A.; Perini, F.; Dell’Aversano, C.; Capellacci, S.; Tartaglione, L.; Giacobbe, M.G.; Casabianca, S.; Fraga, S.; Ciminiello, P.; Scardi, M. The sxt Gene and Paralytic Shellfish Poisoning Toxins as Markers for the Monitoring of Toxic Alexandrium Species Blooms. Environ. Sci. Technol. 2015, 49, 14230–14238. [Google Scholar] [CrossRef] [PubMed]
- Penna, A.; Bertozzini, E.; Battocchi, C.; Galluzzi, L.; Giacobbe, M.G.; Vila, M.; Garcés, E.; Lugliè, A.; Magnani, M. Monitoring of HAB species in the Mediterranean Sea through molecular methods. J. Plank. Res. 2007, 29, 19–38. [Google Scholar] [CrossRef]
- Kai, A.K.L.; Cheung, Y.K.; Yeung, P.K.K.; Wong, J.T.Y. Development of single-cell PCR methods for the Raphidophyceae. Harmful Algae 2006, 5, 649–657. [Google Scholar] [CrossRef]
- Scholin, C.A.; Herzog, M.; Sogin, M.; Anderson, D.M. Identification of group and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). 2. Sequence analysis of a fragment of the LSU rRNA gene. J. Phycol. 1994, 30, 999–1011. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications, 1st ed.; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Orlando, FL, USA, 1989; pp. 315–322. [Google Scholar]
- Ki, J.S.; Han, M.S. Informative characteristics of 12 divergent domain in complete large subunit rDNA sequences from the Harmful Dinoflagellate Genus, Alexandrium (Dinophyceae). J. Eukaryot. Microbiol. 2007, 54, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML-VI-HPC: Maximum Likelihood-based Phylogenetic Analyses with Thousands of Taxa and Mixed Models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Skjæveland, A.; Russel, J.S.; Enger, P.; Ruden, T.; Mevik, B.H.; Burki, F.; Botnen, A.; Shalchian-Tabrizi, K. AIR: A batch-oriented web program package for construction of supermatrices ready for phylogenomic analyses. BMC Bioinform. 2009, 10, 357–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utermhöl, H. Zur vervollkhung der quantitativen phytoplanktonmethodik. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Marchetto, A.; Padedda, B.M.; Mariani, M.A.; Lugliè, A.; Sechi, N. A numerical index for evaluating phytoplankton response to changes in nutrient levels in deep mediterranean reservoirs. J. Limnol. 2009, 68, 106–121. [Google Scholar] [CrossRef]
- Komárek, J.; Komárková, J. Review of the European Microcystis morphospecies (Cyanoprokaryotes) from nature. Fottea 2002, 2, 1–24. [Google Scholar]
- Komarek, J.; Zapomelova, E. Planktic morphospecies of the cyanobacterial genus Anabaena = subg. Dolichospermum-1. part: Coiled types. Fottea 2007, 7, 1–31. [Google Scholar] [CrossRef]
- Komarek, J.; Zapomelova, E. Planktic morphospecies of the cyanobacterial genus Anabaena = subg. Dolichospermum-2. part: Straight types. Fottea 2008, 8, 1–14. [Google Scholar] [CrossRef]
- Suda, S.; Watanabe, M.M.; Otsuka, S.; Mahakahant, A.; Yongmanitchai, W.; Nopartnaraporn, N.; Liu, Y.; Day, J.G. Taxonomic revision of water-bloom-forming species of oscillatorioid cyanobacteria. Int. J. Syst. Evol. Microbiol. 2002, 52, 1577–1595. [Google Scholar] [CrossRef] [PubMed]
- Poikane, S. Water Framework Directive Intercalibration Technical Report. Part 2: Lakes. Available online: http://publications.jrc.ec.europa.eu/repository/bitstream/JRC51340/3009_08-vol-lakes-cover.pdf (accessed on 14 November 2017).
- Faassen, E.J.; Beekman, W.; Lurling, M. Evaluation of a Commercial Enzyme Linked Immunosorbent Assay (ELISA) for the Determination of the Neurotoxin BMAA in Surface Waters. PLoS ONE 2013, 8, e84578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clausi, M.T.; Vita, V.; Bruno, M.; Franchino, C.; Trifirò, G.; Palumbo, M.P.; Floridi, F.; De Pace, R. Validation of ELISA methods for search and quantification of β-N-methylamino-l-alanine in water and fish tissue. Int. J. Environ. Anal. Chem. 2016, 96, 1290–1299. [Google Scholar] [CrossRef]
- Lugliè, A.; Giacobbe, M.G.; Sannio, A.; Fiocca, F.; Sechi, N. First record of the dinoflagellate Alexandrium catenella (Whedon & Kofoid) Balech (Dinophyta), a potential producer of paralytic shellfish poisoning, in Italian waters (Sardinia, Tyrrhenian Sea). Bocconea 2003, 16, 1045–1052. [Google Scholar]
- Vila, M.; Camp, J.; Garcés, E.; Masó, M.; Delgado, M. High resolution spatio-temporal detection of potentially harmful dinoflagellates in confined waters of the NW Mediterranean. J. Plankton Res. 2001, 23, 497–514. [Google Scholar] [CrossRef]
- Vila, M.; Garcés, E.; Masó, M.; Camp, J. Is the distribution of the toxic dinoflagellate Alexandrium catenella expanding along the NW Mediterranean coast. Mar. Ecol. Prog. Ser. 2001, 222, 73–83. [Google Scholar] [CrossRef]
- Bravo, I.; Garces, E.; Diogene, J.; Fraga, S.; Sampedro, N.; Figueroa, R.I. Resting cysts of the toxigenic dinoflagellate genus Alexandrium in recent sediments from the Western Mediterranean Coast, including the first description of cysts of A. kutnerae and A. peruvianum. Eur. J. Phycol. 2006, 41, 293–302. [Google Scholar] [CrossRef]
- Bravo, I.; Vila, M.; Masó, M.; Figueroa, R.I.; Ramilo, I. Alexandrium catenella and Alexandrium minutum blooms in the Mediterranean Sea: Toward the identification of ecological niches. Harmful Algae 2008, 7, 515–522. [Google Scholar] [CrossRef]
- Giacobbe, M.G.; Masó, M.; Milandri, A.; Penna, A.; Poletti, R. Plankton toxicity and shellfish contamination by phycotoxins in a new Mediterranean locality. In Proceedings of the 5th International Conference on Molluscan Shellfish Safety, Galway, Ireland, 14–18 June 2004; Henshilwood, K., Deegan, B., McMahon, T., Cusack, C., Keaveney, S., Silke, J., O’Cinneide, M., Lyons, D., Hess, P., Eds.; Marine Institute: Galway, Ireland, 2006. [Google Scholar]
- Garcés, E.; Bravo, I.; Vila, M.; Figueroa, R.I.; Masó, M.; Sampedro, N. Relationship between vegetative cells and cyst production during Alexandrium minutum bloom in Arenys de mar harbour (NW mediterranean). J. Plankton Res. 2004, 26, 1–9. [Google Scholar] [CrossRef]
- Anglès, S.; Garceś, E.; Reñé, A.; Sampedro, N. Life-cycle alternations in Alexandrium minutum natural populations from the NW Mediterranean Sea. Harmful Algae 2012, 16, 1–11. [Google Scholar] [CrossRef]
- Giacobbe, M.G.; Azzaro, F.; Decembrini, F.; Galletta, M.; Gangemi, E.; Raffa, F.; Ceredi, A.; Milandri, A.; Poletti, R.; Masó, M. Toxic blooms of Alexandrium minutum Dinoflagellate in a coastal area of the Ionian Sea. Biol. Mar. Medit. 2004, 11, 703–707. [Google Scholar]
- Taylor, F.J.R.; Fukuyo, Y.; Larsen, J.; Hallegraeff, G.M. Taxonomy of harmful dinoflagellates. In Manual on Harmful Marine Microalgae, Monographs on Oceanographic Methodology; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; Unesco: Paris, France, 2003; Volume 3, pp. 389–432. ISBN 92-3-103871-0. [Google Scholar]
- Adachi, M.; Sako, Y.; Ishida, Y. Identification of the toxic dinoflagellates Alexandrium catenella and A. tamarense (Dinophyceae) using DNA probes and whole-cell hybridization. J. Phycol. 1996, 32, 1049–1052. [Google Scholar] [CrossRef]
- Scholin, C.A.; Hallegraeff, G.; Anderson, D.M. Molecular evolution of the Alexandrium tamarense ‘species complex’ (Dinophyceae): Dispersal in the North American and West Pacific regions. Phycologia 1995, 34, 472–485. [Google Scholar] [CrossRef]
- John, U.; Medlin, L.K.; Groben, R. Development of specific rRNA probes to distinguish between geographic clades of the Alexandrium tamarense species complex. J. Plankton Res. 2005, 27, 199–204. [Google Scholar] [CrossRef]
- Masseret, E.; Grzebyk, D.; Nagai, S.; Genovesi, B.; Lasserre, B.; Laabir, M.; Collos, Y.; Vaquer, A.; Berrebi, P. Unexpected genetic diversity among and within populations of the toxic dinoflagellate Alexandrium catenella as revealed by nuclear microsatellite markers. Appl. Environ. Microbiol. 2009, 75, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Orr, R.J.S.; Stüken, A.; Rundberget, T.; Eikrem, W.; Jakobsen, K.S. Improved phylogenetic resolution of toxic and non-toxic Alexandrium strains using a concatenated rDNA approach. Harmful Algae 2001, 10, 676–688. [Google Scholar] [CrossRef]
- John, U.; Litaker, W.; Montresor, M.; Murray, S.; Brosnahan, M.L.; Anderson, D.M. Proposal to reject the name Alexandrium catenella (Dinophyceae). Taxon 2014, 63, 932–933. [Google Scholar] [CrossRef] [PubMed]
- John, U.; Litaker, R.W.; Montresor, M.; Murray, S.; Brosnahan, M.L.; Anderson, D.M. Formal Revision of the Alexandrium tamarense Species Complex (Dinophyceae) Taxonomy: The Introduction of Five Species with Emphasis on Molecular-based (rDNA) Classification. Protist 2014, 165, 779–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraga, S.; Sampedro, N.; Larsen, J.; Moestrup, O.; Calado, A.J. Arguments against the proposal 2302 by John & al. to reject the name Gonyaulax catenella (Alexandrium catenella). Taxon 2015, 64, 634–635. [Google Scholar] [CrossRef]
- John, U.; Fensome, R.A.; Medlin, L.K. The application of a molecular clock based on molecular sequences and the fossil record to explain biogeographic distributions within the Alexandrium tamarense “species complex” (Dinophyceae). Mol. Biol. Evol. 2003, 20, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Lilly, E.L.; Halanych, K.M.; Anderson, D.M. Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae). J. Phycol. 2007, 43, 1329–1338. [Google Scholar] [CrossRef]
- Masselin, P.; Amzil, Z.; Abadie, E.; Nézan, E.; Le Bec, C.; Carreras, A.; Chiantella, C.; Truquet, P. Paralytic shellfish poisoning on the French Mediterranean coast in autumn 1998: Alexandrium “tamarense complex” (Dinophyceae) as causative agent. In Proceedings of the 9th International Conference on Harmful Algal Blooms, Hobart, Australia, 7–11 February 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2000. [Google Scholar]
- Milandri, A.; Cangini, M.; Costa, A.; Giacobbe, M.G.; Poletti, R.; Pompei, M.; Riccardi, E.; Rubini, S.; Virgilio, S.; Pigozzi, S. Caratterizzazione delle tossine PSP (Paralytic Shellfish Poisoning) in mitili raccolti in differenti aree marine italiane. Biol. Mar. Medit. 2008, 15, 38–41. [Google Scholar]
- Stüken, A.; Orr, R.J.S.; Kellmann, R.; Murray, S.A.; Neilan, B.A.; Jakobsen, K.S. Discovery of Nuclear-Encoded Genes for the Neurotoxin Saxitoxin in Dinoflagellates. PLoS ONE 2011, 6, e20096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orr, R.J.S.; Stüken, A.; Murray, S.A.; Jakobsen, K.S. Evolutionary acquisition and loss of saxitoxin biosynthesis in dinoflagellates: The second “core” gene, sxtG. Appl. Environ. Microbiol. 2013, 79, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Laabir, M.; Collos, Y.; Masseret, E.; Grzebyk, D.; Abadie, E.; Savar, V.; Sibat, M.; Amzil, Z. Influence of Environmental Factors on the Paralytic Shellfish Toxin Content and Profile of Alexandrium catenella (Dinophyceae) Isolated from the Mediterranean Sea. Mar. Drugs 2013, 11, 1583–1601. [Google Scholar] [CrossRef] [PubMed]
- Tatters, A.O.; Flewelling, L.J.; Fu, F.; Granholm, A.A.; Hutchins, D.A. High CO2 promotes the production of paralytic shellfish poisoning toxins by Alexandrium catenella from Southern California waters. Harmful Algae 2013, 30, 37–43. [Google Scholar] [CrossRef]
- Perini, F.; Galluzzi, L.; Dell’Aversano, C.; Iacovo, E.D.; Tartaglione, L.; Ricci, F.; Forino, M.; Ciminiello, P.; Penna, A. SxtA and sxtG gene expression and toxin production in the Mediterranean Alexandrium minutum (Dinophyceae). Mar. Drugs 2014, 12, 5258–5276. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.M.; Muñoz, M.G.; Contreras, A.M. Temperature as a factor regulating growth and toxin content in the dinoflagellate Alexandrium catenella. Harmful Algae 2006, 5, 762–769. [Google Scholar] [CrossRef]
- Hii, K.S.; Lim, P.T.; Kon, N.F.; Takana, Y.; Usup, G.; Leaw, C.P. Physiological and transcriptional responses to inorganic nutrition in a tropical Pacific strain of Alexandrium minutum: Implications for the saxitoxin genes and toxin production. Harmful Algae 2016, 56, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Van de Waal, D.; Eberlein, T.; John, U.; Wohlrab, S.; Rost, B. Impact of elevated pCO2 on paralytic shellfish poisoning toxin content and composition in Alexandrium tamarense. Toxicon 2014, 78, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Bricelj, B.M.; Shumway, S.E. Paralytic Shellfish Toxins in Bivalve Molluscs: Occurrence, Transfer Kinetics, and Biotransformation. Rev. Fish. Sci. 1998, 6, 315–383. [Google Scholar] [CrossRef]
- Krock, B.; Seguel, C.G.; Cembella, A.D. Toxin profile of Alexandrium catenella from the Chilean coast as determined by liquid chromatography with fluorescence detection and liquid chromatography coupled with tandem mass spectrometry. Harmful Algae 2007, 6, 734–744. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.; Wiese, M.; Stüken, A.; Brett, S.; Kellmann, R.; Hallegraeff, G.; Neilan, B. sxtA-based quantitive molecular assay to identify saxitoxin producing harmful algal blooms in marine waters. Appl. Environ. Microbiol. 2011, 77, 7050–7057. [Google Scholar] [CrossRef] [PubMed]
- Collos, Y.; Bec, B.; Jauzein, C.; Abadie, E.; Laugier, T.; Lautier, J.; Pastoureaud, A.; Souchu, P.; Vaquer, A. Oligotrophication and emergence of picocyanobacteria and a toxic dinoflagellate in Thau lagoon, southern France. J. Sea Res. 2009, 61, 68–75. [Google Scholar] [CrossRef]
- Boero, F.; Belmonte, G.; Fanelli, G.; Piraino, S.; Rubino, F. The continuity of living matter and the discontinuities of its constituents: Do plankton and benthos really exist? Trends Ecol. Evol. 1996, 11, 177–180. [Google Scholar] [CrossRef]
- Rubino, F.; Moscatello, S.; Saracino, O.D.; Fanelli, G.; Belmonte, G.; Boero, F. Plankton derived resting stages in marine coastal sediments along the Salento Peninsula (Apulia, South eastern Italy). Mar. Ecol. 2002, 23, 329–339. [Google Scholar] [CrossRef]
- Satta, C.T.; Anglès, S.; Lugliè, A.; Guillen, J.; Sechi, N.; Camp, J.; Garcés, E. Studies on dinoflagellate cyst assemblages in two estuarine Mediterranean bays: A useful tool for the discovery and mapping of harmful algal species. Harmful Algae 2013, 24, 65–79. [Google Scholar] [CrossRef]
- Satta, C.T.; Anglès, S.; Garcés, E.; Sechi, N.; Pulina, S.; Padedda, B.M.; Stacca, D.; Lugliè, A. Dinoflagellate cyst assemblages in surface sediments from three shallow Mediterranean lagoons (Sardinia, north western Mediterranean Sea. Estuar. Coast. 2014, 37, 646–663. [Google Scholar] [CrossRef]
- Satta, C.T.; University of Sassari. Personal communication, 2017.
- Tillmann, U.; Alpermann, T.L.; da Purificação, R.C.; Krock, B.; Cembella, A. Intra-population clonal variability in allelochemical potency of the toxigenic dinoflagellate Alexandrium tamarense. Harmful Algae 2009, 8, 759–769. [Google Scholar] [CrossRef]
- Zou, C.; Ye, R.; Zheng, J.; Luo, Z.; Gu, H.; Yang, W.; Li, H.; Liu, J. Molecular Phylogeny and PSP Toxin Profile of the Alexandrium tamarense Species Complex Along the Coast of China. Mar. Pollut. Bull. 2014, 89, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Hold, G.L.; Smith, E.A.; Birkbeck, T.H.; Gallacher, S. Comparison of paralytic shellfish toxin (PST) production by the dinoflagellates Alexandrium lusitanicum NEPCC 253 and Alexandrium tamarense NEPCC 407 in the presence and absence of bacteria. FEMS Microbiol. Ecol. 2001, 36, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.M.; Fernandez, P.; Reguera, B. Toxin profiles of natural populations and cultures of Alexandrium minutum Halim from Galician (Spain) coastal waters. J. Appl. Phycol. 1994, 6, 275–279. [Google Scholar] [CrossRef]
- Hansen, G.; Daugbjerg, N.; Franco, J.M. Morphology, toxin composition and LSU rDNA phylogeny of Alexandrium minutum (Dinophyceae) from Denmark, with some morphological observations on other European strains. Harmful Algae 2003, 2, 317–335. [Google Scholar] [CrossRef]
- Lilly, E.L.; Halanych, K.M.; Anderson, D.M. Phylogeny, biogeography, and species boundaries within the Alexandrium minutum group. Harmful Algae 2005, 4, 1004–1020. [Google Scholar] [CrossRef]
- Stüken, A.; Riobó, P.; Franco, J.; Jacobsen, K.S.; Guillou, L.; Figueroa, R.I. Paralytic shellfish toxin content is related to genomic sxtA4 copy number in Alexandrium minutum strains. Front. Microbiol. 2015, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W. Health effects of toxin-producing cyanobacteria: ‘The CyanoHABs’. Hum. Ecol. Risk Assess. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Codd, J.A. Cyanobacteria, neurotoxins and water resources: Are there implications for human neurodegenerative disease? Amyotroph. Lateral Scler. 2009, 10, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Codd, G.A. Cyanotoxins. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer-Verlag: Dordrecht, The Netherlands, 2012; pp. 651–675. [Google Scholar]
- Zegura, B.; Straser, A.; Filipic, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins: A review. Mutat. Res. 2011, 727, 16–41. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Bell, S.G.; Kaya, K.; Ward, C.J.; Beattie, K.A.; Metcalf, J.S. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 1999, 34, 405–415. [Google Scholar] [CrossRef]
- Vasconcelos, V. Eutrophication, toxic cyanobacteria and cyanotoxins: When ecosystems cry for help. Limnetica 2006, 25, 425–432. [Google Scholar]
- Codd, G.A.; Azevedo, S.M.F.O.; Bagchi, S.N.; Burch, M.D.; Carmichael, W.W.; Harding, W.R.; Kaya, K.; Utkilen, H.C. CYANONET: A Global Network for Cyanobacterial Bloom and Toxin Risk Manage; Unesco: Paris, France, 2005. [Google Scholar]
- Masseret, E.; Banack, S.; Boumédiène, F.; Abadie, E.; Brient, L.; Pernet, F.; Juntas-Morales, R.; Pageot, N.; Metcalf, J.; Cox, P.; et al. French Network on ALS Clusters Detection and Investigation. Dietary BMAA Exposure in an Amyotrophic Lateral Sclerosis Cluster from Southern France. PLoS ONE 2013, 8, e83406. [Google Scholar] [CrossRef] [PubMed]
- Réveillon, D.; Séchet, V.; Hess, P.; Amzil, Z. Systematic Detection of BMAA (β-N-Methylamino-l-Alanine) and DAB (2,4-Diaminobutyric Acid) in Mollusks Collected in Shellfish Production Areas Along the French Coasts. Toxicon 2015, 110, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Réveillon, D.; Séchet, V.; Hess, P.; Amzil, Z. Production of BMAA and DAB by diatoms (Phaeodactylum tricornutum, Chaetoceros sp., Chaetoceros calcitrans and, Thalassiosira pseudonana) and bacteria isolated from a diatom culture. Harmful Algae 2016, 58, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Banack, S.A.; Lindsay, J.; Morrison, L.F.; Cox, P.A.; Codd, G.A. Co-occurrence of β-N-methylamino-l-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990–2004. Environ. Microbiol. 2008, 10, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Caller, T.; Henegan, P.; Haney, J.; Murby, A.; Metcalf, J.S.; Powell, J.; Cox, P.A.; Stommel, E. Detection of cyanotoxins, β-N-methylamino-l-alanine and microcystins, from a lake surrounded by cases of amyotrophic lateral sclerosis. Toxins 2015, 7, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Hallegraef, G.M. Harmful algal booms: A global review. In Manual on Harmful Marine Microalgae, Monographs on Oceanographic Methodology; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; Unesco: Paris, France, 2003; Volume 3, pp. 25–49. ISBN 92-3-103871-0. [Google Scholar]
- Borghero, G.; Pugliatti, M.; Marrosu, F.; Marrosu, M.G.; Murru, M.R.; Floris, G.; Cannas, A.; Parish, L.D.; Occhineri, P.; Cau, T.B.; et al. ITALSGEN and SARDINALS Consortia. Genetic architecture of ALS in Sardinia. Neurobiol. Aging 2014, 35, 2882.e7–2882.e12. [Google Scholar] [CrossRef] [PubMed]
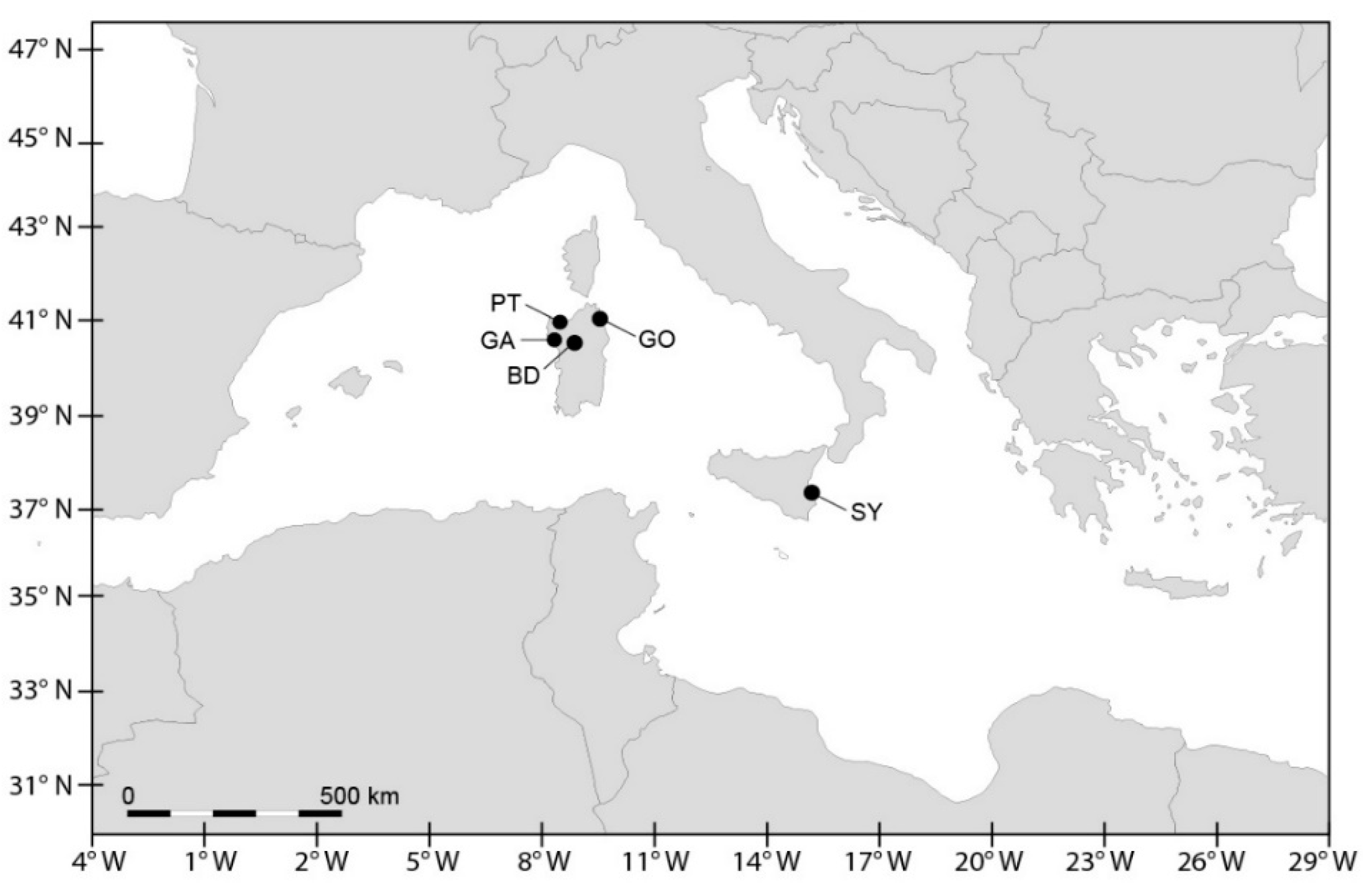
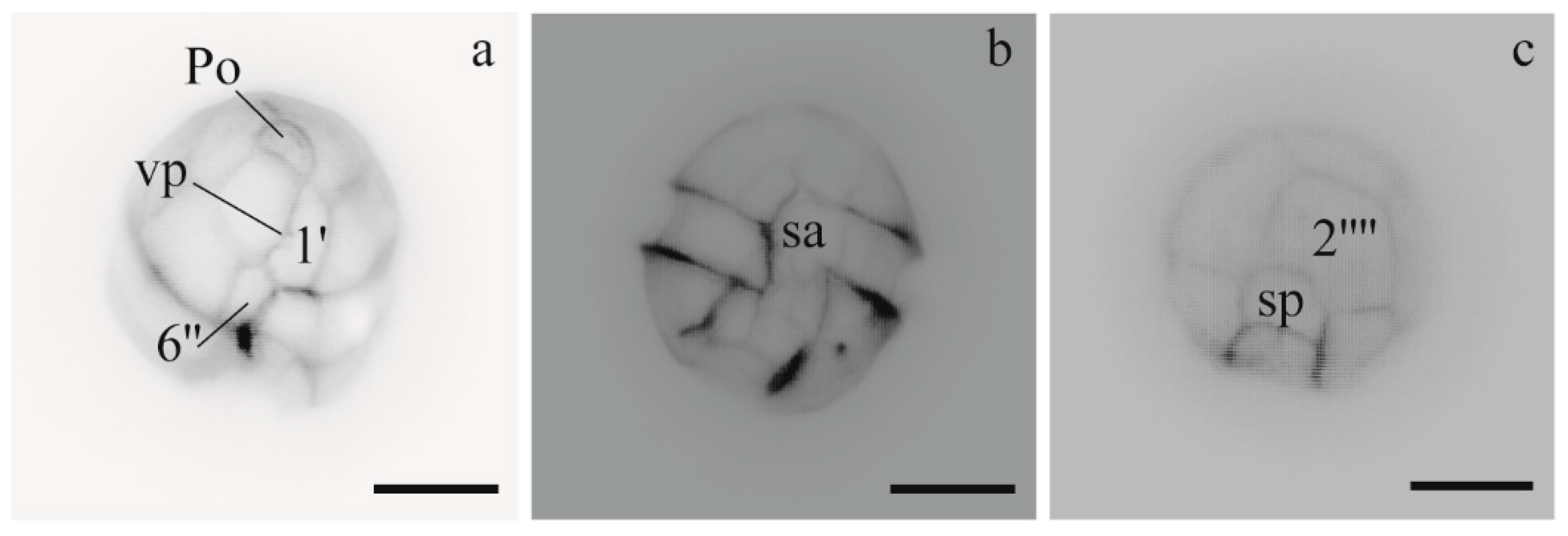
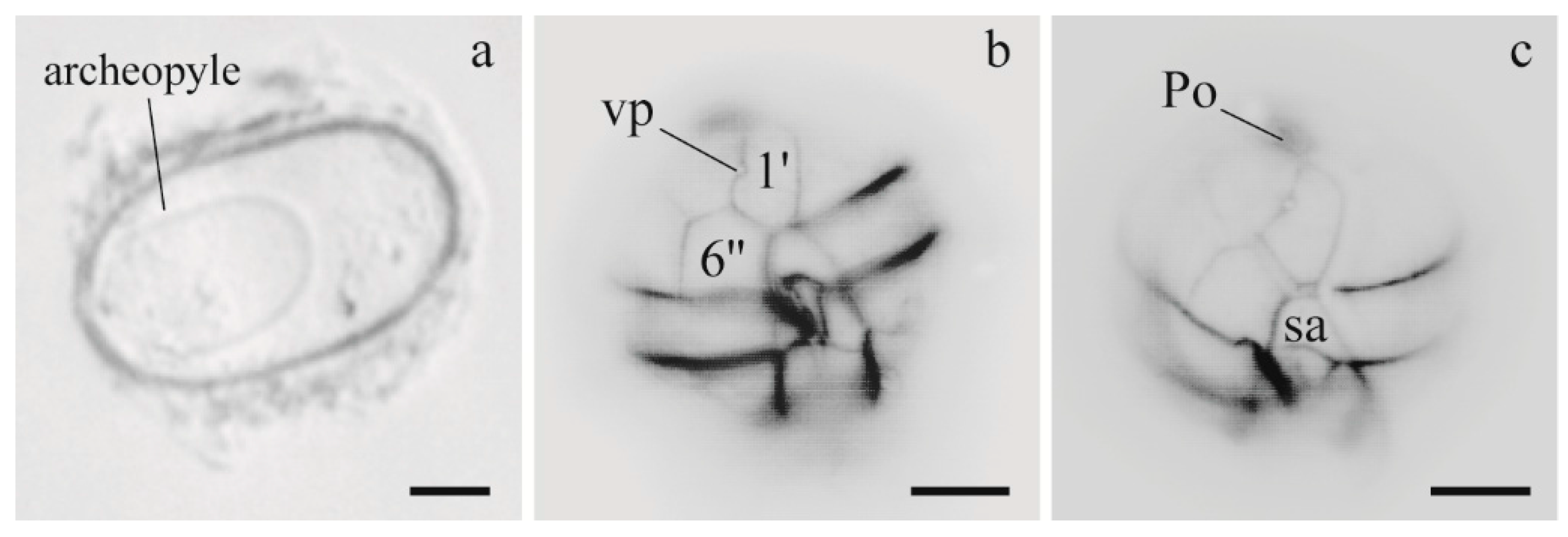
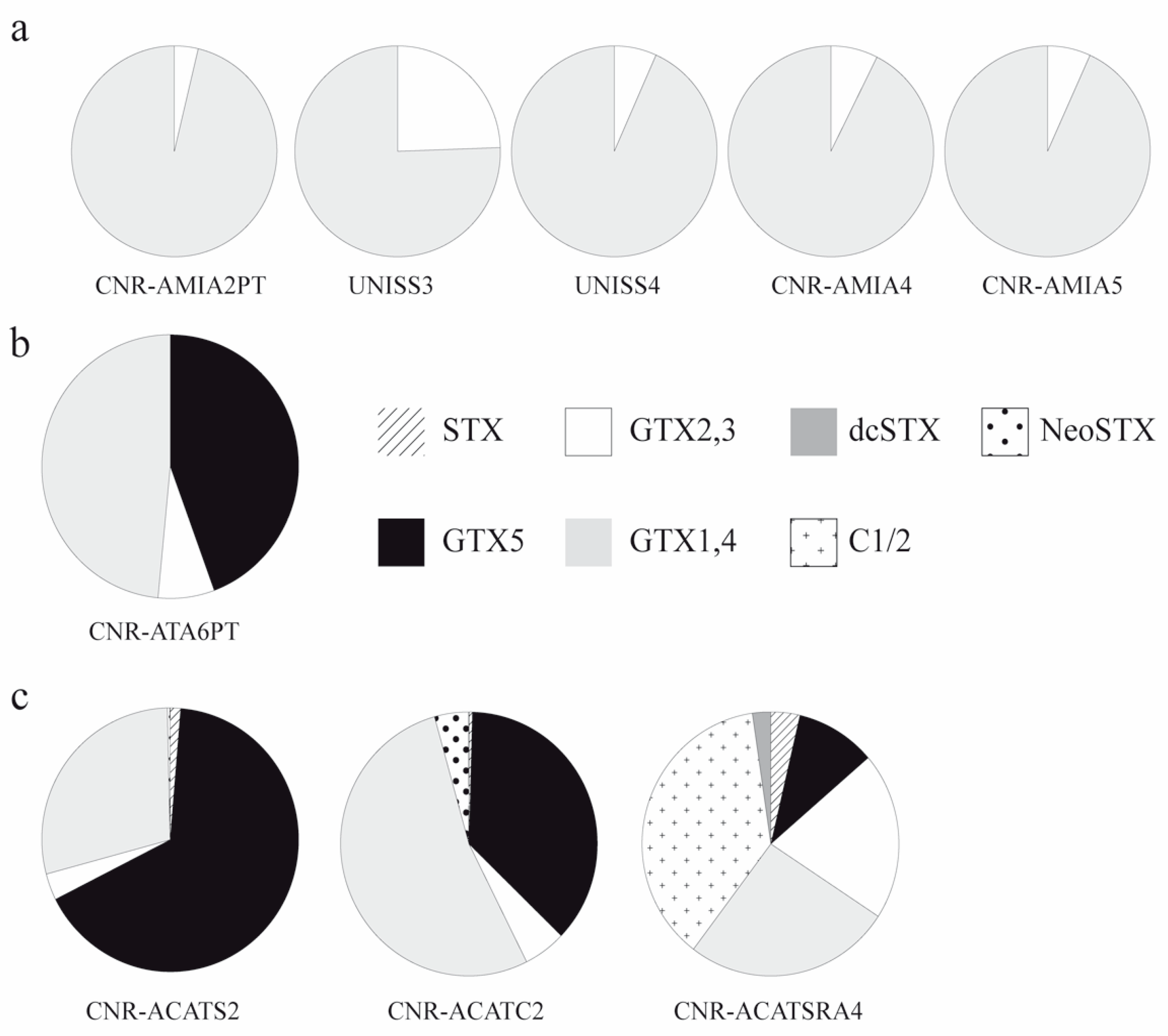
| Species | Strain | Geographic Origin | Year and Source of Culture | Code Site | PSP Toxicity | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Toxins | Morphology | Genetic | ||||||
| A. pacificum | CNR-ACATS2 | Gulf of Olbia, Tyrrhenian | 2002, vegetative cell | GO | yes | † | † | * |
| A. pacificum | CNR-ACATC2 | Gulf of Olbia, Tyrrhenian | 2002, vegetative cell | GO | yes | † | † | * |
| A. pacificum | CNR-ACATSRA4 | Syracuse harbour, Ionian | 2012, vegetative cell | SY | yes | † | † | ** |
| A. minutum | UNISS3 | Gulf of Olbia, Tyrrhenian | 2012, vegetative cell | GO | yes | †,§ | †,§ | †,§ |
| A. minutum | UNISS4 | Gulf of Olbia, Tyrrhenian | 2012, vegetative cell | GO | yes | †,§ | †,§ | †,§ |
| A. minutum | CNR-AMIA2PT | Porto Torres harbour, Asinara Gulf | 2002, vegetative cell | PT | yes | † | † | * |
| A. minutum | CNR-AMID6 | Syracuse harbour, Ionian | 2012, vegetative cell | SY | no | † | n.d. | ** |
| A. minutum | CNR-AMISY1 | Syracuse harbour, Ionian | 2012, vegetative cell | SY | no | † | n.d. | ** |
| A. minutum | CNR-AMIA4 | Syracuse harbour, Ionian | 2001, vegetative cell | SY | yes | † | # | # |
| A. minutum | CNR-AMIA5 | Syracuse harbour, Ionian | 2001, vegetative cell | SY | yes | † | # | # |
| A. tamarense | CNR-ATA6PT | Porto Torres harbour, Asinara Gulf | 2002, vegetative cell | PT | yes | † | † | ## |
| A. tamarense | UNISS5 | Gulf of Alghero, Sardinian | 2010, resting cyst | GA | no | †,§ | †,§ | †,§ |
| Strain | Code | Site | Toxins (fmol cell−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| STX | GTX5 | GTX2,3 | GTX1,4 | C1/2 | dcGTX2,3 | dcSTX | NeoSTX | Total | |||
| A. pacificum | CNR-ACATS2 | GO | 0.229 | 11.808 | 0.579 | 5.129 | --- | --- | --- | 0.066 | 17.811 |
| A. pacificum | CNR-ACATC2 | GO | 0.048 | 4.280 | 0.622 | 6.159 | --- | --- | --- | 0.505 | 11.614 |
| A. pacificum | CNR-ACATSRA4 * | SY | 0.008 | 0.022 | 0.045 | 0.057 | 0.082 | <LOD | 0.005 | <LOD | 0.219 |
| A. minutum | UNISS3 * | GO | <LOQ | <LOD | 0.004 | 0.013 | <LOD | <LOQ | <LOD | <LOD | 0.017 |
| A. minutum | UNISS4 * | GO | <LOQ | <LOQ | 0.001 | 0.018 | <LOD | <LOQ | <LOD | <LOD | 0.020 |
| A. minutum | CNR-AMIA2PT | GPT | <LOD | <LOD | 0.012 | 0.308 | --- | --- | --- | <LOD | 0.320 |
| A. minutum | CNR-AMID6 * | SY | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| A. minutum | CNR-AMISY1 * | SY | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| A. minutum | CNR-AMIA4 | SY | <LOD | <LOD | 0.008 | 0.100 | --- | --- | --- | <LOD | 0.108 |
| A. minutum | CNR-AMIA5 | SY | <LOD | <LOD | 0.007 | 0.096 | --- | --- | --- | <LOD | 0.103 |
| A. tamarense | CNR-ATA6PT | GPT | <LOD | 0.044 | 0.007 | 0.048 | --- | --- | --- | <LOD | 0.099 |
| A. tamarense | UNISS5 * | GA | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Taxa | 2014 | 2015 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| March | April | March | June | July | August | September | October | November | December | January | March | April | |
| Aphanizomenon flos-aquae | 0.00 | 0.09 | 1.32 | 28.14 | 68.78 | 6.98 | 0.00 | 0.00 | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 |
| Aphanizomenon sp. | 1.21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.18 | 0.08 | 0.05 | 0.00 | 0.00 | 0.00 |
| Aphanocapsa incerta | 0.00 | 0.00 | 8.92 | 5.47 | 0.00 | 0.00 | 37.65 | 223.62 | 0.54 | 0.00 | 0.00 | 0.00 | 0.00 |
| Aphanocapsa sp. | 6.70 | 0.27 | 10.86 | 17.81 | 2.82 | 65.24 | 26.17 | 30.71 | 7.41 | 5.92 | 10.09 | 0.10 | 0.00 |
| Aphanothece sp. | 0.00 | 0.00 | 0.00 | 0.00 | 0.34 | 0.38 | 0.00 | 0.00 | 8.46 | 4.62 | 3.02 | 0.38 | 0.00 |
| Chroococcus sp. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.02 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cyanocatena sp. | 0.00 | 0.00 | 0.00 | 0.00 | 48.12 | 21.02 | 308.14 | 190.01 | 1.24 | 0.00 | 0.00 | 0.00 | 0.00 |
| Coelosphaerium sp. | 0.00 | 0.00 | 0.12 | 0.40 | 0.92 | 2.44 | 1.17 | 14.90 | 6.13 | 2.46 | 0.59 | 0.13 | 0.07 |
| Dolichospermum flos-aquae | 0.00 | 0.13 | 1.58 | 39.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.69 | 0.00 | 0.00 |
| Dolichospermum planctonicum | 0.00 | 0.00 | 0.00 | 0.00 | 15.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Dolichospermum sp. | 0.00 | 0.00 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.05 | 0.01 |
| Dolichospermum spiroides | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Dolichospermum viguierii | 0.00 | 0.00 | 0.00 | 0.00 | 0.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Merismopedia punctata | 0.00 | 0.00 | 0.00 | 0.63 | 0.00 | 0.00 | 0.00 | 0.09 | 0.94 | 1.08 | 0.00 | 0.00 | 0.10 |
| Merismopedia tenuissima | 0.05 | 0.00 | 0.50 | 0.93 | 0.13 | 1.22 | 43.10 | 13.20 | 4.68 | 2.01 | 0.04 | 0.00 | 0.00 |
| Microcystis aeruginosa | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.26 | 0.99 | 0.00 | 0.12 | 0.15 | 0.00 | 0.00 |
| Microcystis sp. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 1.74 | 0.70 | 0.29 | 0.00 | 0.00 | 0.00 | 0.00 |
| Planktolyngbya sp. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.92 | 0.00 |
| Planktothrix sp. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.41 | 0.00 | 0.00 |
| Pseudanabaena sp. | 0.12 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.03 | 0.14 | 0.00 | 0.00 | 0.44 | 0.20 | 0.62 |
| Pseudanabaenaceae | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.61 | 7.81 | 0.26 | 0.09 | 0.00 |
| Snowella lacustris | 15.42 | 10.64 | 7.62 | 0.00 | 0.00 | 0.04 | 0.20 | 0.00 | 0.00 | 0.22 | 11.67 | 1.32 | 7.09 |
| Snowella sp. | 0.00 | 0.00 | 0.00 | 0.73 | 0.00 | 0.00 | 0.00 | 0.00 | 0.23 | 0.24 | 0.00 | 0.00 | 0.00 |
| Woronichinia compacta | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.42 | 0.29 | 0.00 |
| Woronichinia naegeliana | 0.00 | 0.18 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.12 | 0.00 |
| Woronichinia sp. | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.07 | 0.00 |
| Other Cyanophyceae | 0.00 | 0.00 | 1.39 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total abundance (cells × 106 L−1) | 23.5 | 11.3 | 32.5 | 93.2 | 136.6 | 97.4 | 421.6 | 474.6 | 34.6 | 24.7 | 27.8 | 6.7 | 7.9 |
| MCs (µg L−1) | 0.09 | 0.11 | 0.05 | 0.31 | 0.25 | 0.30 | 0.38 | 0.59 | 0.95 | 0.67 | 0.53 | 0.42 | 0.21 |
| BMAA (µg L−1) | 5.87 | 1.43 | 2.93 | 17.84 | 5.90 | 2.96 | 14.78 | 17.67 | 5.44 | 1.61 | 9.00 | 10.23 | 10.15 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugliè, A.; Giacobbe, M.G.; Riccardi, E.; Bruno, M.; Pigozzi, S.; Mariani, M.A.; Satta, C.T.; Stacca, D.; Bazzoni, A.M.; Caddeo, T.; et al. Paralytic Shellfish Toxins and Cyanotoxins in the Mediterranean: New Data from Sardinia and Sicily (Italy). Microorganisms 2017, 5, 72. https://doi.org/10.3390/microorganisms5040072
Lugliè A, Giacobbe MG, Riccardi E, Bruno M, Pigozzi S, Mariani MA, Satta CT, Stacca D, Bazzoni AM, Caddeo T, et al. Paralytic Shellfish Toxins and Cyanotoxins in the Mediterranean: New Data from Sardinia and Sicily (Italy). Microorganisms. 2017; 5(4):72. https://doi.org/10.3390/microorganisms5040072
Chicago/Turabian StyleLugliè, Antonella, Maria Grazia Giacobbe, Elena Riccardi, Milena Bruno, Silvia Pigozzi, Maria Antonietta Mariani, Cecilia Teodora Satta, Daniela Stacca, Anna Maria Bazzoni, Tiziana Caddeo, and et al. 2017. "Paralytic Shellfish Toxins and Cyanotoxins in the Mediterranean: New Data from Sardinia and Sicily (Italy)" Microorganisms 5, no. 4: 72. https://doi.org/10.3390/microorganisms5040072





