Core Sulphate-Reducing Microorganisms in Metal-Removing Semi-Passive Biochemical Reactors and the Co-Occurrence of Methanogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Bioreactors and Sampling
2.2. DNA Extraction, Amplification of SSU rRNA Genes and Pyrosequencing
2.3. Bioinformatics
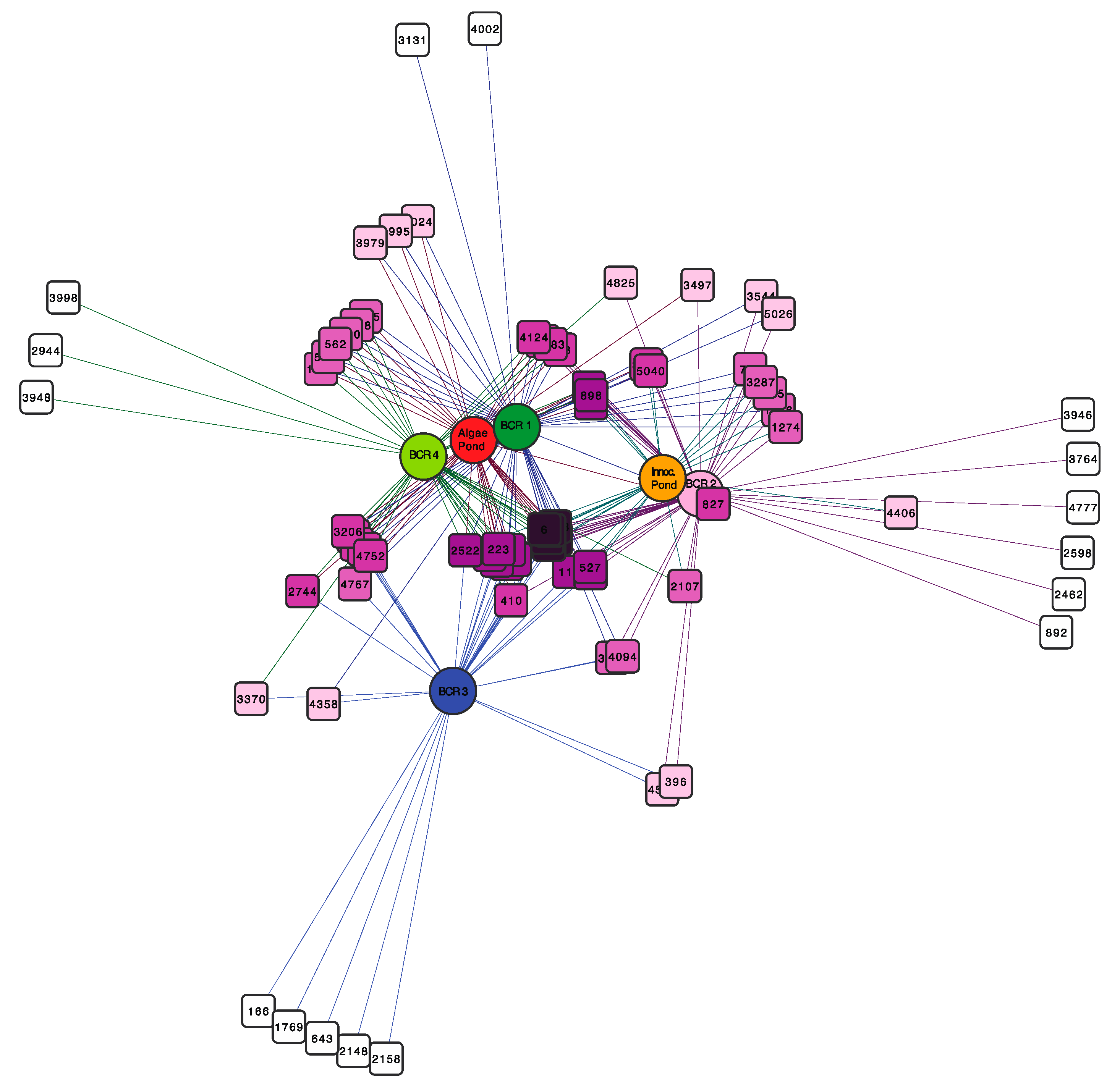
3. Results
3.1. Taxonomic and Phylogenetic Diversity
3.1.1. Overall Microbial Population Composition
3.1.2. Sulphate-Reducing Microorganisms
3.1.3. Methanogens
3.2. RelativeAabundances of SRM- and Methanogen-Related OTUs
4. Discussion
4.1. SRM Common to BCRs Treating Metal-Containing Effluents
4.2. Co-Occurrence or Competition with Methanogens
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wildeman, T.R.; Schmiermund, R. Mining influenced waters: Their chemistry and methods of treatment. In Proceedings of the 2004 National Meeting of the American Society of Mining and Reclamation and The 25th West Virginia Surface Mine Drainage Task Force, Lexington, KY, USA, 18–24 April 2004; pp. 2001–2013. [Google Scholar]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.J.; Scheeren, P.J.M.; Buisman, C.J.N. Microbial removal of heavy metals and sulfate from contaminated groundwaters. In Emerging Technology for Bioremediation of Metals; Means, J.L., Hinchee, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 1994; Volume 2; pp. 1–160. ISBN 978-1-5667-0085-6. [Google Scholar]
- Bratkova, S.; Koumanova, B.; Beschkov, V. Biological treatment of mining wastewaters by fixed-bed bioreactors at high organic loading. Bioresour. Technol. 2013, 137, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Kieu, H.T.Q.; Müller, E.; Horn, H. Heavy metal removal in anaerobic semi-continuous stirred tank reactors by a consortium of sulfate-reducing bacteria. Water Res. 2011, 45, 3863–3870. [Google Scholar] [CrossRef] [PubMed]
- Buisman, C.J.N.; Vellinga., S.H.J.; Janssen, G.H.R.; Dijkman, H. Biological sulfide production for metal recovery. In Proceedings of the 1999 TMS Congress: “Fundamentals Lead Zinc Extraction and Recycling”, San Diego, CA, USA, 28 February–4 March 1999. [Google Scholar]
- Gusek, J.J. Passive Treatment 101: An Overview of the Technologies. In Proceedings of the U.S. EPA/National Groundwater Association Remediation of Abandoned Mine Lands, Denver, CO, USA, 2–3 October 2008; pp. 1–13. [Google Scholar]
- Neculita, C.-M.; Zagury, G.J.; Bussière, B. Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria: Critical review and research needs. J. Environ. Qual. 2007, 36, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.S.; Pugh, C.W.; Segid, Y.T.; Behum, P.T.; Lefticariu, L.; Bender, K.S. Performance and microbial community dynamics of a sulfate-reducing bioreactor treating coal generated acid mine drainage. Biodegradation 2012, 23, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Mattes, A.; Evans, L.J.; Gould, D.W.; Duncan, W.F.A.; Glasauer, S. The long term operation of a biologically based treatment system that removes As, S and Zn from industrial (smelter operation) landfill seepage. Appl. Geochem. 2009, 26, 1886–1896. [Google Scholar] [CrossRef]
- Khoshnoodi, M.; Dipple, G.; Baldwin, S. Mineralogical Study of a Biologically-Based Treatment System that Removes Arsenic, Zinc and Copper from Landfill Leachate. Minerals 2013, 3, 427–449. [Google Scholar] [CrossRef]
- Muyzer, G.; Stams, A.J.M. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K.; Stackebrandt, E.; Hamilton, W.A. Energy metabolism and phylogenetic diversity of sulphate-reducing bacteria. In Sulphate-Reducing Bacteria; Barton, L.L., Hamilton, W.A., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 1–37. ISBN 978-1-4899-1582-5. [Google Scholar]
- Lyew, D.; Knowles, R.; Sheppard, J. Biological treatment of acid mine drainage under continuous flow conditions in a reactor. Process Saf. Environ. Prot. Trans. Inst. Chem. Eng. Part B 1994, 72, 42–47. [Google Scholar]
- Schmidtova, J.; Baldwin, S.A. Correlation of bacterial communities supported by different organic materials with sulfate reduction in metal-rich landfill leachate. Water Res. 2011, 45, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.S.; Meisinger, D.B.; Porter, M.L.; Payn, R.A.; Schmid, M.; Stern, L.A.; Schleifer, K.H.; Lee, N.M. Linking phylogenetic and functional diversity to nutrient spiraling in microbial mats from Lower Kane Cave (USA). ISME J. 2010, 4, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.A.; Yao, L.; Van Dongen, U.; Kuenen, J.G.; Muyzer, G. Analysis of diversity and activity of sulfate-reducing bacterial communities in sulfidogenic bioreactors using 16S rRNA and dsrB genes as molecular markers. Appl. Environ. Microbiol. 2007, 73, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Loy, A.; Lehner, A.; Lee, N.; Adamczyk, J.; Meier, H.; Ernst, J.; Schleifer, K.-H.; Wagner, M. Oligonucleotide microarray for 16S rRNA gene-based detection of all recognized lineages of sulfate-reducing prokaryotes in the environment. Appl. Environ. Microbiol. 2002, 68, 5064–5081. [Google Scholar] [CrossRef] [PubMed]
- Hiibel, S.R.; Pereyra, L.P.; Inman, L.Y.; Tischer, A.; Reisman, D.J.; Reardon, K.F.; Pruden, A. Microbial community analysis of two field-scale sulfate-reducing bioreactors treating mine drainage. Environ. Microbiol. 2008, 10, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.A.; Khoshnoodi, M.; Rezadehbashi, M.; Taupp, M.; Hallam, S.; Mattes, Al.; Sanei, H. The Microbial Community of a Passive Biochemical Reactor Treating Arsenic, Zinc, and Sulfate-Rich Seepage. Front. Bioeng. Biotechnol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Raskin, L.; Rittmann, B.E.; Stahl, D.A. Competition and coexistence of sulfate-reducing and methanogenic populations in anaerobic biofilms. Appl. Environ. Microbiol. 1996, 62, 3847–3857. [Google Scholar] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.A.; Kleerebezem, R.; Stams, A.J.M.; Kuenen, J.G.; Muyzer, G. Competition and coexistence of sulfate-reducing bacteria, acetogens and methanogens in a lab-scale anaerobic bioreactor as affected by changing substrate to sulfate ratio. Appl. Microbiol. Biotechnol. 2008, 78, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Plugge, C.M.; Zhang, W.; Scholten, J.C.M.; Stams, A.J.M. Metabolic flexibility of sulfate-reducing bacteria. Front. Microbiol. 2011, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, L.P.; Hiibel, S.R.; Pruden, A.; Reardon, K.F. Comparison of microbial community composition and activity in sulfate-reducing batch systems remediating mine drainage. Biotechnol. Bioeng. 2008, 101, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Hiibel, S.R.; Pereyra, L.P.; Breazeal, M.V.R.; Reisman, D.J.; Reardon, K.F.; Pruden, A. Effect of Organic Substrate on the Microbial Community Structure in Pilot-Scale Sulfate-Reducing Biochemical Reactors Treating Mine Drainage. Environ. Eng. Sci. 2011, 28, 563–572. [Google Scholar] [CrossRef]
- Stams, A.J.M.; Plugge, C.M.; de Bok, F.A.M.; Van Houten, B.H.G.W.; Lens, P.; Dijkman, H.; Weijma, J. Metabolic interactions in methanogenic and sulfate-reducing bioreactors. Water Sci. Technol. 2005, 52, 13–20. [Google Scholar] [PubMed]
- Logan, M.V.; Reardon, K.F.; Figueroa, L.A.; McLain, J.E.T.; Ahmann, D.M. Microbial community activities during establishment, performance, and decline of bench-scale passive treatment systems for mine drainage. Water Res. 2005, 39, 4537–4551. [Google Scholar] [CrossRef] [PubMed]
- Larratt, H. Passive Sulphate Reducing Bacteria Treatment Ponds: Research and Operation at Highmont Tailings; Highland Valley Copper Annual Reclamation Report Volume II-Report 3; Larrat Aquatic: West Kelowna, BC, Canada, 2008; pp. 1–47. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; Huttley, G.A.; Kelley, S.T.; Knights, D.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, K.T.; Tiedje, J.M. Prokaryotic taxonomy and phylogeny in the genomic era: Advancements and challenges ahead. Curr. Opin. Microbiol. 2007, 10, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, K.D.; Tatusova, T.; Klimke, W.; Maglott, D.R. NCBI Reference Sequences: Current status, policy and new initiatives. Nucleic Acids Res. 2009, 37, D32–D36. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Delsuc, F.; Dufayard, J.-F.; Gascuel, O. Estimating maximum likelihood phylogenies with PhyML. Methods Mol. Biol. 2009, 537, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Siegert, M.; Krüger, M.; Teichert, B.; Wiedicke, M.; Schippers, A. Anaerobic Oxidation of Methane at a Marine Methane Seep in a Forearc Sediment Basin off Sumatra, Indian Ocean. Front. Microbiol. 2011, 2, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, G.; Osman, S.; Vaishampayan, P.A.; Andersen, G.L.; Stetler, L.D.; Sani, R.K. Microbial diversity in uranium mining-impacted soils as revealed by high-density 16S microarray and clone library. Microb. Ecol. 2010, 59, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Abicht, H.K.; Mancini, S.; Karnachuk, O.V.; Solioz, M. Genome sequence of Desulfosporosinus sp. OT, an acidophilic sulfate-reducing bacterium from copper mining waste in Norilsk, Northern Siberia. J. Bacteriol. 2011, 193, 6104–6105. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.W.; Fournelle, J.H.; Banfield, J.F. Quantifying heavy metals sequestration by sulfate-reducing bacteria in an acid mine drainage-contaminated natural wetland. Front. Microbiol. 2013, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Andrea, I.; Rodríguez, N.; Amils, R.; Sanz, J.L. Microbial diversity in anaerobic sediments at rio tinto, a naturally acidic environment with a high heavy metal content. Appl. Environ. Microbiol. 2011, 77, 6085–6093. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Romanek, C.S.; Wiegel, J. Desulfosporosinus youngiae sp. nov., a spore-forming, sulfate-reducing bacterium isolated from a constructed wetland treating acid mine drainage. Int. J. Syst. Evol. Microbiol. 2009, 59, 2743–2746. [Google Scholar] [CrossRef] [PubMed]
- Montoya, L.; Celis, L.B.; Gallegos-García, M.; Razo-Flores, E.; Alpuche-Solís, Á.G. Consortium diversity of a sulfate-reducing biofilm developed at acidic pH influent conditions in a down-flow fluidized bed reactor. Eng. Life Sci. 2013, 13, 302–311. [Google Scholar] [CrossRef]
- Battaglia-Brunet, F.; Crouzet, C.; Burnol, A.; Coulon, S.; Morin, D.; Joulian, C. Precipitation of arsenic sulphide from acidic water in a fixed-film bioreactor. Water Res. 2012, 46, 3923–3933. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Garcia-Dominguez, E.; Rhine, E.D.; Young, L.Y. A novel arsenate respiring isolate that can utilize aromatic substrates. FEMS Microbiol. Ecol. 2004, 48, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; Nannipieri, P.; Rasse, D.P.; Weiner, S.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Pester, M.; Bittner, N.; Deevong, P.; Wagner, M.; Loy, A. A “rare biosphere” microorganism contributes to sulfate reduction in a peatland. ISME J. 2010, 4, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Balk, M.; Altinbaş, M.; Rijpstra, W.I.C.; Sinninghe Damsté, J.S.; Stams, A.J.M. Desulfatirhabdium butyrativorans gen. nov., sp. nov., a butyrate-oxidizing, sulfate-reducing bacterium isolated from an anaerobic bioreactor. Int. J. Syst. Evol. Microbiol. 2008, 58, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Padrón, E.B.; Bordenave, S.; Lin, S.; Bhaskar, I.M.; Dong, X.; Sensen, C.W.; Fournier, J.; Voordouw, G.; Gieg, L.M. Carbon and sulfur cycling by microbial communities in a gypsum-treated oil sands tailings pond. Environ. Sci. Technol. 2011, 45, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, K.U.; Jakobsen, T.F.; Glastrup, J.; Ingvorsen, K. Desulfosalsimonas propionicica gen. nov., sp. nov., a halophilic, sulfate-reducing member of the family Desulfobacteraceae isolated from a salt-lake sediment. Int. J. Syst. Evol. Microbiol. 2010, 60, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Phillips, E.J. Novel processes for anaerobic sulfate production from elemental sulfur by sulfate-reducing bacteria. Appl. Environ. Microbiol. 1994, 60, 2394–2399. [Google Scholar] [PubMed]
- Finster, K.W.; Kjeldsen, K.U.; Kube, M.; Reinhardt, R.; Mussmann, M.; Amann, R.; Schreiber, L. Complete genome sequence of Desulfocapsa sulfexigens, a marine deltaproteobacterium specialized in disproportionating inorganic sulfur compounds. Stand. Genom. Sci. 2013, 8, 58–68. [Google Scholar] [CrossRef]
- Finster, K. Microbiological disproportionation of inorganic sulfur compounds. J. Sulfur Chem. 2008, 29, 281–292. [Google Scholar] [CrossRef]
- Kuever, J. The Family Desulfobulbaceae. In The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 75–86. [Google Scholar]
- Dann, A.L.; Cooper, R.S.; Bowman, J.P. Investigation and optimization of a passively operated compost-based system for remediation of acidic, highly iron- and sulfate-rich industrial waste water. Water Res. 2009, 43, 2302–2316. [Google Scholar] [CrossRef] [PubMed]
- Röling, W.F.; van Breukelen, B.M.; Braster, M.; Lin, B.; van Verseveld, H.W. Relationships between microbial community structure and hydrochemistry in a landfill leachate-polluted aquifer. Appl. Environ. Microbiol. 2001, 67, 4619–4629. [Google Scholar] [CrossRef] [PubMed]
- Methe, B.A.; Nelson, K.E.; Eisen, J.A.; Paulsen, I.T.; Nelson, W.; Heidelberg, J.F.; Wu, D.; Wu, M.; Ward, N.; Beanan, M.J. Genome of Geobacter sulfurreducens: Metal reduction in subsurface environments. Science 2003, 80, 1967–1969. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Ueki, T.; Zhang, T.; Aklujkar, M.; Butler, J.E.; Giloteaux, L.; Rotaru, A.-E.; Holmes, D.E.; Franks, A.E.; Orellana, R. Geobacter: The microbe electric’s physiology, ecology, and practical applications. Adv. Microb. Physiol. 2011, 59, 1–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Balkwill, D.L.; Aldrich, H.C.; Drake, G.R.; Boone, D.R. Characterization of the anaerobic propionate-degrading syntrophs Smithella propionica gen. nov., sp. nov. and Syntrophobacter wolinii. Int. J. Syst. Bacteriol. 1999, 49, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Stams, A.J.M.; Plugge, C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 2009, 7, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.; Abicht, H.K.; Karnachuk, O.V.; Solioz, M. Genome sequence of Desulfovibrio sp. A2, a highly copper resistant, sulfate-reducing bacterium isolated from effluents of a zinc smelter at the Urals. J. Bacteriol. 2011, 193, 6793–6794. [Google Scholar] [CrossRef] [PubMed]
- Colleran, E.; Pender, S. Mesophilic and thermophilic anaerobic digestion of sulphate-containing wastewaters. Water Sci. Technol. 2002, 45, 231–235. [Google Scholar] [PubMed]
- Strocchi, A.; Furne, J.; Ellis, C.; Levitt, M.D. Methanogens outcompete sulphate reducing bacteria for H2 in the human colon. Gut 1994, 35, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Salo, M.; Bomberg, M.; Grewar, T.; Seepei, L.; Gericke, M.; Arnold, M. Compositions of the Microbial Consortia Present in Biological Sulphate Reduction Processes During Mine Effluent Treatment. In Proceedings of the 13th International Mine Water Association Congress—“Mine Water & Circular Economy—A Green Congress”, Rauha, Lappeentanta, Finland, 25–30 June 2017. [Google Scholar]
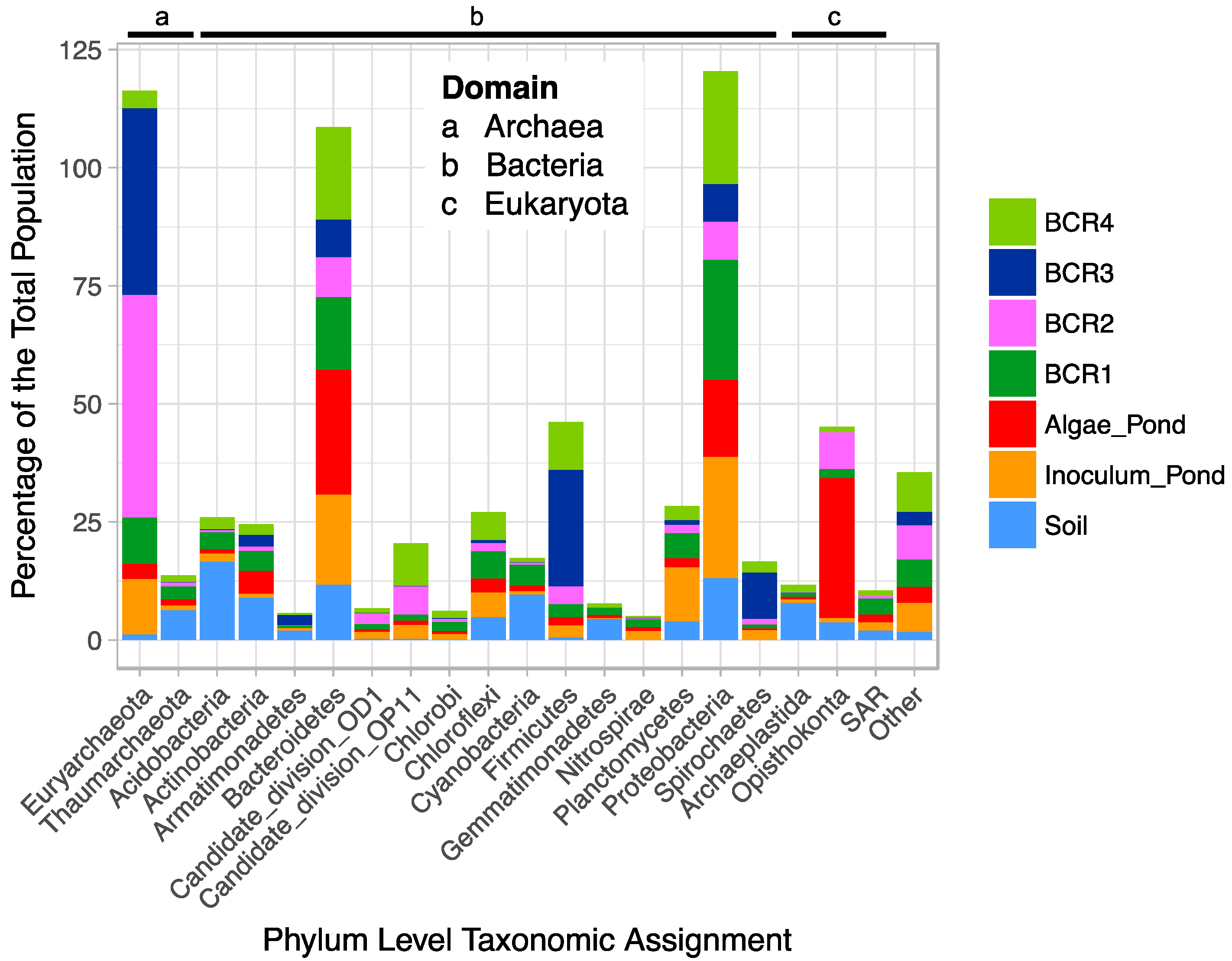
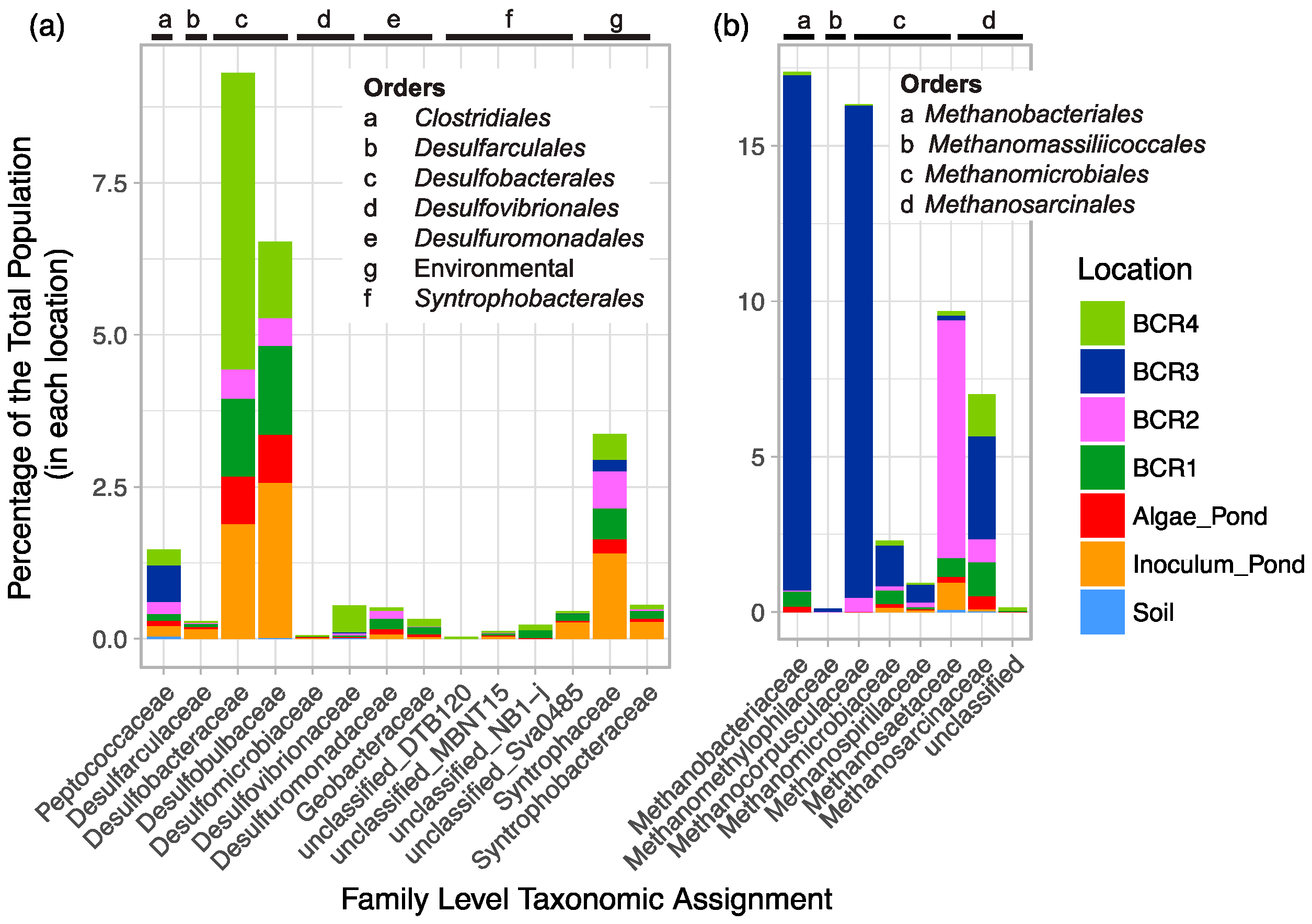
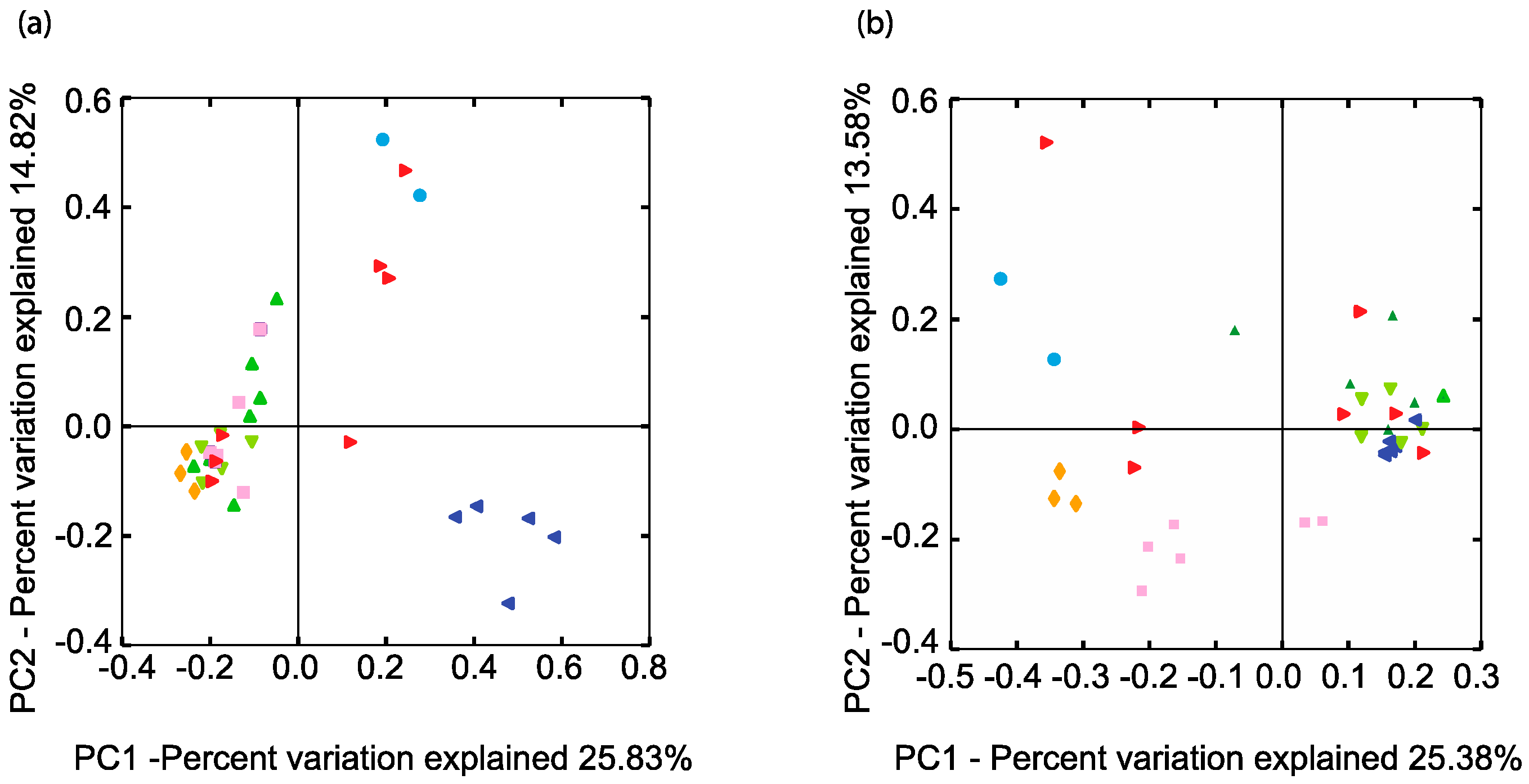
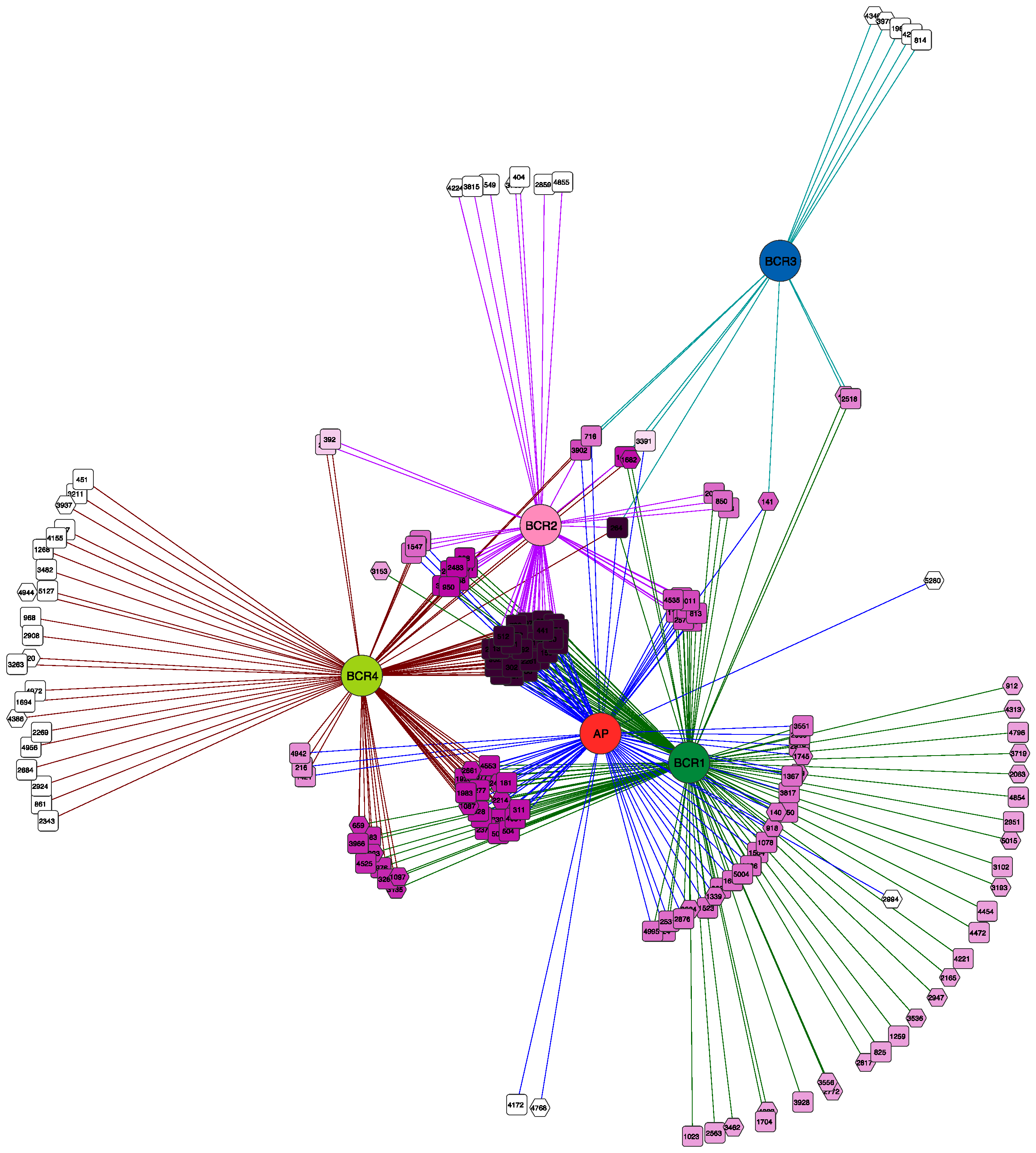
| Property | Site 1 | Site 2 | Site 3 | ||||
|---|---|---|---|---|---|---|---|
| Algae Pond | BCR1 | BCR2 | Inoculum Pond | Soil | BCR3 | BCR4 | |
| Pore water pH | NA | 7.8 | 6.7 | NA | NA | 5.6–7.5 | 7.6 |
| Pore water dissolved oxygen (mg/L) | NA | 0.08 | 0.36 | NA | NA | 0–1.25 | 3.17 |
| Pore water oxidation-reduction potential (mV reference Ag/AgCl) | NA | −518 | −504 | NA | NA | less than −112 | −21.4 |
| Metals in the influent | Cu; Mo | Cu; Mo | Cu; Mo | NA | NA | Zn; As; Cd | Cu; Mo; Se |
| Sulfate concentration in the influent | NA | 321 | 448 | NA | NA | 80–600 | 450 |
| Organic materials | Algae | Wood chips/Manure | Wood chips/Manure | Natural | Natural | Pulp mill biosolids | Wood/Hay/Manure |
| Date of commissioning | 1999 | 1999 | 2002 | Natural | Natural | 2002 | 2010 |
| Orientation of flow | NA | Horizontal plug flow | Vertical flow (up or down) | NA | NA | Vertical upflow | Vertical upflow |
| Location | Total Number Genus-Level Taxa | Number SRM Genus-Level Taxa | Percentage SRM |
|---|---|---|---|
| BCR1 | 2376 | 70 | 5.5 |
| BCR2 | 1637 | 57 | 2.2 |
| BCR3 | 662 | 10 | 0.3 |
| BCR4 | 1687 | 67 | 8.3 |
| Algae Pond | 2037 | 69 | 3.3 |
| Inoculum Pond | 1122 | 56 | 9.0 |
| Soil | 668 | 6 | 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezadehbashi, M.; Baldwin, S.A. Core Sulphate-Reducing Microorganisms in Metal-Removing Semi-Passive Biochemical Reactors and the Co-Occurrence of Methanogens. Microorganisms 2018, 6, 16. https://doi.org/10.3390/microorganisms6010016
Rezadehbashi M, Baldwin SA. Core Sulphate-Reducing Microorganisms in Metal-Removing Semi-Passive Biochemical Reactors and the Co-Occurrence of Methanogens. Microorganisms. 2018; 6(1):16. https://doi.org/10.3390/microorganisms6010016
Chicago/Turabian StyleRezadehbashi, Maryam, and Susan A. Baldwin. 2018. "Core Sulphate-Reducing Microorganisms in Metal-Removing Semi-Passive Biochemical Reactors and the Co-Occurrence of Methanogens" Microorganisms 6, no. 1: 16. https://doi.org/10.3390/microorganisms6010016




