Adaptations and Predispositions of Different Middle European Arthropod Taxa (Collembola, Araneae, Chilopoda, Diplopoda) to Flooding and Drought Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Collembola (Springtails)
2.1. Flood Adaptations and Predispositions
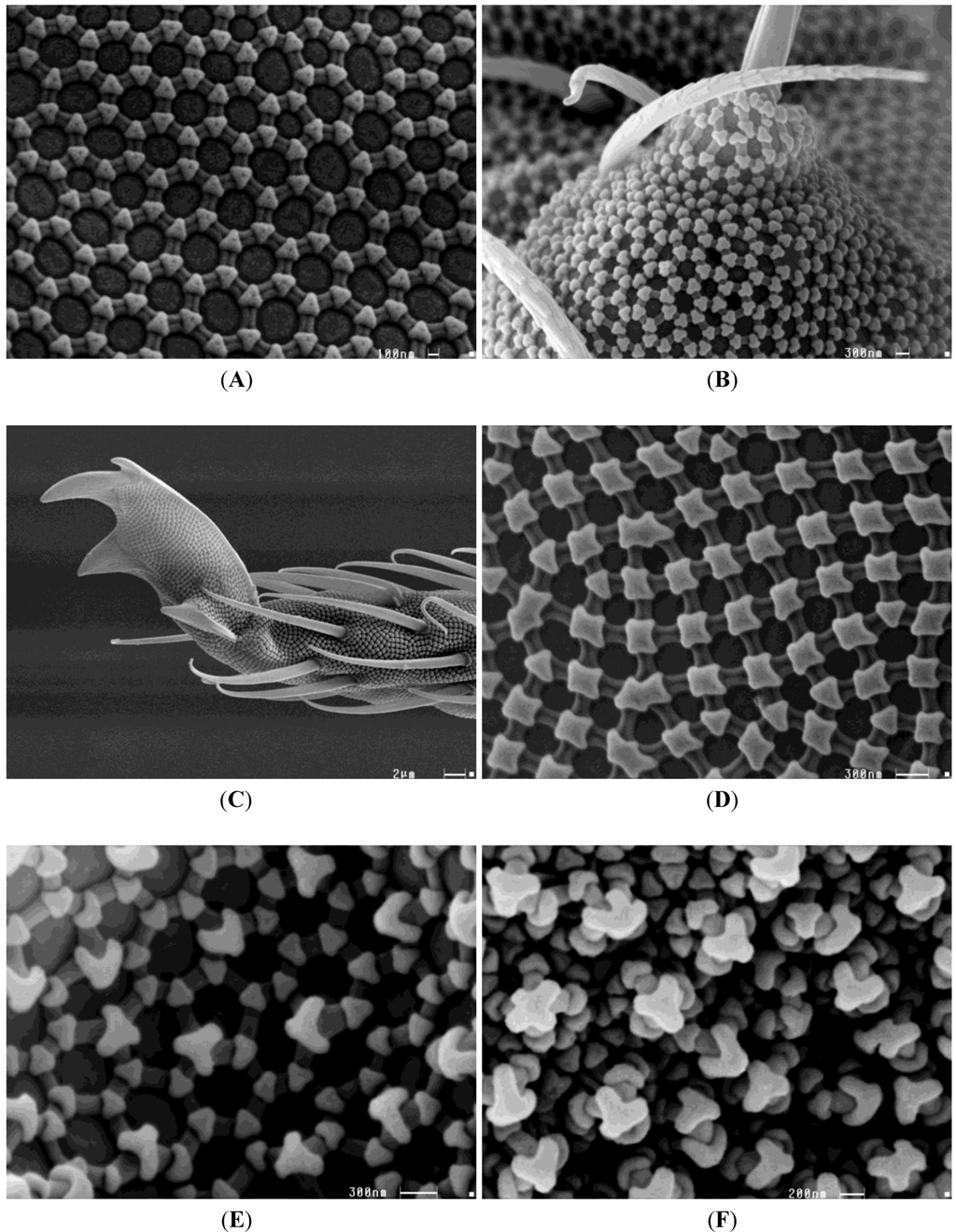
2.2. Drought Adaptations and Predispositions
| Type of Adaptation | Periodical flood | Aperiodical flood | Drought |
|---|---|---|---|
| Morphological Adaptation |
|
|
|
| Physiological Adaptation |
|
|
|
| Behavioral and phenological Adaptation |
|
|
|
3. Araneae (Spiders)
3.1. Flood Adaptations and Predispositions
3.2. Drought Adaptations and Predispositions
| Type of Adaptation | Periodical flood | Aperiodical flood | Drought |
|---|---|---|---|
| Morphological Adaptation |
|
|
|
| Physiological Adaptation |
|
|
|
| Behavioral and phenological Adaptation |
|
|
|
| Type of Adaptation | Periodical flood | Aperiodical flood | Drought |
|---|---|---|---|
| Morphological Adaptation |
|
|
|
| Physiological Adaptation |
|
|
|
| Behavioral and phenological Adaptation |
|
|
|
4. Diplopoda and Chilopoda (Myriapoda: Millipedes and Centipedes)
4.1. Flood Adaptations and Predispositions
4.2. Drought Adaptations and Predispositions
5. General Aspects of the Bioindication Value and Impacts of the Predicted Climate Change Scenario
6. Conclusions
Acknowledgments
Conflict of interest
References
- Adis, J. Überlebensstrategien terrestrischer Invertebraten in Überschwemmungswäldern Zentralamazoniens. Verhandlungen des Naturwissenschaftlichen Vereins Hamburg (NF) 1992, 33, 21–114. [Google Scholar]
- Adis, J.; Junk, W.J. Terrestrial invertebrates inhabiting lowland river floodplains of Central Amazonia and Central Europe: A review. Freshw. Biol. 2002, 47, 711–731. [Google Scholar] [CrossRef]
- Junk, W.J. Ecological Studies 126—The Central Amazon Floodplain. Ecology of a Pulsing System, 1st ed.; Springer: Berlin, Germany, 1997. [Google Scholar]
- Junk, W.J.; Nunes da Cunha, N.; Wantzen, K.M.; Petermann, P.; Strüssmann, C.; Marques, M.I.; Adis, J. Biodiversity and its conservation in the Pantanal of Mato Grosso, Brazil. Aquat. Sci. 2006, 68, 278–309. [Google Scholar] [CrossRef]
- Weigmann, G.; Wohlgemuth-von Reiche, D. Vergleichende Betrachtungen zu den Bodentieren im Überflutungsbereich von Tieflandauen. In Limnologie Aktuell Band 9, Das Untere Odertal, 1st ed.; Dohle, W., Bornkamm, R., Weigmann, G., Eds.; Schweizerbart'sche Verlagsbuchhandlung (Nägele u. Obermiller): Stuttgart, Germany, 1999; pp. 229–240. [Google Scholar]
- Deharveng, L.; D’Haese, C.A.; Bedos, A. Global diversity of springtails (Collembola; Hexapoda) in freshwater. Hydrobiologia 2008, 595, 329–338. [Google Scholar] [CrossRef]
- Deharveng, L.; Lek, S. High diversity and community permeability: The riparian Collembola (Insecta) of a Pyrenean massif. Hydrobiologia 1995, 312, 59–74. [Google Scholar] [CrossRef]
- Hering, D.; Gerhard, M.; Manderbach, R.; Reich, M. Impact of a 100-year flood on vegetation, benthic invertebrates, riparian fauna and large woody debris standing stock in an alpine floodplai. River Res. Appl. 2004, 20, 445–457. [Google Scholar] [CrossRef]
- Marx, M.T.; Wild, A.-K.; Knollmann, U.; Kamp, G.; Wegener, G.; Eisenbeis, G. Responses and adaptations of collembolan communities (Hexapoda: Collembola) to flooding and hypoxic conditions. Pesquisa Agropecuária Brasileira 2009, 44, 1002–1010. [Google Scholar] [CrossRef]
- Plum, N. Terrestrial invertebrates in flooded grassland: A literature review. Wetlands 2005, 25, 721–737. [Google Scholar] [CrossRef]
- Rothenbücher, J.; Schaefer, M. Conservation of leafhoppers in floodplain grasslands—Trade-off between diversity and naturalness in a northern German national park. J. Insect Conserv. 2005, 9, 335–349. [Google Scholar] [CrossRef]
- Rothenbücher, J.; Schaefer, M. Submersion tolerance in floodplain arthropod communities. Basic Appl. Ecol. 2006, 7, 398–408. [Google Scholar] [CrossRef]
- Tamm, J.C. Das jahresperiodisch trockenliegende Eulitoral der Edertalsperre als Lebens-und Ersatzlebensraum; eine Ökosystemstudie mit terrestrischem Schwerpunkt. Arch. Hydrobiol. Suppl. Algol. Stud. 1982, 64, 484–553. [Google Scholar]
- Tamm, J.C. Surviving long submergence in the egg stage—A successful strategy of terrestrial arthropods living on floodplains (Collembola, Acari, Diptera). Oecologia 1984, 61, 417–419. [Google Scholar] [CrossRef]
- Tamm, J.C. Temperature-controlled under-water egg dormancy and post-flood hatching in Isotoma viridis (Collembola) as forms of adaptation to annual long-term flooding. Oecologia 1986, 68, 241–245. [Google Scholar] [CrossRef]
- Tamm, J.C.; Mittmann, H.W.; Woas, S. Zur Landmilbenfauna eines jahresperiodisch trockenfallenden Stauseebodens. Pedobiologia 1984, 27, 395–404. [Google Scholar]
- Jucevica, E.; Melecis, V. Global warming affect Collembola community: A long-term study. Pedobiologia 2005, 50, 177–184. [Google Scholar] [CrossRef]
- Archaux, F.; Wolters, V. Impact of summer drought on forest biodiversity: What do we know? Ann. For. Sci. 2006, 63, 645–652. [Google Scholar] [CrossRef]
- McMahon, T.A.; Finlayson, B.L. Droughts and anti-droughts: The low-flow hydrology of Australian rivers. Freshw. Biol. 2003, 48, 1147–1160. [Google Scholar] [CrossRef]
- Humphries, P.; Baldwin, D.S. Drought and aquatic ecosystems: An introduction. Freshw. Biol. 2003, 48, 1141–1146. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W.; Ulbrich, U.; Brücher, T.; Graczyk, D.; Krüger, A.; Leckebusch, G.C.; Menzel, L.; Pinskwar, I.; Radziejewski, M.; Szwed, M. Summer floods in Central Europe—Climate Change Track? Natural Hazards 2005, 36, 165–189. [Google Scholar] [CrossRef]
- Christensen, O.B.; Goodess, C.M.; Ciscar, J.-C. Methodological framework of the PESETA project on the impacts of climate change in Europe. Clim. Change 2012, 112, 7–28. [Google Scholar] [CrossRef]
- Jentsch, A.; Kreyling, J.; Boettcher-Treschkow, J.; Beierkuhnlein, C. Beyond gradual warming: Extreme weather events alter flower phenology of European grassland and heath species. Glob. Chang. Biol. 2009, 15, 837–849. [Google Scholar] [CrossRef]
- Jöhnk, K.D.; Huisman, J.; Sharples, J.; Sommeijer, B.; Visser, P.M.; Stroom, J.M. Summer heatwaves promote blooms of harmful cyanobacteria. Glob. Chang. Biol. 2008, 14, 495–512. [Google Scholar] [CrossRef]
- Kyselý, J.; Gaál, L.; Beranová, R.; Plavcová, E. Climate change scenarios of precipitation extremes in Central Europe from ENSEMBLES regional climate models. Theor. Appl. Climatol. 2011, 104, 529–542. [Google Scholar] [CrossRef]
- Milad, M.; Schaich, H.; Bürgi, M.; Konold, B. Climate change and nature conservation in Central European forests: A review of consequences, concepts and challenges. For. Ecol. Manage. 2011, 261, 829–843. [Google Scholar] [CrossRef]
- Nikulin, G.; Kjellström, E.; Hansson, U.; Strandberg, G.; Ullerstig, A. Evaluation and future projections of temperature, precipitation and wind extremes over Europe in an ensemble of regional climate simulations. Tellus (A) 2011, 63, 41–55. [Google Scholar] [CrossRef]
- Schär, C.; Vidale, P.L.; Lüthi, D.; Frei, C.; Häberli, C.; Liniger, M.A.; Appenzeller, C. The role of increasing temperature variability in European summer heatwaves. Nature 2004, 427, 332–336. [Google Scholar]
- Verzano, K.; Bärlund, I.; Flörke, M.; Lehner, B.; Kynast, E.; Voß, F.; Alcamo, J. Modeling variable river flow velocity on continental scale: Current situation and climate change impacts in Europe. J. Hydrol. 2012, 424-425, 238–251. [Google Scholar] [CrossRef]
- Luterbacher, J.; Dietrich, D.; Xoplaki, E.; Grosjean, M.; Wanner, H. European seasonal and annual variability, trends and extremes since 1500. Science 2004, 303, 1499–1503. [Google Scholar] [CrossRef]
- Schindler, U.; Steidl, J.; Müller, L.; Eulenstein, F.; Thiere, J. Drought risk to agricultural land in Northeast and Central Germany. J. Plant Nutr. Soil Sci. 2007, 170, 357–362. [Google Scholar] [CrossRef]
- Das Abflussregime des Rheins und seiner Nebenflüsse im 20. Jahrhundert—Analyse, Veränderungen, Trends; Final Report, Internationale Kommission für die Hydrologie des Rheingebietes (IKHR): Koblenz, Germany, 2007; 1–377.
- Klein Tank, A.M.G.; Können, P.G. Trends in indices of daily temperature and precipitation extremes in Europe, 1946–99. J. Clim. 2003, 16, 3665–3680. [Google Scholar] [CrossRef]
- Moberg, A.; Jones, P.D. Trends in indices for extremes in daily temperature and precipitation in Central and Western Europe, 1901–99. Int. J. Climatol. 2005, 25, 1149–1171. [Google Scholar] [CrossRef]
- Schröter, D.; Zebisch, M.; Grothmann, T. Climate Change in Germany—Vulnerability and Adaptation of Climate-Sensitive Sectors; Annual Report; DWD: Offenbach, Germany, 2005; pp. 44–56. [Google Scholar]
- Molnar, P.; Favre, V.; Perona, P.; Burlando, P.; Randin, C.; Ruf, W. Floodplain forest dynamics in a hydrologically altered mountain river. Peckiana 2008, 5, 17–24. [Google Scholar]
- Hirst, S.; Maulik, S. On some arthropod remains from the Rhynie chert (old red sandstone). Geol. Mag. 1926, 63, 69–71. [Google Scholar] [CrossRef]
- Whalley, P.; Jarzembowski, E.A. A new assessment of Rhyniella, the earliest known insect, from the Devonian of Rhynie, Scotland. Nature 1981, 291. [Google Scholar]
- Habgood, K.S.; Hass, H.; Kerp, H. Evidence for an early terrestrial food web: Coprolites from the early Devonian Rhynie chert. Trans. R. Soc. Edinb. Earth Sci. 2004, 94, 371–389. [Google Scholar]
- Bauer, R.; Christian, E. Adaptations of three springtail species to granite boulder habitats (Collembola). Pedobiologia 1993, 37, 280–290. [Google Scholar]
- Brand, R.H. The effect of prescribed burning on epigeic springtails (Insecta: Collembola) of woodland litter. Am. Midl. Nat. 2002, 148, 383–393. [Google Scholar] [CrossRef]
- Elnitsky, M.A.; Benoit, J.B.; Denlinger, D.L.; Lee, R.E., Jr. Desiccation tolerance and drought acclimation in the Antarctic collembolan Cryptopygus antarcticus. J. Insect Physiol. 2008, 54, 1432–1439. [Google Scholar] [CrossRef]
- Greenslade, P. Survival of Collembola in arid environments: Observations in South Australia and the Sudan. J. Arid Environ. 1981, 4, 219–228. [Google Scholar]
- Hawes, T.C.; Couldridge, C.E.; Bale, J.S.; Worland, M.R.; Convey, P. Habitat temperature and the temporal scaling of cold hardening in the high Arctic collembolan, Hypogastrura tullbergi (Schäffer). Ecol. Entomol. 2006, 31, 450–459. [Google Scholar] [CrossRef]
- Hawes, T.C.; Worland, M.R.; Convey, P.; Bale, J.S. Aerial dispersal of springtails on the Antarctic Peninsula: Implications for local distribution and demography. Antarct. Sci. 2007, 19, 3–10. [Google Scholar]
- Palissa, A. Collembola. In Süßwasserfauna von Mitteleuropa 10, 1st ed.; Schwoerbel, J., Zwick, P., Eds.; Spektrum Verlag: Heidelberg/Berlin, Germany, 2000. [Google Scholar]
- Shaw, P.C.A.; Ozanne, C.; Speight, M.; Palmer, I. Edge effects and arboreal Collembola in coniferous plantations. Pedobiologia 2007, 51, 287–293. [Google Scholar] [CrossRef]
- Rusek, J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 1998, 7, 1207–1219. [Google Scholar] [CrossRef]
- Hopkin, S.P. Biology of the Springtails, 1st ed.; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Russell, D.J.; Schick, H.; Nährig, D. Reactions of Soil Collembolan Communities to Inundation in Floodplain Ecosystems of the Upper Rhine Valley. In Wetlands in Central Europe, 1st ed.; Broll, G., Merbach, W., Pfeiffer, E.M., Eds.; Springer Verlag: Berlin, Germany, 2002; pp. 35–70. [Google Scholar]
- Griegel, A. Räumliche Verteilung und jahreszeitliche Dynamik von Kleinarthropoden (Collembola, Gamasida) in den Auen des Unteren Odertals. In Limnologie Aktuell Band 9, Das Untere Odertal, 1st ed.; Dohle, W., Bornkamm, R., Weigmann, G., Eds.; Schweizerbart´sche Verlagsbuchhandlung (Nägele u. Obermiller): Stuttgart, Germany, 1999; pp. 211–228. [Google Scholar]
- Griegel, A. Auswirkungen von Überflutungen auf die Zönosen der Collembolen und der Gamasiden (Insecta: Collembola, Acari: Gamasida) in der Flußaue des unteren Odertals, 1st ed.; Dissertation.de-Verlag im Internet GmbH: Berlin, Germany, 2000. [Google Scholar]
- Lessel, T.; Marx, M.T.; Eisenbeis, G. Effects of ecological flooding on the temporal and spatial dynamics of carabid beetles (Coleoptera: Carabidae) and springtails (Collembola) in a polder habitat. ZooKeys 2011, 100, 421–446. [Google Scholar]
- Marx, M.T. The collembolan population of a river bank reinforcement system in front of a middle Rhine region floodplain under influence of inundation and extreme drought. Peckiana 2008, 5, 115–125. [Google Scholar]
- Russell, D.J.; Griegel, A. Influence of variable inundation regimes on soil Collembola. Pedobiologia 2006, 50, 165–175. [Google Scholar] [CrossRef]
- Russell, D.J. Metacommunity responses of soil Collembola to inundation intensity in the Upper Rhine Valley. Peckiana 2008, 5, 127–143. [Google Scholar]
- Sterzyńska, M.; Ehrnsberger, R. Diversity and structure of collembolan communities in wetlands. In Proceedings of the 5th Central European Workshop on Soil Zoology, České Budějovice, Czech Republic, 27-30 April 1999; pp. 325–334.
- Eisenbeis, G.; Wichard, W. Atlas zur Biologie der Bodenarthropoden, 1st ed.; Verlag G. Fischer: Stuttgart, Germany, 1985. [Google Scholar]
- Helbig, R.; Nickerl, J.; Neinhuis, C.; Werner, C. Smart skin pattern protect springtails. PLoS ONE 2011, 6, e25105. [Google Scholar]
- Marx, M.T.; Messner, B. A general definition of the term “plastron” in terrestrial and aquatic arthropods. Org. Divers. Evol. 2012. [Google Scholar]
- Lawrence, P.N.; Massoud, Z. Cuticle structures in the Collembola (Insecta). Revue d’Écologie et de Biologie de Sol 1973, 10, 77–101. [Google Scholar]
- Hale, W.G.; Smith, A.L. Scanning electron microscope studies of cuticular structures in the genus Onychiurus (Collembola). Revue d’Écologie et de Biologie de Sol 1966, 3, 343–354. [Google Scholar]
- Ghiradella, H.; Radigan, W. Collembolan cuticle: Wax layer and anti-wetting properties. J. Insect Physiol. 1974, 20, 301–306. [Google Scholar] [CrossRef]
- Messner, B. Vorschlag für die Neufassung des Begriffes “Plastron” bei den Arthropoden. Deutsche Entomologische Zeitschrift (N.F.) 1988, 35, 379–381. [Google Scholar] [CrossRef]
- Coulson, S.J.; Hodkinson, I.D.; Webb, N.R.; Harrison, J.A. Survival of terrestrial soil-dwelling arthropods on and in seawater: Implications for trans-oceanic dispersal. Funct. Ecol. 2002, 16, 353–356. [Google Scholar] [CrossRef]
- Moore, P.D. Springboards for springtails. Nature 2002, 418, 381. [Google Scholar] [CrossRef]
- Coulson, S.J.; Birkemoe, T. Long-term cold tolerance in Arctic invertebrates: Recovery after 4 years at below −20 °C. Can. J. Zool. 2000, 78, 2055–2058. [Google Scholar] [CrossRef]
- Fridriksson, S. Surtsey, Evolution of Life on a Volcanic Island, 1st ed.; Butterworth: London, UK, 1975. [Google Scholar]
- Zinkler, D.; Platthaeus, J. Tolerance of soil-dwelling Collembola to high carbon dioxide concentrations. Eur. J. Entomol. 1996, 93, 443–450. [Google Scholar]
- Zinkler, D. Vergleichende Untersuchungen zur Atmungsphysiologie von Collembolen (Apterygota) und anderen Kleinarthropoden. Z. Vgl. Physiol. 1966, 52, 99–144. [Google Scholar] [CrossRef]
- Zinkler, D.; Rüssbeck, R. Ecophysiological adaptations of Collembola to low oxygen concentrations. In Proceedings of 2nd International Seminar on Apterygota, Siena, Italy, 4–6 September 1986; pp. 123–127.
- Paul, R.J.; Colmorgen, M.; Hüller, S.; Tyroller, F.; Zinkler, D. Circulation and respiration control in millimeter-sized animals (Daphnia magna, Folsomia candida) studied by optical methods. J. Comp. Physiol. B 1997, 167, 399–408. [Google Scholar] [CrossRef]
- Zinkler, D.; Rüssbeck, R.; Biefang, M.; Baumgärtl, H. Intertidal respiration of Anurida maritima (Collembola: Neanuridae). Eur. J. Entomol. 1999, 96, 205–209. [Google Scholar]
- Joosse, E.N.G. Some observations on the biology of Anurida maritima (Collembola). Zeitschrift für Morphologie und Ökologie der Tiere 1966, 57, 320–328. [Google Scholar] [CrossRef]
- Blancquaert, J.P.; Coessens, R.; Mertens, J. Life history of some Symphypleona (Collembola) under experimental conditions. I. Embryonal development and Diapause. Revue d’Écologie et de Biologie de Sol 1981, 18, 115–126. [Google Scholar]
- Gauer, U. Collembola in Central Amazon inundation forests—Strategies for surviving floods. Pedobiologia 1997, 41, 69–73. [Google Scholar]
- Thibaud, J.M. Biologie et écologie des Collemboles Hypogastruridae édaphiques et cavernicoles. Mémoires du Muséum National d‘Histoire Naturelle A 1970, 51, 86–201. [Google Scholar]
- Beck, L. Der Einfluss der jahresperiodischen Überflutungen auf den Massenwechsel der Bodenarthropoden im zentralamazonischen Regenwaldgebiet. Pedobiologia 1972, 12, 133–148. [Google Scholar]
- Alvarez, T.; Frampton, G.K.; Goulson, D. The effects of drought upon epigeal Collembola from arable soils. Agric. For. Entomol. 1999, 1, 243–248. [Google Scholar] [CrossRef]
- Lindberg, N.; Bengtsson, J. Population responses of oribatid mites and collembolans after drought. Appl. Soil Ecol. 2005, 28, 163–174. [Google Scholar] [CrossRef]
- Lindberg, N.; Bengtsson, J. Recovery of forest soil fauna diversity and composition after repeated summer droughts. Oikos 2006, 114, 494–506. [Google Scholar] [CrossRef]
- Meier, P.; Zettel, J. Cold hardiness in Entomobrya nivalis (Collembola, Entomobryidae): Annual cycle of polyols and antifreeze proteins, and antifreeze triggering by temperature and photoperiod. J. Comp. Physiol. B 1997, 167, 297–304. [Google Scholar] [CrossRef]
- Simon, H.R. Entomobrya nivalis (Linnaeus, 1758) als dominante Art im Nahrungssystem von Apfelbaumkronen—Zwischenergebnisse aus dem Projekt „Monitoring von Arthropoden in Apfelanlagen“ (Collembola). Entomol. Z. Insektenbörse 2007, 117, 184–189. [Google Scholar]
- Vegter, J.J. Phenology and seasonal resource partitioning in forest floor Collembola. Oikos 1987, 48, 175–185. [Google Scholar] [CrossRef]
- Massoud, Z.N.; Poinsot, N.; Poivre, C. Contribution à l’étude du comportement constructeur chez les Collemboles. Revue d’Écologie et de Biologie de Sol 1968, 5, 283–286. [Google Scholar]
- Poinsot, N. Nouveaux exemples de comportement constructeur chez les collemboles Isotomidae. Revue du Comportement Animal 1970, 4, 59–63. [Google Scholar]
- Poinsot, N. Contribution à l’étude du comportement constructeur chez les Collemboles. Revue d’Écologie et de Biologie de Sol 1971, 8, 163–165. [Google Scholar]
- Belgnaoui, S.; Barra, J.A. Water loss and survival in anhydrobiotic Collembola Folsomides angularis (Insecta). Revue d’Écologie et de Biologie de Sol 1989, 26, 123–132. [Google Scholar]
- Poinsot-Balaguer, N.; Barra, J.A. L’anhydrobiose: un problème biologique nouveau chez les Collemboles (Insecta). Revue d’Écologie et de Biologie de Sol 1991, 28, 197–205. [Google Scholar]
- Block, W. Cold or drought—The lesser of two evils for terrestrial arthropods. Eur. J. Entomol. 1996, 93, 325–339. [Google Scholar]
- Holmstrup, M.; Hedlund, K.; Boriss, H. Drought acclimation and lipid composition in Folsomia candida: implications for cold shock, heat shock and acute desiccation stress. J. Insect Physiol. 2002, 48, 961–970. [Google Scholar] [CrossRef]
- Poinsot-Balaguer, N.; Barra, J.A. Experimental and ultrastructural data on freezing resistance of Folsomides angularis (Insecta, Collembola). Pedobiologia 1983, 25, 357–363. [Google Scholar]
- Worland, M.R.; Grubor-Lajsic, G.; Montiel, P.O. Partial desiccation induced by sub-zero temperatures as a component of the survival strategy of the Arctic collembolan Onychiurus arcticus (Tullberg). J. Insect Physiol. 1998, 44, 211–219. [Google Scholar] [CrossRef]
- Hinton, H.E. Cryptobiosis in the larva of Polypedilum vanderplanki Hint. (Chironomidae). J. Insect Physiol. 1960, 5, 286–300. [Google Scholar] [CrossRef]
- Cassagnau, P. Les différents types d’écomorphose chez les collemboles Isotomidae. Revue d’Écologie et de Biologie de Sol 1971, 8, 55–57. [Google Scholar]
- Fountain, M.T.; Hopkin, S.P. Folsomia candida (Collembola): A “standard” soil arthropod. Annu. Rev. Entomol. 2005, 50, 201–222. [Google Scholar] [CrossRef]
- Sjursen, H.; Bayley, M.; Holmstrup, M. Enhanced drought tolerance of a soil-dwelling springtail by pre-acclimation to a mild drought stress. J. Insect Physiol. 2001, 47, 1021–1027. [Google Scholar] [CrossRef]
- Pflug, A.; Wolters, V. Influence of drought and litter age on Collembola communities. Eur. J. Soil Biol. 2001, 37, 305–308. [Google Scholar] [CrossRef]
- Sjursen, H.; Holmstrup, M. Cold and drought stress in combination with pyrene exposure: studies with Protaphorura armata (Collembola: Onychiuridae). Ecotoxicol. Environ. Saf. 2004, 57, 145–152. [Google Scholar] [CrossRef]
- Kaersgaard, C.W.; Holmstrup, M.; Malte, H.; Bayley, M. The importance of cuticular permeability, osmolyte production and body size for the desiccation resistance of nine species of Collembola. J. Insect Physiol. 2004, 50, 5–15. [Google Scholar] [CrossRef]
- Bayley, M.; Holmstrup, M. Water vapour absorption in arthropods by accumulation of myoinositol and glucose. Science 1999, 285, 1909–1911. [Google Scholar] [CrossRef]
- Duffey, E. Spider ecology and habitat structure (Arach., Araneae). Senckenb. Biol. 1966, 47, 45–49. [Google Scholar]
- Foelix, R.F. Biology of Spiders, 2nd ed.; Oxford University Press: New York, NY, USA, 1996. [Google Scholar]
- Beyer, W.; Grube, R. Einfluss des Überflutungsregimes auf die epigäische Spinnen-und Laufkäferfauna an Uferabschnitten im Nationalpark “Unteres Odertal”. Verhandlungen der Gesellschaft für Ökologie 1997, 27, 349–356. [Google Scholar]
- Bonn, A.; Hagen, K.; Wohlgemuth-von Reiche, D. The significance of flood regimes for carabid beetle and spider communities in riparian habitats—A comparison of tree major rivers in Germany. River Res. Appl. 2002, 18, 43–64. [Google Scholar] [CrossRef]
- Thaler, K.; Pintar, M.; Steiner, H.M. Fallenfänge von Spinnen in den östlichen Donauauen (Stockerau, Niederösterreich). Spixiana 1984, 7, 97–103. [Google Scholar]
- Siepe, A. Einfluss häufiger Überflutungen auf die Spinnen-Besiedlung am Oberrhein-Ufer. Mitt. Dtsch. Ges. Allg. Angew. Ent. 1985, 4, 281–284. [Google Scholar]
- Wohlgemuth-von Reiche, D.; Grube, R. Zur Lebensraumbindung der Laufkäfer und Webspinnen (Coleoptera, Carabidae; Araneae) im Überflutungsbereich der Odertal-Auen. In Limnologie Aktuell Band 9, Das Untere Odertal, 1st ed.; Dohle, W., Bornkamm, R., Weigmann, G., Eds.; Schweizerbartsche Verlagsbuchhandlung (Nägele u. Obermiller): Stuttgart, Germany, 1999; pp. 147–169. [Google Scholar]
- Richter, C.J.J. Aerial dispersal in relation to habitat in eight wolf spider species (Pardosa, Araneae, Lycosidae). Oecologia 1970, 5, 200–214. [Google Scholar] [CrossRef]
- Suter, R.B. An aerial lottery: The physics of ballooning in a chaotic atmosphere. J. Arachnol. 1999, 27, 281–293. [Google Scholar]
- Thomas, C.F.G.; Jepson, P.C. Differential aerial dispersal of linyphiid spiders from a grass and a cereal field. J. Arachnol. 1999, 27, 294–300. [Google Scholar]
- Kiss, B.; Samu, F. Comparison of autumn and winter development of two wolf spider species (Pardosa, Lycosidae, Araneae) having different life history patterns. J. Arachnol. 2002, 30, 409–415. [Google Scholar] [CrossRef]
- Bauchhenss, E. Die epigäische Spinnenfauna eines Auwaldgebietes der Donau im Landkreis Dillingen/Donau (Deutschland, Bayern). Arachnologische Mitteilungen 1991, 2, 20–30. [Google Scholar]
- Manderbach, R. Der Stellenwert des Lebenszyklus für das Überleben der Ufer bewohnenden Wolfspinnenarten Pardosa wagleri (Hahn, 1822) und Pirata knorri (Scopoli, 1763. Arachnologische Mitteilungen 2001, 21, 1–13. [Google Scholar]
- Krumpálová, Z. Floods—As the factor of degradation and recovery of araneocoenoses. In Proceedings of the 7th Central European Workshop on Soil Zoology, České Budějovice, Czech Republic, 14–16 April 2003; pp. 77–83.
- Kubcová, L.; Schlaghamerský, J. Zur Spinnenfauna der Stammregion stehenden Totholzes in südmährischen Auenwäldern. Arachnologische Mitteilungen 2002, 24, 36–61. [Google Scholar]
- Zulka, K.P. Einfluss der Hochwässer auf die epigäische Arthropodenfauna im Überschwemmungsbereich der March (Niederösterreich). Mitt. Dtsch. Ges. Allg. Angew. Ent. 1989, 7, 74–75. [Google Scholar]
- Stratton, G.E.; Suter, R.B.; Miller, P.R. Evolution of water surface locomotion by spiders: A comparative approach. Biol. J. Linn. Soc. Lond. 2004, 81, 63–78. [Google Scholar] [CrossRef]
- Seymour, R.S.; Hetz, S.K. The diving bell and the spider: The physical gill of Argyroneta aquatic. J. Exp. Biol. 2011, 214, 2175–2181. [Google Scholar] [CrossRef]
- Pétillon, J.; Montaigne, W.; Renault, D. Hypoxic coma as a strategy to survive inundation in a salt-marsh inhabiting spider. Biol. Lett. 2009, 5, 442–445. [Google Scholar] [CrossRef]
- Steinberger, K.H. Zur Spinnenfauna der Parndorfer Platte, einer Trockenlandschaft im Osten Österreichs (Burgenland) (Arachnida: Araneae, Opiliones). Denisia 2004, 12, 419–440. [Google Scholar]
- Edney, E.B. Water balance in land arthropods. In Zoophysiology and Ecology, 1st ed.; Farner, D.S., Hoar, W.S., Hoelldobler, B., Langer, H., Lindauer, M., Eds.; Springer-Verlag: Heidelberg, Germany, 1977. [Google Scholar]
- Barth, F.G. Die Feinstruktur des Spinneninteguments. 1. Die Cuticula des Laufbeins adulter häutungsferner Tiere (Cupiennius salei, Keys.). Z. Zellforsch. Mikrosk. Anat. 1969, 97, 137–159. [Google Scholar] [CrossRef]
- Barth, F.G. Die Feinstruktur des Spinneninteguments. II. Die räumliche Anordnung der Mikrofasern in der lamellierten Cuticula und ihre Beziehung zur Gestalt der Porenkanäle (Cupiennius salei, Keys., adult, häutungsfern, Tarsus). Z. Zellforsch. Mikrosk. Anat. 1970, 104, 87–106. [Google Scholar] [CrossRef]
- Hadley, N.F.; Quinlan, M.C. Cuticular permeability of the black widow spider Latrodectus hesperus. J. Comp. Physiol. B 1989, 159, 243–248. [Google Scholar] [CrossRef]
- Hadley, N.F.; Ahearn, G.A.; Howarth, G. Water and metabolic relations of cave-adapted and epigean lycosid spiders in Hawaii. J. Arachnol. 1981, 9, 215–222. [Google Scholar]
- Humphreys, W.F. The influence of burrowing and thermoregulatory behaviour on the water relations of Geolycosa godeffroyi (Araneae: Lycosidae), an Australian wolf spider. Oecologia 1975, 21, 291–311. [Google Scholar] [CrossRef]
- Ehn, R.; Tichy, H. Hygro- and thermoreceptive tarsal organ in the spider Cupiennius salei. J. Comp. Physiol. A 1994, 174, 345–350. [Google Scholar]
- Tichy, H.; Loftus, R. Hygroreceptors in insects and a spider: Humidity Transduction Models. Naturwissenschaften 1996, 83, 255–263. [Google Scholar]
- Machin, J.; Lampert, G.J. A passive two layer permeability-water model for Periplaneta cuticle. J. Exp. Biol. 1985, 117, 171–179. [Google Scholar]
- Opell, B.D. The respiratory complementarity of spider book lung and tracheal systems. J. Morphol. 1998, 236, 57–64. [Google Scholar] [CrossRef]
- Cloudsley-Thompson, J. Nocturnal ecology and water regulation of British cribellate spiders of the genus Ciniflo. Biol. J. Linn. Soc. Lond. 1957, 43, 133–152. [Google Scholar]
- Levi, H.W. Adaptations of respiratory systems of spiders. Evolution 1967, 21, 571–583. [Google Scholar] [CrossRef]
- Levi, H.W.; Kirber, W.M. On the evolution of tracheae in arachnids. Bull. Br. Arachnol. Soc. 1976, 3, 187–188. [Google Scholar]
- Finke, T.; Paul, R. Book lung function in arachnids III. The function of the spiracles. J. Comp. Physiol. B 1989, 159, 433–441. [Google Scholar] [CrossRef]
- Maddrell, S.H.P. The functional design of the insect excretory system. J. Exp. Biol. 1981, 90, 1–15. [Google Scholar]
- Parry, D.A. On the drinking of soil capillary water by spiders. J. Exp. Biol. 1954, 31, 218–227. [Google Scholar]
- Butt, A.G.; Taylor, H.H. Regulatory responses of the coxal organs and the anal excretory system to dehydration and feeding in the spider Porrhothele antipodiana (Mygalomorha: Dipluridae). J. Exp. Biol. 1995, 198, 1137–1149. [Google Scholar]
- Hieber, C.S. The role of spider cocoons in controlling desiccation. Oecologia 1992, 89, 442–448. [Google Scholar]
- Austin, A.D. Life history of Clubiona robusta L. Koch and related species (Araneae: Clubionidae) in South Australia. J. Arachnol. 1984, 12, 87–104. [Google Scholar]
- Schaefer, M. An analysis of diapause and resistance in the egg stage of Floronia bucculenta (Araneae: Linyphiidae). Oecologia 1976, 25, 155–174. [Google Scholar] [CrossRef]
- Austin, A.D.; Anderson, D.T. Reproduction and development of the spider Nephila edulis (Koch) (Araneae: Araneidae). Aust. J. Zool. 1978, 26, 501–518. [Google Scholar] [CrossRef]
- Humphreys, W.F. Thermal behaviour of a small spider (Araneae: Araneidae: Araneinae) on horizontal webs in semi-arid Western Australia. Behav. Ecol. Sociobiol. 1991, 28, 47–54. [Google Scholar]
- Adis, J. An “aquatic” millipede from a Central Amazonian inundation forest. Oecologia 1986, 68, 347–349. [Google Scholar] [CrossRef]
- Adis, J. On the survival strategy of Mestosoma hylaeicum Jeekel, a millipede from central Amazonian floodplains (Paradoxosomatidae, Polydesmida, Diplopoda). Berichte des naturwissenschaftlich-medizinischen Vereins in Innsbruck. Supplementum 1992, 10, 183–187. [Google Scholar]
- Zerm, M. Vorkommen und Verteilung von Tausendfüßern, Hundertfüßern, Zwergfüßern (Myriapoda: Diplopoda, Chilopoda, Symphyla) und Landasseln (Isopoda: Oniscidea) in den Auen des Unteren Odertals. In Limnologie Aktuell Band 9, Das Untere Odertal, 1st ed.; Dohle, W., Bornkamm, R., Weigmann, G., Eds.; Schweizerbartsche Verlagsbuchhandlung (Nägele u. Obermiller): Stuttgart, Germany, 1999; pp. 197–210. [Google Scholar]
- Zerm, M. Distribution and phenology of Lamyctes fulvicornis and other lithobiomorph centipedes in the floodplain of the Lower Oder Valley, Germany (Chilopoda: Henicopidae, Lithobiidae. Entomol. Scand. Suppl. 1997, 51, 125–132. [Google Scholar]
- Zulka, K.P. Überflutung als ökologischer Faktor Verteilung, Phänologie und Anpassung der Diplopoda, Lithobiomorpha und Isopoda in den Flußauen der March. Ph.D. Thesis, University of Vienna, Vienna, Austria, 1991. [Google Scholar]
- Tufova, J.; Tuf, I.H. Survival under water—Comparative study of millipedes (Diplopoda), centipedes (Chilopoda) and terrestrial isopods (Oniscidea). In Proceedings of the 7th Central European Workshop on Soil Zoology, České Budějovice, Czech Republic, 2003; Tajovský, K., Schlaghamerský, J., Pižl, V., Eds.; Institute of Soil Biology: České Budějovice, Czech Republic, 2005; pp. 195–198. [Google Scholar]
- Verhoeff, K.W. Vom Einflusse unbewegten Wassers auf Tausendfüßler. 104. Diplopoden-Aufsatz. Zool. Anz. 1926, 68, 193–201. [Google Scholar]
- Schubart, O. Tausendfüßler oder Myriapoda. I: Diplopoda. In Die Tierwelt Deutschlands und der angrenzenden Meeresteile, 1st ed.; Gustav Fischer Verlag: Jena, Germany, 1934; pp. 1–318. [Google Scholar]
- Thiele, H.U. Die Diplopoden des Rheinlandes. Decheniana 1968, 120, 343–366. [Google Scholar]
- Eason, E.H. The Centipedes of the British Isles, 1st ed.; Frederick Warne & Co. Ltd.: London, UK, 1964. [Google Scholar]
- Keay, A.N.; Forman, R.I. An experimental study of the tolerance of Hapolophilus subterraneus (Shaw) and Henia vesuviana (Newport) to low humidity levels. Bull. British Myriapod Group 1987, 4, 16–21. [Google Scholar]
- Hopkin, S.P.; Read, H.J. The Biology of Millipedes, 1st ed.; Oxford University Press: New York, NY, USA, 1992. [Google Scholar]
- Krishnan, G. The millipede Thyropygus with special reference to Indian species. CSIR Zool. Mem. Indian Anim. Types 1968, 1, 1–84. [Google Scholar]
- Blower, J.G. Millipedes and centipedes as soil animals. In Soil Zoology, 1st ed.; Kevan, D.K.M., Ed.; Academic Press Inc.: New York, NY, USA, 1955; pp. 138–151. [Google Scholar]
- Demange, J.M.; Mauriès, J.P. Données de morphologie, tératologie, développement postembryonnaire, faunistique et écologie des Myriapodes Diplopodes nuisibles aux cultures du Sénégal. Bulletin du Muséum national d'histoire naturelle, 3eme série, Zoologie 1975, 225, 1243–1255. [Google Scholar]
- Haacker, U. Deskriptive, experimentelle und vergleichende Untersuchungen zur Autökologie rhein-mainischer Diplopoden. Oecologia 1968, 1, 87–129. [Google Scholar] [CrossRef]
- Lewis, J.G.E. The ecology of centipedes and millipedes in Northern Nigeria. Symp. Zool. Soc. London 1974, 32, 423–431. [Google Scholar]
- Crawford, C.S. Desert millipedes a rationale for their distribution. In Myriapod Biology; Camatini, M., Ed.; Academic Press Inc.: New York, NY, USA, 1979; pp. 171–181. [Google Scholar]
- Crawford, C.S.; Matlack, M.C. Water relations of desert millipede larvae, larva-containing pellets and surrounding soil. Pedobiologia 1979, 19, 48–55. [Google Scholar]
- Bercovitz, K.; Warburg, M.R. Factors affecting egg-laying and clutch size of Archispirostreptus tumuliporus judaicus (Attems) (Myriapoda), Diplopoda in Israel. Soil Biol. Biochem. 1988, 20, 869–874. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Marx, M.T.; Guhmann, P.; Decker, P. Adaptations and Predispositions of Different Middle European Arthropod Taxa (Collembola, Araneae, Chilopoda, Diplopoda) to Flooding and Drought Conditions. Animals 2012, 2, 564-590. https://doi.org/10.3390/ani2040564
Marx MT, Guhmann P, Decker P. Adaptations and Predispositions of Different Middle European Arthropod Taxa (Collembola, Araneae, Chilopoda, Diplopoda) to Flooding and Drought Conditions. Animals. 2012; 2(4):564-590. https://doi.org/10.3390/ani2040564
Chicago/Turabian StyleMarx, Michael Thomas, Patrick Guhmann, and Peter Decker. 2012. "Adaptations and Predispositions of Different Middle European Arthropod Taxa (Collembola, Araneae, Chilopoda, Diplopoda) to Flooding and Drought Conditions" Animals 2, no. 4: 564-590. https://doi.org/10.3390/ani2040564




