Congenital Malformations in River Buffalo (Bubalus bubalis)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Musculoskeletal Defects
2.1. Transverse Hemimelia
2.2. Arthrogryposis
2.3. Other Musculoskeletal Defects
2.4. Umbilical Hernia
2.5. Schistosoma Reflexum Syndrome
3. Gastrointestinal Defects
Atresia Ani
4. Craniofacial Abnormalities
4.1. Hydrocephalus
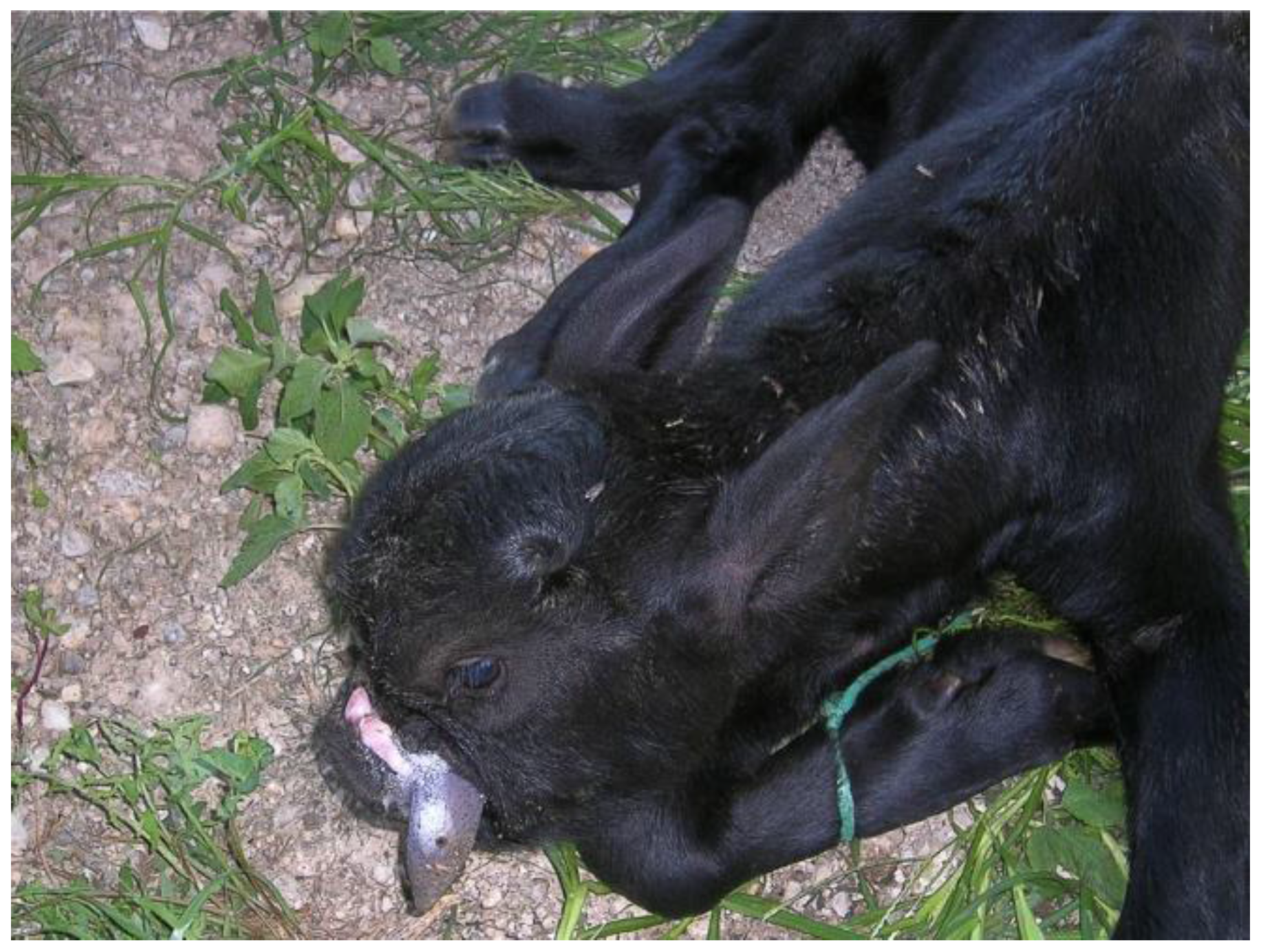
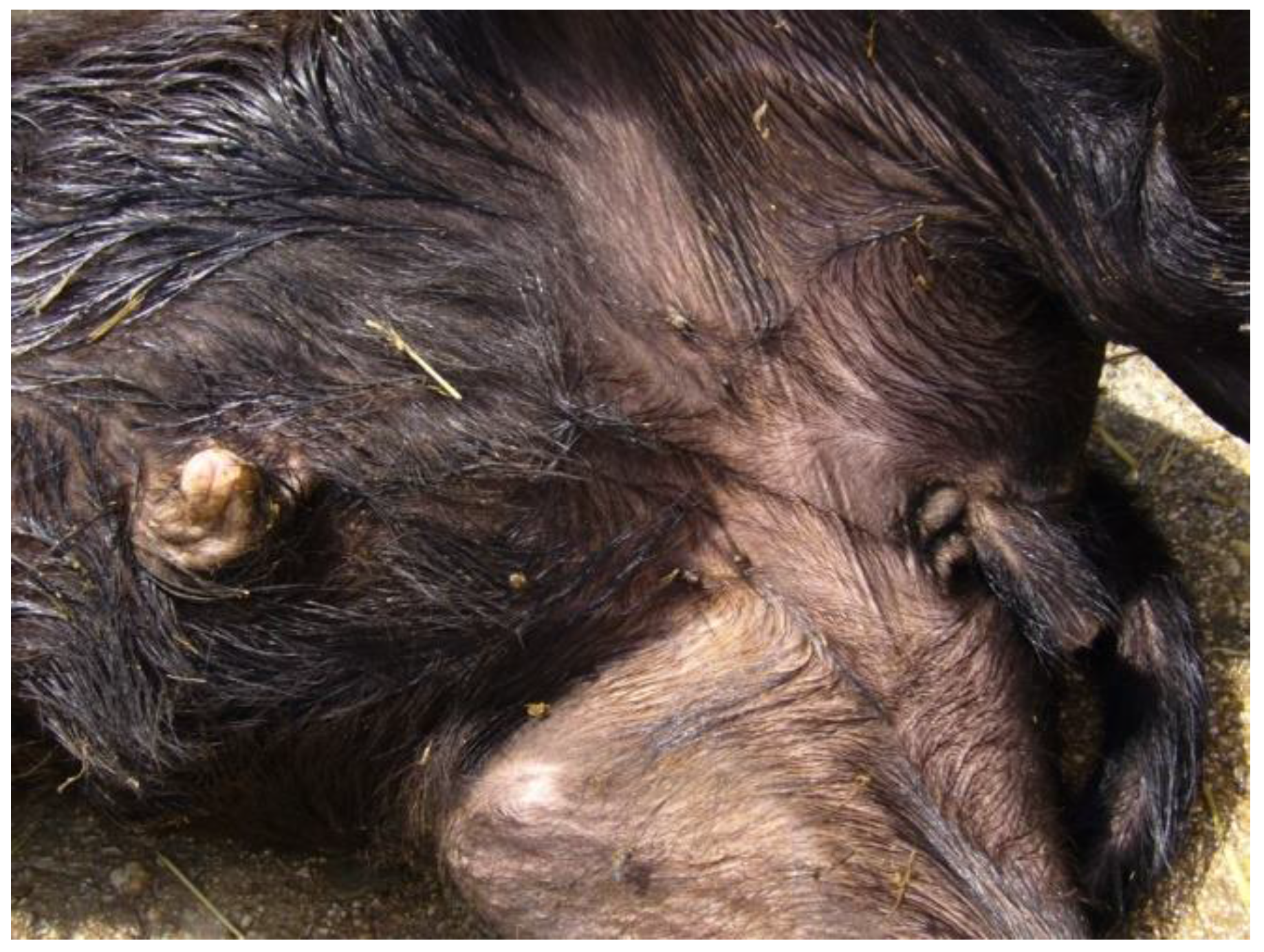
4.2. Polycephaly
4.3. Other Craniofacial Malformations
5. Disorders of Sexual Development
6. Other Malformations
Meccanobullosus Acantholytic Dermatosis
7. Conclusions
Author Contributions
Conflicts of Interest
References
- FAO. Water Buffaloes. Available online: http://www.fao.org/agriculture/dairy-gateway/milk-production/dairy-animals/water-buffaloes/en/#.WJsMcLFE_aY (accessed on 15 March 2016).
- Gholap, P.N.; Kale, D.S.; Sirothia, A.R. Genetic diseases in cattle: A review. Res. J. Anim. Vet. Fish. Sci. 2014, 2, 24–33. [Google Scholar]
- Purohit, G.N.; Kumar, P.; Solanki, K.; Shekher, C.; Yadav, S.P. Perspectives of fetal dystocia in cattle and buffalo. Vet. Sci. Dev. 2012, 2, 31–42. [Google Scholar] [CrossRef]
- Marcolongo-Pereira, C.; Schild, A.L.; Pereira-Soares, M.; Vargas, S.F., Jr.; Riet-Correa, F. Congenital defects in ruminants in southern Brazil. Pesq. Vet. Bras. 2010, 30, 816–826. [Google Scholar] [CrossRef]
- Damé, M.C.F.; Riet-Correa, F.; Schild, A.L. Hereditary diseases and congenital defects diagnosed in water buffalo (Bubalus bubalis) in Brazil. Pesq. Vet. Bras. 2013, 33, 831–839. [Google Scholar] [CrossRef]
- Peretti, V.; Ciotola, F.; Albarella, S.; Restucci, B.; Meomartino, L.; Ferretti, L.; Barbieri, V.; Iannuzzi, L. Increased SCE levels in Mediterranean Italian buffaloes affected by limb malformation (transversal hemimelia). Cytogenet. Genome Res. 2008, 120, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, G.P.; Perucatti, A.; Di Palo, R.; Iannuzzi, A.; Ciotola, F.; Peretti, V.; Neglia, G.; Campanile, G.; Zicarelli, L.; Iannuzzi, L. Sex chromosomeabnormalities and sterility in river buffalo. Cytogenet. Genome Res. 2008, 120, 127–131. [Google Scholar] [CrossRef] [PubMed]
- WHO. Congenital Anomalies. Available online: http://www.who.int/mediacentre/factsheets/fs370/en/ (accessed on 15 March 2016).
- Correale, E.; Consalvo, F. About some congenital malformation in buffaloes bred in the Salerno province (Italy). Bubalus Bubalis 2003, 9, 22–29. [Google Scholar]
- Albarella, S.; Ciotola, F.; Dario, C.; Iannuzzi, L.; Barbieri, V.; Peretti, V. Chromosome instability in Mediterranean Italian buffaloes affected by limb malformation (transversal hemimelia). Mutagenesis 2009, 24, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Whitacre, L.K.; Hoff, J.L.; Schnabel, R.D.; Albarella, S.; Ciotola, F.; Peretti, V.; Strozzi, F.; Ferrandi, C.; Ramunno, L.; Sonstegard, T.D.; et al. Elucidating the genetic basis of an oligogenic birth defect using whole genome sequence data in a non-model organism, Bubalus bubalis. Sci. Rep. 2017, 7, 39719. [Google Scholar] [CrossRef] [PubMed]
- Jethva, P.C.; Patel, S.B.; Manohar, B.K. Amelia of hind limbs in a buffalo calf: A case report. Indian J. Fields Vet. 2014, 9, 73–74. [Google Scholar]
- Honparkhe, M.; Lochan, S.; Shiv, K.; Kumar, N.; Kumar, A. Transversal tetra-hemimelia with multiple craniofacial anomalies in a buffalo calf. Indian J. Anim. Reprod. 2016, 37, 61–62. [Google Scholar]
- Agerholm, J.S.; Hewicker-Trautwein, M.; Peperkamp, K.; Windsor, P.A. Virus-induced congenital malformations in cattle. Acta Vet. Scand. 2015, 57, 54. [Google Scholar] [CrossRef] [PubMed]
- Buoen, L.C.; Zhang, T.Q.; Weber, A.F.; Turner, T.; Bellamy, J.; Ruth, G.R. Arthrogryposis in the foal and its possible relation to autosomal trisomy. Equine Vet. J. 1997, 29, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.M.; MacHugh, D.E.; Park, S.D.; Scraggs, E.; Haley, C.S.; Lynn, D.J.; Boland, M.P.; Doherty, M.L. Linkage mapping of the locus for inherited ovine arthrogryposis (IOA) to sheep chromosome 5. Mamm. Genome 2007, 18, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Beever, J.E.; Marron, B.M. Screening for Arthrogryposis Multiplex in Bovines. U.S. Patent 20110151440 A1, 23 Jun 2011. Available online: http://patft.uspto.gov/netacgi/nphParser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearchbool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=%22Screening+arthrogryposis+multiplex+bovines%22.TI.&OS=TTL/ (accessed on 15 March 2016). [Google Scholar]
- Agerholm, J.S.; McEvoy, F.J.; Menzi, F.; Jagannathan, V.; Drögemüller, C. A CHRNB1 frameshift mutation is associated with familial arthrogryposis multiplex congenita in Red dairy cattle. BMC Genom. 2016, 17, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannuzzi, L.; Di Meo, G.P.; Perucatti, A.; Rullo, R.; Incarnato, D.; Longeri, M.; Bongioni, G.; Molteni, L.; Galli, A.; Zanotti, M.; et al. Comparative FISH-mapping of the survival of motor neuron gene (SMN) in domestic bovids. Cytogenet. Genome Res. 2003, 102, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Schild, A.L.; Soares, M.P.; Damé, M.C.; Portianski, E.L.; Riet-Correa, F. Arthrogryposis in Murrah buffaloes in southern Brazil. Pesq. Vet. Bras. 2003, 23, 13–16. [Google Scholar] [CrossRef]
- Saini, G.S.; Pandey, A.K.; Chaudhary, R.N.; Kumar, A.; Sharma, S. Arthrogryposis in a Murrah buffalo calf: A case report. Buffalo Bull. 2010, 29, 318–320. [Google Scholar]
- Mehmood, M.U.; Qamar, A.; Raza, S.; Khan, H.; Shahzad, Q.; Sattar, A. Dystocia due to Perosomus Elumbis (Acaudatus) in a buffalo. Pak. J. Zool. 2014, 46, 1468–1470. [Google Scholar]
- Agerholm, J.S.; Holm, W.; Schmidt, M.; Hyttel, P.; Fredholm, M.; McEvoy, F.J. Perosomus elumbis in Danish Holstein cattle. BMC Vet. Res. 2014, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Avedillo, L.J.; Camón, J. Perosomus elumbis in a pig. Vet. Rec. 2007, 160, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, I.; Geburek, F.; Wohlsein, P. Perosomus elumbis, cerebral aplasia, and spina bifida in an aborted thoroughbred foal. Res. Vet. Sci. 2012, 92, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Kumar, W.P.; Sharma, G.C.; Krishnappa, B.; Rakesh, H.B.; Soni, Y.K. A rare case of Campylorrhacchis contorta in a buffalo: A case report. Buffalo Bull. 2014, 33, 355–357. [Google Scholar]
- Lossin, C.; George, A.L., Jr. Myotonia congenita. Adv. Gen. San. 2008, 63, 25–55. [Google Scholar]
- Borges, A.S.; Barbosa, J.D.; Resende, L.A.; Mota, L.S.; Amorim, R.M.; Carvalho, T.L.; Garcia, J.F.; Oliveira-Filho, J.P.; Oliveira, C.M.; Souza, J.E.; et al. Clinical and molecular study of a new form of hereditary myotonia in Murrah water buffalo. Neuromuscul. Disord. 2013, 23, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, B.; Grahn, R.A.; Creighton, E.K.; Williams, D.C.; Dickinson, P.J.; Sturges, B.K.; Guo, L.T.; Shelton, G.D.; Leegwater, P.A.; Longeri, M.; et al. COLQ variant associated with Devon Rex and Sphynx feline hereditary myopathy. Anim. Genet. 2015, 46, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.L.; Noorai, R.E.; Starr-Moss, A.N.; Quignon, P.; Rinz, C.J.; Ostrander, E.A.; Steiner, J.M.; Murphy, K.E.; Clark, L.A. Genome-wide association studies for multiple diseases of the German Shepherd Dog. Mamm. Genome 2012, 23, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Komine, M.; Langohr, I.M.; Kiupel, M. Megaesophagus in Friesian horses associated with muscular hypertrophy of the caudal esophagus. Vet. Pathol. 2014, 51, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Ulutas, B.; Sarierler, M.; Bayramli, G.; Ocal, K. Macroscopic findings of idiopathic congenital megaoesophagus in a calf. Vet. Rec. 2006, 158, 26. [Google Scholar] [CrossRef] [PubMed]
- Brenig, B.; Schütz, E.; Hardt, M.; Scheuermann, P.; Freick, M. A 20 bp Duplication in Exon 2 of the Aristaless-Like Homeobox 4 Gene (ALX4) Is the Candidate Causative Mutation for Tibial Hemimelia Syndrome in Galloway Cattle. PLoS ONE 2015, 10, e0129208. [Google Scholar] [CrossRef] [PubMed]
- Steinman, A.; Kelmer, G.; Avni, G.; Johnston, D.E. Omphalocele in a foal. Vet. Rec. 2000, 146, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.S.; Mao, H.R.; Guo, Y.M.; Ren, J.; Xiao, S.J.; Wu, G.Z.; Shen, H.Q.; Wu, L.H.; Ruan, G.F.; Brenig, B.; et al. A genome-wide scan reveals candidate susceptibility loci for pig hernias in an intercross between White Duroc and Erhualian. J. Anim. Sci. 2009, 87, 2469–2474. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Long, Y.; Ruan, G.; Wu, L.; Zhang, Z.; Xiao, S.; Deng, W.; Lv, X.; Hu, D.; Wu, G.; et al. Identification of susceptibility gene for pig umbilical hernia in different populations using transmission disequilibrium test. Yi Chuan 2014, 36, 995–1005. [Google Scholar] [PubMed]
- Čítek, J.; Řehout, V.; Hájková, J. Congenital disorders in the cattle population of the Czech Republic. Czech J. Anim. Sci. 2009, 54, 55–64. [Google Scholar]
- Ron, M.; Tager-Cohen, I.; Feldmesser, E.; Ezra, E.; Kalay, D.; Roe, B.; Seroussi, E.; Weller, J.I. Bovine umbilical hernia maps to the centromeric end of Bos taurus autosome 8. Anim. Genet. 2004, 35, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Amle, M.B.; Shelar, R.R.; Thorat, M.G.; Zope, A.N. Congenital umbilical hernia and cryptorchidism in a Pandharpuri buffalo calf. Buffalo Bull. 2004, 23, 82–83. [Google Scholar]
- Iannuzzi, L.; Di Meo, G.P.; Perucatti, A.; Schibler, L.; Incarnato, D.; Gallagher, D.; Eggen, A.; Ferretti, L.; Cribiu, E.P.; Womack, J. The river buffalo (Bubalus bubalis, 2n = 50) cytogenetic map: Assignment of 64 loci by fluorescence in situ hybridization and R-banding. Cytogenet. Genome Res. 2003, 102, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Laughton, K.W.; Fisher, K.R.; Halina, W.G.; Partlow, G.D. Schistosomus reflexus syndrome: A heritable defect in ruminants. Anat. Histol. Embryol. 2005, 34, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Citek, J. Pedigree analysis of Czech Holstein calves with schistosoma reflexum. Acta Vet. Scand. 2012, 54, 22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, S.; Sharma, U.; Kushwaha, R.B.; Pandey, A.K. Dystocia due to schistosomus reflexus in a Murrah buffalo. Indian J. Anim. Reprod. 2012, 33, 84–85. [Google Scholar]
- Ozalp, G.R.; Celikler, S.; Simsek, G.; Ozyigit, M.O.; Inan, S. A case of schistosoma reflexum in a cat with chromosomal aberrations. Reprod. Domest. Anim. 2011, 46, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Binanti, D.; Prati, I.; Locatelli, V.; Pravettoni, D.; Sironi, G.; Riccaboni, P. Perineal choristoma and atresia ani in 2 female Holstein Friesian calves. Vet. Pathol. 2013, 50, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Greber, D.; Doherr, M.; Drögemüller, C.; Steiner, A. Occurrence of congenital disorders in Swiss sheep. Acta Vet. Scand. 2013, 55, 27. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.B.; Ferris, R.A.; McCue, P.M.; Leise, B.S. Surgical management of atresia ani and perineal hypospadias in a miniature donkey foal. Equine Vet. Educ. 2015. [Google Scholar] [CrossRef]
- Zimmerman, J.J.; Karriker, L.A.; Ramirez, A.; Schwartz, K.J.; Stevenson, G.W. Diseases of Swine, 10th ed.; Wiley-Blackwell: Ames, IA, USA, 2012; pp. 119–141. [Google Scholar]
- Cassini, P.; Montironi, A.; Botti, S.; Hori, T.; Okhawa, H.; Stella, A.; Andersson, L.; Giuffra, E. Genetic analysis of anal atresia in pigs: Evidence for segregation at two main loci. Mamm. Genome 2005, 16, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, S.; Fries, R.; Thaller, G. Genome wide scan for anal atresia in swine identifies linkage and association with a chromosome region on Sus scrofa chromosome 1. Genetics 2005, 171, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Wang, C.; Li, X.; Yu, M.; Zhao, S.H.; Li, X. Molecular characterization and genome-wide mutations in porcine anal atresia candidate gene GLI2. Mamm. Genome 2013, 24, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Mohammadi, R.; Mohammadpour, I. Surgical repair and management of congenital intestinal atresia in 68 calves. Vet. Surg. 2010, 39, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.; Yoshida, C.; Isobe, N.; Nakao, T.; Yamashiro, H.; Kubota, H.; Miyake, Y.; Nakada, K. Atresia ani with diphallus and separate scrota in a calf: A case report. Theriogenology 2004, 61, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.E.; Yoshida, C.; Nishibori, M.; Nakao, T.; Yamashiro, H. A case of freemartin with atresia recti and ani in Japanese Black calf. Anim. Reprod. Sci. 2005, 85, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Gülbahar, M.Y.; Kabak, M.; Yarim, M.; Guvenc, T.; Kabak, Y.B. Persistent cloaca, fused kidney, female pseudohermaphroditism and skeletal anomalies in a simmenthal calf. Anat. Histol. Embryol. 2009, 38, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, L.; Cheng, W. Anorectal malformation: The etiological factors. Pediatr. Surg. Int. 2015, 31, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.B. Hydrocephalus in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Saini, G.S.; Chander, S.; Chaudhary, R.N.; Jakhar, P.; Singh, M.; Sundar, S.; Yadav, S. Dystocia due to abnormal calf in a buffalo: A case report. Buffalo Bull. 2010, 29, 315–317. [Google Scholar]
- Sharma, S.K.; Joshi, M.; Khosa, J.S.; Singh, D. An unusual case of dystocia due to hydrocephalic monster in a buffalo. Int. J. Sci. Environ. Technol. 2015, 4, 300–304. [Google Scholar]
- El-Sheikh, H.; Hegab, A.O.; Zaabel, S.M. Dicephalicatlodymus monster associated with hydropsamnii in a buffalo cow: A case report. Vet. Res. 2010, 3, 46–48. [Google Scholar]
- Gülbahar, M.Y.; Yüksel, H.; Soygüder, Z.; Erçin, Ö.F. Dicephalus, Arnold-Chiari malformation, spinal dysraphism and other associated anomalies in a Newborn Holstein Calf. Turk. J. Vet. Anim. Sci. 2005, 29, 565–570. [Google Scholar]
- Hind Osman, E.; Badawi, M.N.; Musa, M.B.; Hala Ali, M.; RababAhamed, M. A case of diprosopia in anomalus cross-bred bovine calf. Vet. Res. 2007, 1, 61–64. [Google Scholar]
- Hussein, R.M.N. Congenital anomalies in cattle and buffalo within Mudaina city in Basrah province between period 2007–2009. Kufa J. Vet. Med. Sci. 2010, 1, 207–218. [Google Scholar]
- Wakuri, H.; Mori, T.; Mutoh, K. Arnold-Chiari malformation and associated anomalies in a dicephalic newborn calf. Okajimas Folia Anat. Jpn. 1990, 67, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Mazzullo, G.; Germanà, A.; De Vico, G.; Germanà, G. Diprosopiasis in a lamb. A casereport. Anat. Histol. Embryol. 2003, 32, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, R.O. A dicephalic goat with other defects. Zentral Vet. A 1996, 43, 337–343. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Vasishta, N.K. A diprosopus buffalo neonate: A case report: A case report. Buffalo Bull. 2010, 29, 62–64. [Google Scholar]
- Kumar, P.; Sharma, A.; Singh, M.; Sood, P.; Barman, P. Dystocia due to a dichephalus monster fetus in a buffalo. Buffalo Bull. 2014, 33, 13–15. [Google Scholar]
- Mehmood, M.U.; Abbas, W.; Jabbar, A.; Khan, J.; Riaz, A.; Sattar, A. An Iniodymus Dicephalic Buffalo Neonate. J. Anim. Plant Sci. 2014, 24, 973–975. [Google Scholar]
- Srivastava, S.; Kumar, A.; Maurya, S.K.; Singh, A.; Singh, K. A dicephalus monster in murrah buffalo. Buffalo Bull. 2008, 27, 231–232. [Google Scholar]
- Schalles, R.R.; Leipold, H.W.; McCraw, R.L. Congenital defect in cattle. In Beef Cattle Handbook; Iowa State University: Ames, IA, USA, 1999; pp. 1–5. [Google Scholar]
- Kerkmann, A.; Kuiper, H.; Ganter, M.; Distl, O. Review of literature and results from test matings of East Friesian milk sheep affected with brachygnathia inferior. Berl. Münch. Tierärztl. Wochenschr. 2008, 121, 292–305. [Google Scholar] [PubMed]
- Davies, S.J.; Wise, C.; Venkatesh, B.; Mirza, G.; Jefferson, A.; Volpi, E.V.; Ragoussis, J. Mapping of three translocation breakpoints associated with orofacial clefting within 6p24 and identification of new transcripts within the region. Cytogenet. Genome Res. 2004, 105, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Z.T.; Leslie, E.J.; Arzi, B.; Jayashankar, K.; Karmi, N.; Jia, Z.; Rowland, D.J.; Young, A.; Safra, N.; Sliskovic, S.; et al. A LINE-1 insertion in DLX6 is responsible for cleft palate and mandibular abnormalities in a canine model of Pierre Robin sequence. PLoS Genet. 2014, 10, e1004257. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.P.; Nema, S.P.; Pandey, A.K.; Garg, U.K. Dystocia due to bull dog calf in a she buffalo. Buffalo Bull. 2007, 26, 104–105. [Google Scholar]
- Singh, G.; Pandey, A.K.; Kumar, S.; Sunder, S.; Kumar, R.; Dutt, R. Lipomatous bull dog calf monster in a Murrah buffalo. Indian J. Anim. Reprod. 2012, 33, 94–95. [Google Scholar]
- Lee, P.A.; Houk, C.P.; Ahmed, S.F.; Hughes, I.A. Consensus statement on management of intersex disorders. Pediatrics 2006, 118, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.R.; Kumar, P.; Tomer, O.S.; Kumar, S.; Balain, D.S. Monosomy X and gonadal dysgenesis in a buffalo heifer (Bubalus bubalis). Theriogenology 1990, 34, 99–105. [Google Scholar] [CrossRef]
- Prakash, B.; Balain, D.S.; Lathwal, S.S. A 49, XO sterile Murrah buffalo (Bubalus bubalis). Vet. Rec. 1992, 130, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Balain, D.S.; Lathwal, S.S.; Malik, R.K. Trisomy-X in a sterile river buffalo. Vet. Rec. 1994, 134, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Villagómez, D.A.; Parma, P.; Radi, O.; Di Meo, G.P.; Pinton, A.; Iannuzzi, L.; King, W.A. Classical and molecular cytogenetics of disorders of sex development in domestic animals. Cytogenet. Genome Res. 2009, 126, 110–131. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Singh, K.M.; Soni, K.J.; Chauhan, J.B. Novel cytogenetic finding: An unusual X;X-translocation in Mehsana buffalo (Bubalus bubalis). Cytogenet. Genome Res. 2006, 115, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, L. Evolutionary, Clinical and Molecular Cytogenetics in Water Buffalo: An update. Buffalo Bull. 2013, 32, 244–256. [Google Scholar] [CrossRef]
- Anaya, G.; Moreno-Millán, M.; Bugno-Poniewierska, M.; Pawlina, K.; Membrillo, A.; Molina, A.; Demyda-Peyrás, S. Sex reversal syndrome in the horse: Four new cases of feminization in individuals carrying a 64,XY SRY negative chromosomal complement. Anim. Reprod. Sci. 2014, 151, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Villagómez, D.A.; Lear, T.L.; Chenier, T.; Lee, S.; McGee, R.B.; Cahill, J.; Foster, R.A.; Reyes, E.; St John, E.; King, W.A. Equine disorders of sexual development in 17 mares including XX, SRY-negative, XY, SRY-negative and XY, SRY-positive genotypes. Sex Dev. 2011, 5, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Switonski, M.; Chmurzynska, A.; Szczerbal, I.; Lipczynski, A.; Yang, F.; Nowicka-Posłuszna, A. Sex reversal syndrome (64,XY; SRY-positive) in a mare demonstrating masculine behaviour. J. Anim. Breed. Genet. 2005, 122, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, C.; Parma, P.; De Lorenzi, L.; Di Ianni, F.; Bertocchi, M.; Bertani, V.; Cantoni, A.M.; Parmigiani, E. A Clinical Case of an SRY-Positive Intersex/Hermaphrodite Holstein Cattle. Sex Dev. 2015, 9, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Pinton, A.; Pailhoux, E.; Piumi, F.; Rogel-Gaillard, C.; Darré, R.; Yerle, M.; Ducos, A.; Cotinot, C. A case of intersexuality in pigs associated with a de novo paracentric inversion 9 (p1.2; p2.2). Anim. Genet. 2002, 33, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Meyers-Wallen, V.N. Review and update: Genomic and molecular advances in sex determination and differentiation in small animals. Reprod. Domest. Anim. 2009, 44, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Schlafer, D.H.; Valentine, B.; Fahnestock, G.; Froenicke, L.; Grahn, R.A.; Lyons, L.A.; Meyers-Wallen, V.N. A case of SRY-positive 38,XY true hermaphroditism (XY sex reversal) in a cat. Vet. Pathol. 2011, 48, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Urh, K.; Kunej, T. Molecular mechanisms of cryptorchidism development: Update of the database, disease comorbidity, and initiative for standardization of reporting in scientific literature. Andrology 2016, 4, 894–902. [Google Scholar] [CrossRef] [PubMed]

| Congenital Malformations | Breed | Cases (n) | References |
|---|---|---|---|
| Musculoskeletal defects | |||
| Transverse Hemimelia | MIRB | 10 | [6] |
| MIRB | 13 | [10] | |
| Indian Meshana | 1 | [12] | |
| MIRB | 91 | Present study | |
| unknown | 1 | [13] | |
| Artrogryphosis | MIRB | 1 | [9] |
| Murrah | 6 | [20] | |
| Murrah | 1 | [21] | |
| Other musculoskeletal defects | |||
| Perosomus elumbis | Nili Ravi | 1 | [22] |
| Campylorrhacchis contorta | unknown | 1 | [26] |
| Myotonia congenita | Murrah | 29 | [4] |
| Megaesophagus | Murrah | 9 | [5] |
| Other limb malformations | MIRB | 2 | Present study |
| Omphalocele | MIRB | 72 | [9] |
| Pandharpuri | 1 | [39] | |
| Schistosoma Reflexum | MIRB | 2 | [9] |
| Murrah | 1 | [44] | |
| Gastrointestinal defects | |||
| Atresia ani | MIRB | 2 | [9] |
| Craniofacial abnormality | |||
| Hydrocephalus | MIRB | 3 | [9] |
| Murrah | 7 | [4] | |
| Surti | 1 | [58] | |
| – | 1 | [59] | |
| Polycephaly | MIRB | 1 | Present study |
| – | 1 | [68] | |
| – | 1 | [60] | |
| – | 1 | [67] | |
| Nili Ravi | 1 | [69] | |
| Other Craniofacial malformations | Murrah | 2 | [75,76] |
| Surti | 1 | [58] | |
| MIRB | 1 | Present study | |
| Disorders of sexual development | |||
| X-monosomy | MIRB | 2 | [7] |
| X-Trisomy | MIRB | 1 | [7] |
| Sex reversal | MIRB | 2 | [7] |
| Cryptorchidism | MIRB | 1 | Present study |
| Other malformations | |||
| Meccanobullosus acantholytic dermatosis | Murrah | 4 | [5] |
| Malformed Limb | Anatomic Structures Involved | Frequency (%) |
|---|---|---|
| Left hind limb | Proximal epiphysis tibia | 6 |
| Distal epiphysis tibia | 2 | |
| Tarsus | 10 | |
| Proximal epiphysis metatarsus | 8 | |
| Distal epiphysis metatarsus | 10 | |
| Proximal epiphysis of first phalanx | 8 | |
| Right hind limb | Proximal epiphysis tibia | 12 |
| Tarsus | 2 | |
| Proximal epiphysis metatarsus | 6 | |
| Distal epiphysis metatarsus | 6 | |
| Both hind limbs | r. distal epiphysis tibia l. proximal epiphysis metatarsus | 6 |
| r. distal epiphysis tibia l. distal epiphysis metatarsus | 2 | |
| r. metatarsus l. metatarsus | 8 | |
| r. second tarsus bones l. second phalanx | 2 | |
| r. knee l. twisted | 2 | |
| Both hind limbs and one forelimb | r. hind limb second tarsus bones l. hind limb diaphysis tibia l. forelimb loss of the third phalanx | 2 |
| r. hind limb second tarsus bones l. hind limb proximal epiphysis metatarsus r. forelimb hypoplasia | 2 | |
| Amelia of both hind limbs r. forelimb longitudinal ectromelia of metacarpus | 2 | |
| All limbs involved | 4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albarella, S.; Ciotola, F.; D’Anza, E.; Coletta, A.; Zicarelli, L.; Peretti, V. Congenital Malformations in River Buffalo (Bubalus bubalis). Animals 2017, 7, 9. https://doi.org/10.3390/ani7020009
Albarella S, Ciotola F, D’Anza E, Coletta A, Zicarelli L, Peretti V. Congenital Malformations in River Buffalo (Bubalus bubalis). Animals. 2017; 7(2):9. https://doi.org/10.3390/ani7020009
Chicago/Turabian StyleAlbarella, Sara, Francesca Ciotola, Emanuele D’Anza, Angelo Coletta, Luigi Zicarelli, and Vincenzo Peretti. 2017. "Congenital Malformations in River Buffalo (Bubalus bubalis)" Animals 7, no. 2: 9. https://doi.org/10.3390/ani7020009






