Predicting Lameness in Sheep Activity Using Tri-Axial Acceleration Signals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials & Methods
2.1. Study Site and Animals
2.2. Instrumentation
2.3. Observations
2.4. Lameness Simulation
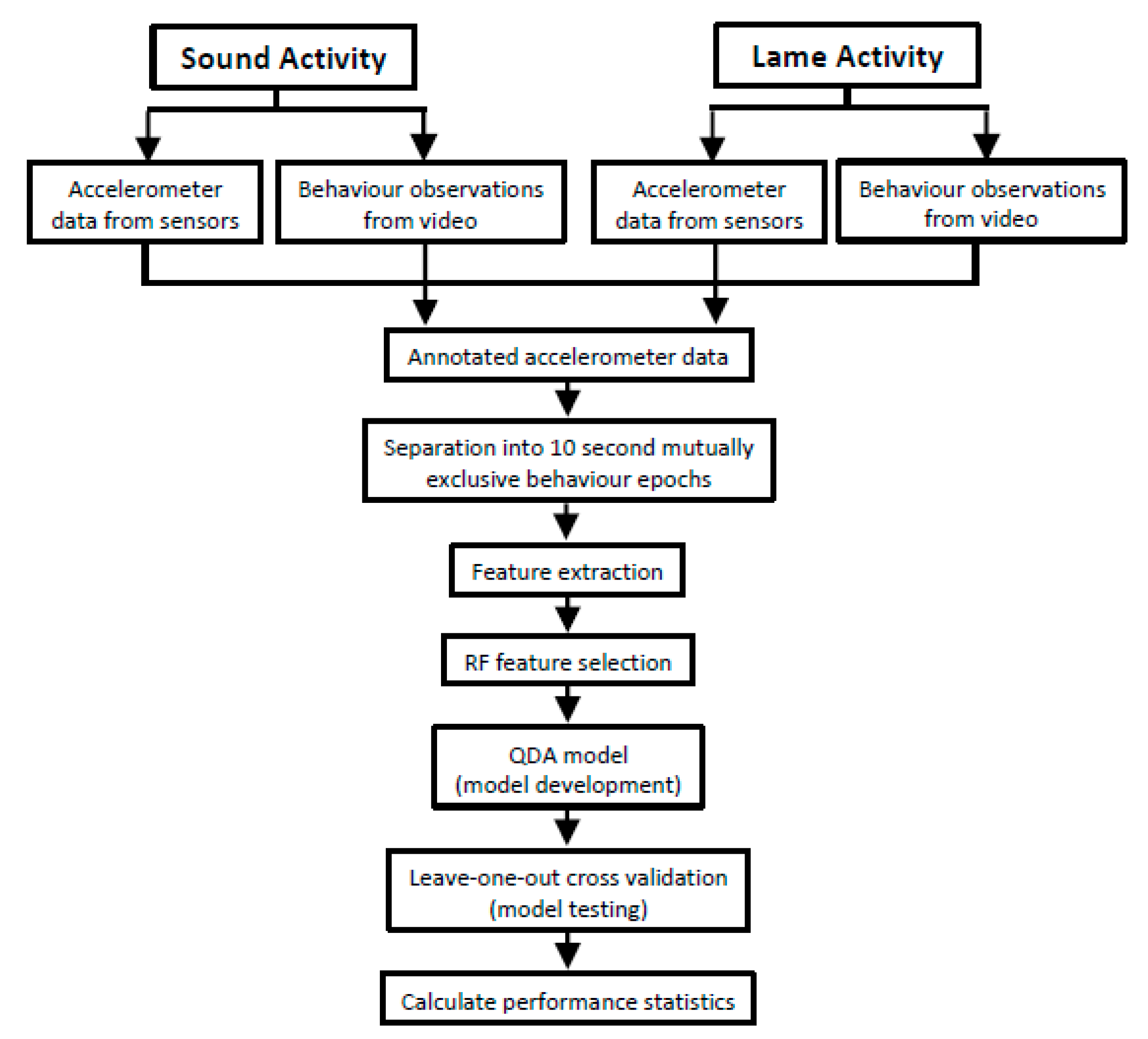
2.5. Developing the Behaviour Classification Model
2.6. Feature Relative Importance and Behaviour Classification
2.7. Model Validation
3. Results & Discussion
3.1. Observations
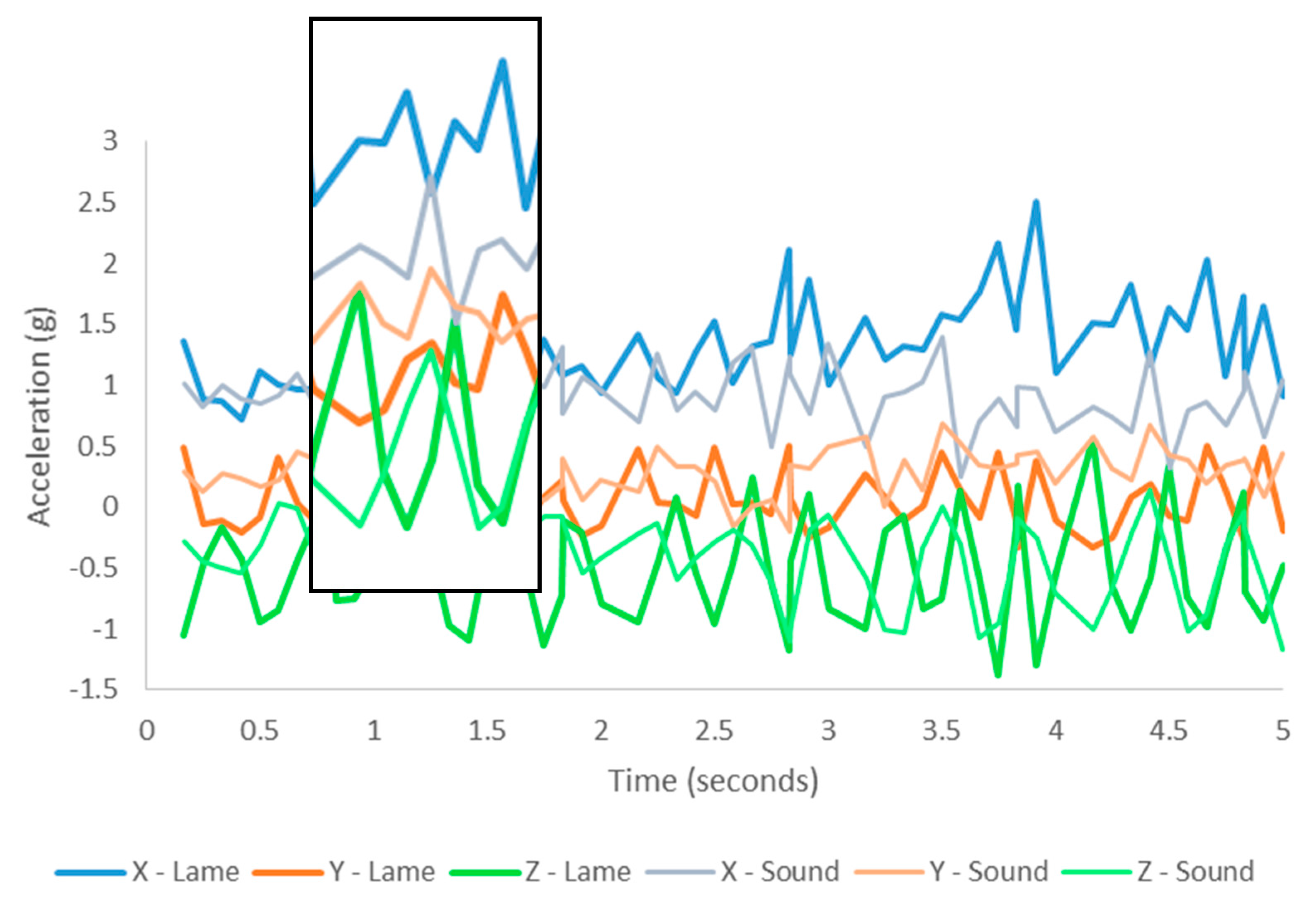
3.2. Visual Description of Behaviour—Ear as an Example
3.3. Feature Selection
3.4. Behaviour Prediction Algorithm
3.4.1. Analysis I
3.4.2. Analysis II
Ear
Collar
Leg
3.5. Classification Algorithm Performance
- Sensitivity = 283/(283 + 13 + 2) = 95%
- Specificity = 241/(241 + 28) = 90%
- Accuracy = (283 + 241)/(298 + 269) = 92%
- Precision = 283/(283 + 26 + 2) = 91%
Ear
Collar
Leg
3.6. General Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abbott, K.; Lewis, C. Current approaches to the management of ovine footrot. Vet. J. 2005, 169, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Winter, A. Lameness in sheep. Small Rumin. Res. 2008, 76, 149–153. [Google Scholar] [CrossRef]
- Hodgkinson, O. The importance of feet examination in sheep health management. Small Rumin. Res. 2010, 92, 67–71. [Google Scholar] [CrossRef]
- Anil, S.; Anil, L.; Deen, J. Challenges of pain assessment in domestic animals. J. Am. Vet. Med. Assoc. 2002, 220, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Animal Health Australia. Available online: www.animalhealthaustralia.com.au/wp-content/uploads/2015/09/LBN_Arthritis_Fact_Sheet.pdf (accessed on 6 November 2017).
- Brightling, T. Livestock Diseases in Australia: Diseases of Cattle, Sheep, Goats and Farm Dogs; Jerram, C.H., Ed.; Mt. Waverly: Victoria, Australia, 2006. [Google Scholar]
- Weary, D.; Huzzey, J.; Von Keyserlingk, M. Board-Invited Review: Using behavior to predict and identify ill health in animals. J. Anim. Sci. 2009, 87, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Lascelles, B.; Roe, S.; Smith, E.; Reynolds, L.; Marcellin-Little, J.; Bergh, M.; Budsberg, S. Evaluation of a pressure walkway system for measurement of vertical limb forces in clinically normal dogs. Am. J. Vet. Res. 2006, 67, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Besancon, M.; Conzemius, M.; Derrick, T.; Ritter, M. Comparison of vertical forces in normal greyhounds between force platform and pressure walkway measurement systems. VCOT Arch. 2003, 16, 153. [Google Scholar]
- Oosterlinck, M.; Pille, F.; Huppes, T.; Gasthuys, F.; Back, W. Comparison of pressure plate and force plate gait kinetics in sound Warmbloods at walk and trot. Vet. J. 2010, 186, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Breur, G. Temporospatial and kinetic characteristics of sheep walking on a pressure sensing walkway. Can. J. Vet. Res. 2008, 72, 50–55. [Google Scholar] [PubMed]
- Seebeck, P.; Thompson, M.; Parwani, A.; Taylor, W.; Schell, H.; Duda, G. Gait evaluation: A tool to monitor bone healing? Clin. Biomech. 2005, 20, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Rajkondawar, P.; Liu, M.; Dyer, R.; Neerchal, N.; Tasch, U.; Lefcourt, A.; Erez, B.; Varner, M. Comparison of models to identify lame cows based on gait and lesion scores, and limb movement variables. J. Dairy Sci. 2006, 89, 4267–4275. [Google Scholar] [CrossRef]
- Maertens, W.; Baert, J.; Vangayte, J.; Vranken, E.; Berckmans, D.; Sonck, B. Acquisition techniques for dairy cow gait analysis. Precis. Livest. Farming 2007, 7, 33–140. [Google Scholar]
- Pluk, A.; Bahr, C.; Maernets, W.; Veermäe, I.; Kokin, E.; Praks, J.; Poikalainen, V.; Pastell, M.; Ahokas, J.; van Nuffel, A.; Vangeyte, J.; Snock, B.; Berckmans, D. Approach to model based motion scoring for lameness detection in dairy cattle. Precis. Livest. Farming 2009, 9, 357–362. [Google Scholar]
- Pastell, M.; Tiusanen, J.; Hakojärvi, M.; Hänninen, L. A wireless accelerometer system with wavelet analysis for assessing lameness in cattle. Biosyst. Eng. 2009, 104, 545–551. [Google Scholar] [CrossRef]
- Van Nuffel, A.; Zwertvaegher, I.; Pluym, L.; Van Weyenberg, S.; Thorup, V.; Pastell, M.; Sonck, B.; Saeys, W. Lameness detection in dairy cows: Part 1. How to distinguish between non-lame and lame cows based on differences in locomotion or behavior. Animals 2015, 5, 838–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprecher, D.; Hostetler, D.; Kaneene, J. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology 1997, 47, 1179–1187. [Google Scholar] [CrossRef]
- Robert, B.; White, B.; Renter, D.; Larson, R. Evaluation of three-dimensional accelerometers to monitor and classify behavior patterns in cattle. Comput. Electron. Agric. 2009, 67, 80–84. [Google Scholar] [CrossRef]
- Martiskainen, P.; Ja¨rvinen, M.; Sko¨n, J.; Tiirikainen, J.; Kolehmainen, M.; Mononen, J. Cow behaviour pattern recognition using a three-dimensional accelerometer and support vector machines. Appl. Anim. Behav. Sci. 2009, 119, 32–38. [Google Scholar] [CrossRef]
- De Passille, A.; Jensen, M.; Chapinal, N.; Rushen, J. Technical note: Use of accelerometers to describe gait patterns in dairy calves. J. Dairy Sci. 2010, 93, 3287–3293. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.; Valencia, P.; Overs, L.; Paull, D.; Purvis, I. New ways of measuring intake, efficiency and behaviour of grazing livestock. Anim. Prod. Sci. 2014, 54, 1796–1804. [Google Scholar] [CrossRef]
- Scheibe, K.; Gromann, C. Application testing of a new three-dimensional acceleration measuring system with wireless data transfer (WAS) for behavior analysis. Behav. Res. Methods 2006, 38, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Keegan, K.; Yonezawa, Y.; Pai, P.; Wilson, D. Accelerometer-based system for the detection of lameness in horses. Biomed. Sci. Instrum. 2001, 38, 107–112. [Google Scholar]
- Keegan, K.; Yonezawa, Y.; Pai, F.; Wilson, D.; Kramer, J. Evaluation of a sensor-based system of motion analysis for detection and quantification of forelimb and hind limb lameness in horses. Am. J. Vet. Res. 2004, 65, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Kokin, E.; Praks, J.; Veermäe, I.; Poikalainen, V.; Vallas, M. IceTag3D™ accelerometric device in cattle lameness detection. Agron. Res. 2014, 12, 223–230. [Google Scholar]
- Chapinal, N.; de Passillé, A.; Rushen, J.; Wagner, S. Automated methods for detecting lameness and measuring analgesia in dairy cattle. J. Dairy Sci. 2010, 93, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Higginson, J.; Millman, S.; Leslie, K.; Kelton, D. Validation of a new pedometry system for use in behavioural research and lameness detection in dairy cattle. In Proceedings of the First North America Conference of Precision Dairy Management, Toronto, ON, Canada, 2–5 March 2010; Progressive Dairy Operators: Elora, ON, Canada, 2010. [Google Scholar]
- O’Callaghan, K.; Cripps, P.; Downham, D.; Murray, R. Subjective and objective assessment of pain and discomfort due to lameness in dairy cattle. In Proceedings of the 2nd Intermational Workshop on the Assessment of Animal Welfare at Farm and Group Level, Bristol, UK, 4–6 September 2002; Federation for Animal Welfare: Hertfordshire, UK, 2003. [Google Scholar]
- Blackie, N.; Amory, J.; Bleach, E.; Scaife, J. The effect of lameness on lying behaviour of zero grazed Holstein dairy cattle. Appl. Anim. Behav. Sci. 2011, 134, 85–91. [Google Scholar] [CrossRef]
- Blomberg, K. Automatic Registration of Dairy Cows Grazing Behaviour on Pasture; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2011. [Google Scholar]
- Alvarenga, F.; Borges, I.; Palkoviˇc, L.; Rodina, J.; Oddy, V.; Dobos, R. Using a three-axis accelerometer to identify and classify sheep behaviour at pasture. Appl. Anim. Behav. Sci. 2016, 181, 91–99. [Google Scholar] [CrossRef]
- Mclennan, K.; Skillings, E.; Rebelo, C.; Corke, M.; Moreira, M.; Morton, J.; Constantino-Casas, F. Technical note: Validation of an automatic recording system to assess behavioural activity level in sheep (Ovis aries). Small Rumin. Res. 2015, 127, 92–96. [Google Scholar] [CrossRef]
- Umstatter, C.; Waterhouse, A.; Holland, J. An automated sensor-based method of simple behavioural classification of sheep in extensive systems. Comput. Electron. Agric. 2008, 64, 19–26. [Google Scholar] [CrossRef]
- Mason, A.; Sneddon, J. Automated monitoring of foraging behaviour in free ranging sheep grazing a biodiverse pasture. In Proceedings of the Seventh International Conference on Sensing Technology, Wellington, New Zealand, 3–5 December 2013. [Google Scholar]
- Trotter, M.; Lamb, D.; Hinch, G.; Guppy, C. Global navigation satellite system livestock tracking: System development and data interpretation. Anim. Prod. Sci. 2010, 50, 616–623. [Google Scholar] [CrossRef]
- Luu, J.; Johnsen, J.; de Passille, A.; Rushen, J. Which measures of acceleration best estimate the duration of locomotor play by dairy calves? Appl. Anim. Behav. Sci. 2013, 148, 21–27. [Google Scholar] [CrossRef]
- Campbell, H.; Gao, L.; Bidder, O.; Hunter, J.; Franklin, C. Creating a behavioural classification module for acceleration data: Using a captive surrogate for difficult to observe species. J. Exp. Boil. 2013, 216, 4501–4506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sawchuk, A. A feature selection-based framework for human activity recognition using wearable multimodal sensors. In Proceedings of the 6th International Conference on Body Area Networks, Beijing, China, 7–10 November 2011; Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering: Beijing, China, 2011. [Google Scholar]
- Trotter, M.; Falzon, G.; Dobos, R.; Lamb, D.; Schneider, D. Accelerometer Based Inference of Livestock Behaviour; In Science and Innovation Awards for Young People in Agriculture, Fisheries, and Forestry; Australian Government: NSW, Australia, 2012.
- Marais, J.; Le Roux, S.; Wolhuter, R.; Niesler, T. Automatic classification of sheep behaviour using 3-axis accelerometer data. In Proceedings of the 2014 PRASA, RobMech and AfLaT International Joint Symposium, Cape Town, South Africa, 27–28 November 2014. [Google Scholar]
- Liaw, A.; Wiener, M. Classification and Regression by random Forest. R News 2002, 2, 18–22. [Google Scholar]
- Diaz-Uriate, R. varSelRF: Variable Selection Using Random Forests. 2014. Available online: http://CRAN.R-project.org/package=varSelRF (accessed on 22 December 2017).
- Pober, D.M.; Staudenmayer, J.; Raphael, C.; Freedson, P. Development of novel techniques to classify physical activity mode using accelerometers. Med. Sci. Sports Exerc. 2006, 38, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Stone, M. Cross-validatory choice and assessment of statistical predictions. J. R. Stat. Soc. 1974, 36, 111–147. [Google Scholar]
- Flower, F.; Sanderson, D.; Weary, D. Effects of milking on dairy cow gait. J. Dairy Sci. 2006, 89, 2084–2089. [Google Scholar] [CrossRef]
- Distl, O.; Mair, A. Computerized analysis of pedobarometric forces in cattle at the ground surface/floor interface. Comput. Electron. Agric. 1993, 8, 237–250. [Google Scholar] [CrossRef]
- Nordlund, K.; Cook, N.B.; Oetzel, G.R. Investigation strategies for laminitis problem herds. J. Dairy Sci. 2004, 87, 27–35. [Google Scholar] [CrossRef]
- Ford, L.; Brian, K. Reducing Lameness for Better Returns 2016. Available online: http://beefandlamb.ahdb.org.uk/wp-content/uploads/2016/03/BRP-Reducing-lameness-manual-7-080316.pdf (accessed on 22 December 2017).
- Kaler, J. Epidemiological Investigations into Lameness in Sheep; University of Warwick: Coventry, UK, 2008. [Google Scholar]
- Colditz, I.; Paull, D.; Hervault, G.; Aubriot, D.; Lee, C. Development of a lameness model in sheep for assessing efficacy of analgesics. Aust. Vet. J. 2011, 89, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Krohn, C.; Munksgaard, L. Behaviour of dairy cows kept in extensive (loose housing/pasture) or intensive (tie stall) environments II. Lying and lying-down behaviour. Appl. Anim. Behave. Sci. 1993, 37, 1–16. [Google Scholar] [CrossRef]
- Kubat, M.; Holte, R.C.; Matwin, S. Machine learning for the detection of oil spills in satellite radar images. Mach. Learn. 1998, 30, 195–215. [Google Scholar] [CrossRef]
- Mazrier, H.; Tal, S.; Aizinbud, E.; Bargai, U. A field investigation of the use of the pedometer for the early detection of lameness in cattle. Can. Vet. J. 2006, 47, 883–886. [Google Scholar] [PubMed]
- Ito, K.; Weary, D.; von Keyserlingk, M. Extreme lying times predict lameness. In Proceedings of the 9th ISAE North-American Regional Meeting, Montreal, QC, Canada, 17–18 July 2009. [Google Scholar]
- King, E.M.; Green, L.E. Assessment of farmer recognition and reporting of lameness in adults in 35 lowland sheep flocks in England. Anim. Welf. 2011, 20, 321–328. [Google Scholar]
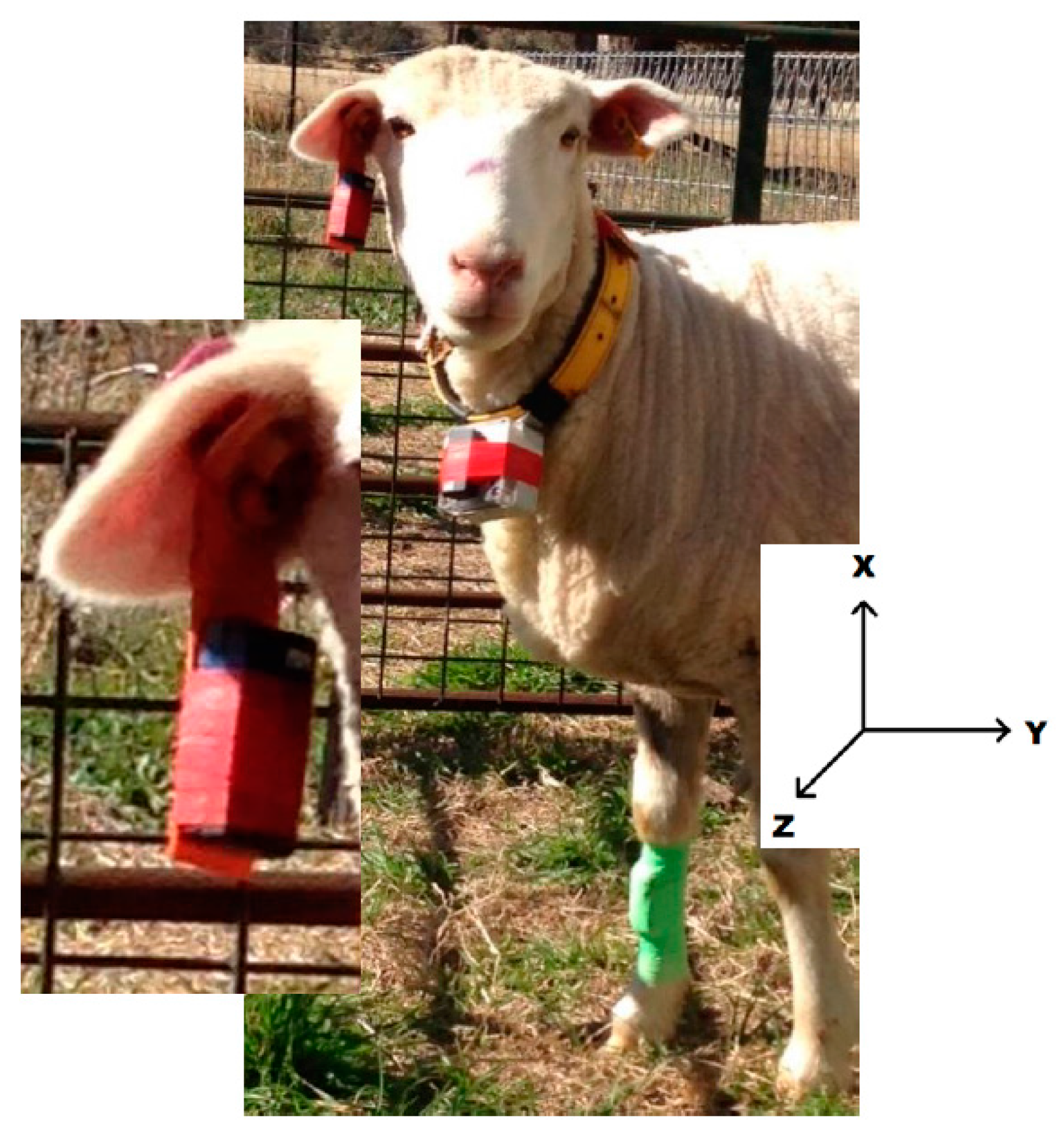

| Behaviour | Classification Description |
|---|---|
| Grazing | Grazing with head down or chewing with head up either standing still or moving. Rumination was classed as standing or lying. |
| Walking | Minimum of two progressive steps either forward/back or sideways. |
| Standing | Static standing with minor limb and head movements. Animal is in a standing posture whilst idle or inactive. Head may be up or down. |
| Lying | Animal is in a lying posture whilst idle or inactive assuming a recumbent position with minor head movements. |
| Feature | Equation | Feature Discussed In |
|---|---|---|
| Average X-axis (Ax) | [32] | |
| Average Y-axis (Ay) | [32] | |
| Average Z-axis (Az) | [32] | |
| Movement Variation (MV) | [38] | |
| Signal Magnitude Area (SMA) | [38] | |
| Average Intensity (AI) | where | [39] |
| Entropy | where n is the number of records in the burst and Ts = Az + Ay + Az | [40] |
| Energy | [40] | |
| Maximum X (MaxX) | The maximum X-axis acceleration value within the epoch | [20,41] |
| Maximum Y (MaxY) | The maximum Y-axis acceleration value within the epoch | [20,41] |
| Maximum Z (MaxZ) | The maximum Z-axis acceleration value within the epoch | [20,41] |
| Minimum X (MinX) | The minimum X-axis acceleration value within the epoch | [20,41] |
| Minimum Y (MinY) | The minimum Y-axis acceleration value within the epoch | [20,41] |
| Minimum Z (MinZ) | The minimum Z-axis acceleration value within the epoch | [20,41] |
| Behaviour | Collar | Leg | Ear |
|---|---|---|---|
| Sound walking | 95 (3) | 94 (3) | 274 (5) |
| Sound standing | 106 (3) | 106 (3) | 862 (5) |
| Sound grazing | 298 (4) | 298 (4) | 342 (5) |
| Sound lying | 40 (1) | 46 (1) | 0 (0) |
| Lame walking | 88 (3) | 92 (4) | 98 (4) |
| lame standing | 62 (3) | 93 (4) | 97 (4) |
| Lame grazing | 171 (3) | 181 (4) | 182 (4) |
| Lame lying | 236 (3) | 279 (4) | 279 (4) |
| RF Variable Selection | |||||
|---|---|---|---|---|---|
| Analysis I | Analysis II | ||||
| Ear | Front Leg | Collar | Ear | Front Leg | Collar |
| MV | Ax | Ax | MV | Ax | Entropy |
| Ay | SMA | Az | AI | SMA | Az |
| Energy | Az | Entropy | Ay | AI | Max-Z |
| SMA | AI | AI | SMA | Max-X | Energy |
| AI | MV | Energy | Energy | Az | AI |
| Min-X | Max-Y | Max-Z | Min-Z | MV | MV |
| Max-Y | Max-X | MV | Min-X | Energy | Ax |
| Max-X | Max-Z | Max-X | Az | Max-Y | Min-X |
| Min-Z | Energy | Min-Z | Max-Y | Entropy | Min-Z |
| Az | Entropy | Min-X | Min-Y | Min-X | Max-X |
| Min-Y | Ay | SMA | Ax | Ay | Min-Y |
| Max-Z | Min-Y | Min-Y | Max-Z | Max-Z | SMA |
| Ax | Min-X | Ay | Entropy | Min-Y | Ay |
| Entropy | Min-Z | Max-Y | Max-X | Min-Z | Max-Y |
| Deployment Location | Predicted Behaviour (Events) | Observed Behaviour (Events) | |||||
|---|---|---|---|---|---|---|---|
| Sound Grazing | Sound Standing | Sound Walking | Sound Lying | Lame Walking | Lame Grazing | ||
| Ear | Sound grazing | 287 | 42 | 5 | 6 | 131 | |
| Sound standing | 44 | 812 | 2 | 3 | 11 | ||
| Sound walking | 1 | 4 | 241 | 9 | 3 | ||
| Sound lying | |||||||
| Lame walking | 0 | 0 | 14 | 78 | 3 | ||
| Lame grazing | 10 | 3 | 11 | 2 | 34 | ||
| Prediction accuracy | 84% | 94% | 88% | 80% | 19% | ||
| Collar | Sound grazing | 281 | 26 | 0 | 0 | 0 | 39 |
| Sound standing | 12 | 53 | 12 | 3 | 29 | 14 | |
| Sound walking | 0 | 22 | 77 | 13 | 13 | 2 | |
| Sound lying | 2 | 5 | 5 | 22 | 4 | 4 | |
| Lame walking | 0 | 0 | 0 | 1 | 11 | 4 | |
| Lame grazing | 3 | 0 | 0 | 0 | 20 | 108 | |
| Prediction accuracy | 94% | 50% | 82% | 56% | 14% | 63% | |
| Leg | Sound grazing | 262 | 34 | 0 | 0 | 5 | 55 |
| Sound standing | 33 | 71 | 0 | 0 | 6 | 105 | |
| Sound walking | 0 | 0 | 81 | 0 | 2 | 0 | |
| Sound lying | 2 | 0 | 0 | 46 | 0 | 8 | |
| Lame walking | 1 | 0 | 13 | 0 | 80 | 6 | |
| Lame grazing | 0 | 1 | 0 | 0 | 0 | 7 | |
| Prediction accuracy | 88% | 67% | 86% | 100% | 86% | 4% | |
| Deployment Location | Predicted Behaviour (Events) | Observed Behaviour (Events) | ||||
|---|---|---|---|---|---|---|
| Sound Grazing | Sound Standing | Sound Walking | Sound Lying | Lame Walking | ||
| Ear | Sound grazing | 321 | 26 | 1 | 6 | |
| Sound standing | 15 | 830 | 1 | 3 | ||
| Sound walking | 2 | 5 | 262 | 9 | ||
| Sound lying | ||||||
| Lame walking | 4 | 0 | 9 | 80 | ||
| Prediction accuracy | 94% | 96% | 96% | 82% | ||
| Collar | Sound grazing | 283 | 26 | 2 | 0 | 0 |
| Sound standing | 13 | 53 | 10 | 12 | 9 | |
| Sound walking | 2 | 15 | 60 | 8 | 42 | |
| Sound lying | 0 | 12 | 23 | 18 | 6 | |
| Lame walking | 0 | 0 | 0 | 2 | 31 | |
| Prediction accuracy | 95% | 50% | 63% | 45% | 35% | |
| Leg | Sound grazing | 266 | 44 | 0 | 0 | 3 |
| Sound standing | 26 | 61 | 0 | 0 | 6 | |
| Sound walking | 0 | 0 | 60 | 0 | 3 | |
| Sound lying | 0 | 0 | 0 | 46 | 0 | |
| Lame walking | 6 | 1 | 34 | 0 | 81 | |
| Prediction accuracy | 89% | 58% | 64% | 100% | 87% | |
| Deployment Location | Predicted Behaviour (Events) | Observed Behaviour (Events) | ||||
|---|---|---|---|---|---|---|
| Sound Grazing | Sound Standing | Sound Walking | Sound Lying | Lame Walking | ||
| Ear | MV, AI, Ay | |||||
| Sensitivity | 94% | 96% | 96% | 82% | ||
| Specificity | 97% | 97% | 99% | 99% | ||
| Accuracy | 96% | 97% | 98% | 98% | ||
| Precision | 91% | 98% | 94% | 82% | ||
| Collar | Entropy, Az, Max-Z | |||||
| Sensitivity | 95% | 50% | 63% | 45% | 35% | |
| Specificity | 90% | 92% | 87% | 96% | 90% | |
| Accuracy | 92% | 85% | 84% | 93% | 83% | |
| Precision | 91% | 55% | 47% | 45% | 35% | |
| Leg | Ax, SMA, AI | |||||
| Sensitivity | 89% | 58% | 64% | 100% | 87% | |
| Specificity | 83% | 94% | 99% | 100% | 98% | |
| Accuracy | 86% | 88% | 94% | 100% | 96% | |
| Precision | 85% | 66% | 95% | 100% | 87% | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barwick, J.; Lamb, D.; Dobos, R.; Schneider, D.; Welch, M.; Trotter, M. Predicting Lameness in Sheep Activity Using Tri-Axial Acceleration Signals. Animals 2018, 8, 12. https://doi.org/10.3390/ani8010012
Barwick J, Lamb D, Dobos R, Schneider D, Welch M, Trotter M. Predicting Lameness in Sheep Activity Using Tri-Axial Acceleration Signals. Animals. 2018; 8(1):12. https://doi.org/10.3390/ani8010012
Chicago/Turabian StyleBarwick, Jamie, David Lamb, Robin Dobos, Derek Schneider, Mitchell Welch, and Mark Trotter. 2018. "Predicting Lameness in Sheep Activity Using Tri-Axial Acceleration Signals" Animals 8, no. 1: 12. https://doi.org/10.3390/ani8010012




