1. Introduction
Genetic selection of chickens for either egg or meat production has benefitted the poultry industry and its consumers. The broiler industry raises both males and females for meat production, while the layer industry rears females only for egg output. The global egg industry was comprised of 7.3 billion hens in 2015, which produced 70.8 million metric tons of eggs [
1]. In the US alone, 365 million laying hens produced 88.4 billion table eggs in 2016 [
2]. For each laying hen that is hatched, a male chick will also be produced. However, male layers do not have any economic value. These birds do not produce eggs, grow slowly, have a poor feed conversion ratio, incur high fattening costs to producers, and yield inferior quality meat [
3,
4]. Therefore, recently hatched male layer chicks are euthanized after being sexed in hatcheries. In the European Union (EU), that number is approximately 280 million chicks per year [
5].
The American Veterinary Medical Association (AVMA) has approved maceration and carbon dioxide (CO
2) gassing as acceptable methods for euthanasia of chicks up to 72 h of age [
6]. Maceration results in the instantaneous death of chicks, poults, and embryonated eggs by physical disruption of the brain [
6]. The method requires special equipment, called macerators, which have rotating blades for rapid fragmentation and death. Carbon dioxide inhalation quickly reduces intracellular pH, resulting in respiratory acidosis and anesthesia [
7]. Continued exposure to the gas leads to hypercapnic hypoxia, respiratory depression, and ultimately death [
8]. Raj and Whittington [
9] studied the efficacy of either a 90% CO
2 treatment or mixture of 20, 30, 40% CO
2 in argon. Their study [
9] reported that day-old chicks subjected to 90% CO
2 in the air died within two minutes of exposure. European Union guidelines of Council Directive 93/119/EC on animal protection at the time of slaughter or killing, requires chicks to be exposed to the highest possible concentration of CO
2 dispensed from a 100% source and requires that the gas concentration be maintained until the death of the chicks [
10]. Nitrogen (N
2) and argon (Ar) are tasteless, odorless gases that displace oxygen from breathing air, resulting in a loss of consciousness from anoxia, which finally leads to bird death [
11]. Residual oxygen concentration should be maintained below 2% while using these anoxic gases for euthanasia [
9,
12]. Raj and Whittington [
9] reported that the recovery rates of chicks exposed to mixtures of 20 to 40% CO
2 in Ar, with a 5% residual oxygen concentration, ranged from 15 to 100%.
An alternative method for poultry stunning is by the reduction in atmospheric pressure [
13]. The method is known as low atmospheric pressure stunning (LAPS) [
14,
15]. This process induces unconsciousness in subjects by decreasing air pressure inside a chamber, subsequently decreasing partial oxygen pressure and ultimately causing hypoxia [
14,
16,
17]. The LAPS method received a ‘no objection’ status from the United States Department of Agriculture (USDA) in 2010 [
13,
17]. Purswell et al. [
15] stated that during LAPS, the anatomy of the avian respiratory system makes it unlikely for gases to be trapped in the body cavities of chickens, unless the trachea is blocked. Additionally, newly hatched male chicks do not have access to feed and have minimal microbial gut colonization, making potential buildup of gases in the gut unlikely.
Methods of humane killing such as gas inhalation, maceration, and LAPS have led to welfare concerns by the public. Inhalation of CO
2 gas at concentrations between 40% and 55% can cause painful sensations in humans due to acidification of the nasal mucosa [
18]. Chickens have arterial and central chemoreceptors that respond to CO
2 upon inhalation. These birds are likely to experience distress when exposed to high CO
2 levels [
12]. Hens exposed to CO
2 gas concentrations of 40 to 50% may experience pain or discomfort [
19]. Raj et al. [
20] reported that a 47% CO
2 gas composition in air is aversive to laying hens. Poultry subjected to CO
2 demonstrate signs of respiratory discomfort such as sneezing, gasping and head shaking [
21]. Chicken embryos are exposed to CO
2 concentrations as high as 14% [
22] and, therefore, may require higher concentrations of CO
2 and longer exposure times to be euthanized when compared to adult chickens [
6]. However, birds do not exhibit aversive behavior when initially exposed to inert gases [
11,
12]. Sandilands et al. [
23] reported that broilers found the three gas mixtures—50% CO
2 in air, 90% N
2 in CO
2, and 80% Argon in CO
2—aversive but, comparatively, inert gas mixtures induced fewer signs of respiratory discomfort than CO
2 in air. Nevertheless, birds subjected to inert gases exhibit severe wing flapping and convulsions [
16]. Exposure to air pressure that has been reduced to 17.8 kPa for two minutes has been shown to be lethal to broilers [
15]. However, the requirement for day-old chicks and poults may be different. Therefore, scientific studies concerning potential applications of LAPS for euthanizing recently hatched chicks and poults are needed.
Scientific studies that have evaluated the current euthanasia procedures for day-old chicks are limited [
24]. Raj and Whittington’s [
9] paper entitled “Euthanasia of day-old chicks with carbon dioxide and argon” is the single research article found in the scientific record that addresses day-old chick euthanasia. To the authors’ knowledge, studies on behavioral and physiological responses of day-old male layers to these different euthanasia methods have not yet been published in the scientific literature. The poultry industry is also looking for humane and viable alternative methods to replace maceration of neonatal chickens. The objective of this study was to develop other viable alternative methods for chick euthanasia. The specific objectives were to evaluate physiological responses of male layer neonates to gas inhalation and LAPS by measuring serum corticosterone (CORT) and serotonin (5-HT) concentrations, and to assess behavioral responses of chicks such as latencies to loss of posture and motionlessness.
2. Materials and Methods
2.1. Experimental Design
Alternative methods to maceration were evaluated and compared in this study. Along with a negative control (Air), experimental treatments included 25% CO2, 50% CO2, 75% CO2, 90% CO2, 100% N2, and LAPS. Since it was practically unfeasible to achieve a 100% CO2 concentration inside the chamber, a 90% CO2 treatment was used as one of the treatments. Chicks were subjected to air as the negative control treatment. This treatment was used to assess potential effects of the experimental chamber, handling process, and flow of gases on the experimental birds. In each treatment, a batch of 10 day-of-hatch Hy-Line W-36 male chicks were used. Each treatment was replicated on 10 different days. The 25% CO2 and 50% CO2 treatments were discontinued after the first replicate day, due to a high number of survivors. A total of 520 male layer chicks were used during the entire study. The chicks were provided with clean drinking water, and a 250 Watt heating lamp was used to maintain optimal temperature during the trial. All birds were cared for under an approved Texas A&M University Institutional Animal Care and Use Committee protocol (IACUC 2016-0200).
2.2. Experimental Chamber
A custom-built vacuum system mounted on a cart was procured from a manufacturer (LACO Technologies, Salt Lake City, UT, USA). It included a horizontal cylindrical vacuum chamber, with an internal diameter of 0.45 m, length of 0.5 m, and internal volume of 80 L. The system had a clear acrylic lid, a vacuum pump of flow rate of 201 L/m (7.1 cfm) and a vacuum controller unit. The vacuum chamber was also used as the experimental chamber for all seven treatments. A thermocouple measured the temperature of the chamber during each treatment. The temperature of the chamber did not change during application of any of the gases—CO2, N2, and air, as well as during LAPS. It was unlikely that the chicks experienced any cold stress, since the temperature remained within a fairly constant range of 20.5 °C to 23.3 °C between treatments.
2.3. Experimental Procedure
A batch of ten chicks was exposed to each treatment gas or a reduction in air pressure until the desired concentration or final air pressure was achieved inside the chamber. The chicks were then held inside the chamber for an additional five minutes. Gas tanks containing 100% CO
2, 100% N
2, and breathing air were procured from a local supplier for the study. A uniform gas delivery pressure of 103.4 kPa was used in all gas treatments. A variable area flow meter (Cole-Parmer, Vernon Hills, IL, USA) was used to control the flow rates of the gases into the chamber. Flow rates of all CO
2 treatments were set at 42 L/m, while that of the 100% N
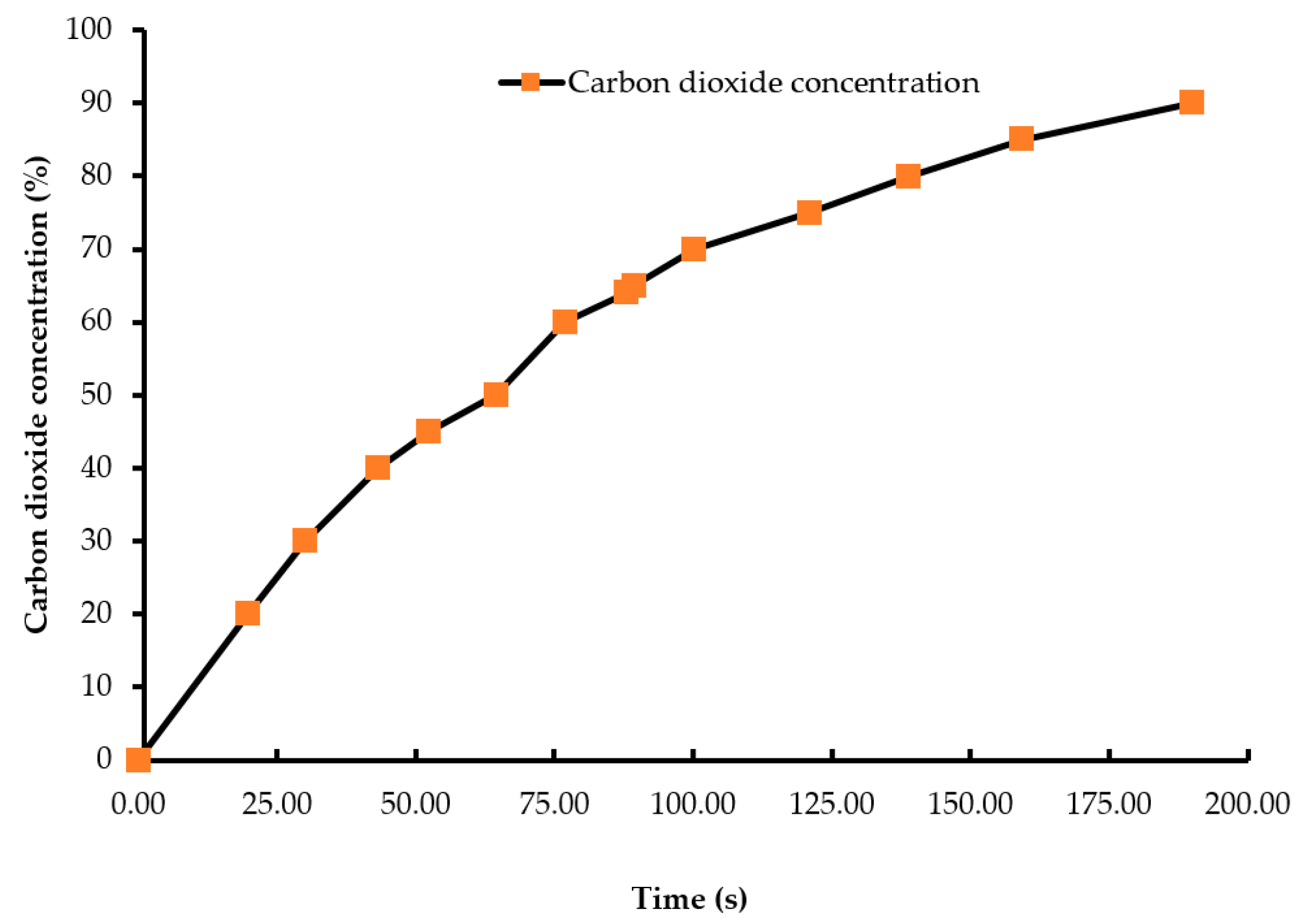
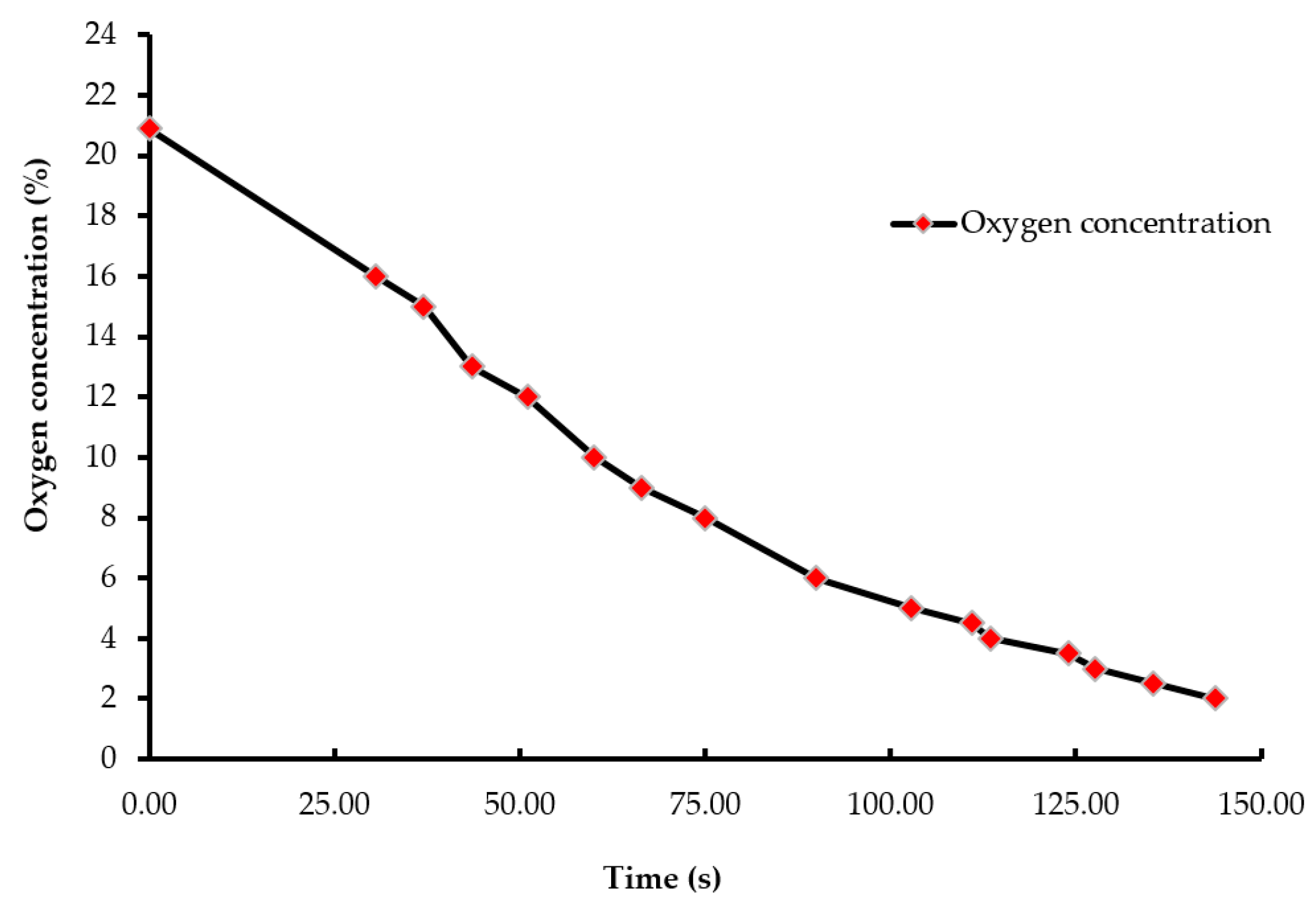
2 and negative control (air) treatments were set at 60 L/m. The time taken to achieve 90% CO
2 in the CO
2 treatment was 195 s, and 2% O
2 took 144 s in the N
2 treatment (
Figure 1 and
Figure 2). However, in the N
2 treatment, gas was allowed to fill the chamber for a total time of 195 s. A higher flow rate was used in the N
2 treatment to achieve a 100% concentration of gas in a similar amount of time as the other treatments. The negative control flow rate of breathing air was set at the same flow rate as the N
2 treatment, for later comparisons. Carbon dioxide (100%) was delivered to the chicks until the desired concentration of CO
2 was achieved inside the chamber. An infrared CO
2 sensor (Servomex, Crowborough, UK) was used to measure and confirm the gas concentration. Nitrogen gas flowed into the chamber until the oxygen concentration was reduced to less than 2%. The oxygen concentration inside the chamber was measured using an electrochemical sensor (Sper Scientific, Tucson, AZ, USA).
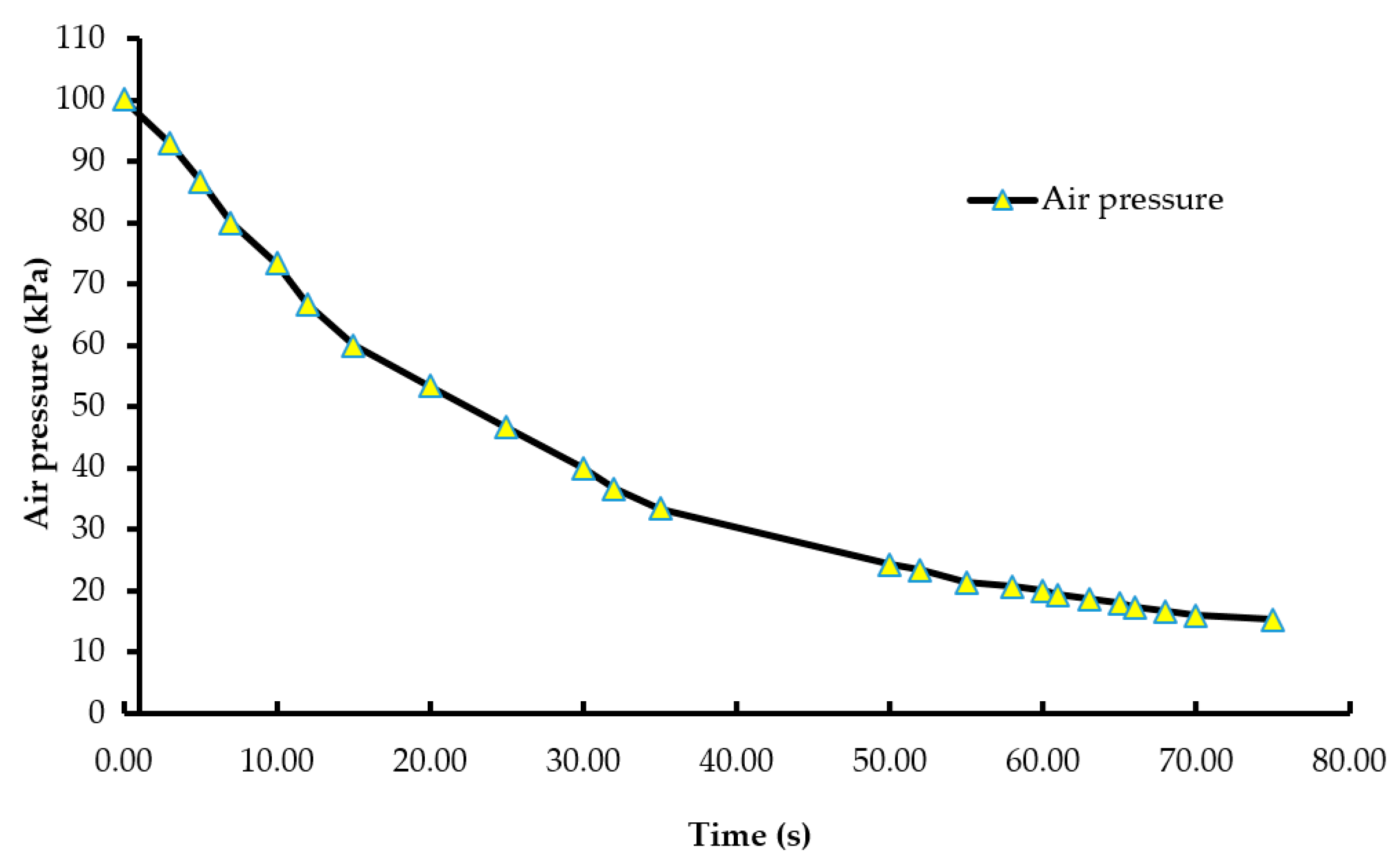
Figure 2 shows the oxygen washout process as nitrogen was filling the chamber. In the LAPS treatment, the air pressure inside the chamber was decreased gradually, which subsequently reduced the partial pressure of oxygen. Purswell et al. [
15] estimated that a negative atmospheric pressure of 19.4 kPa would result in 99.9% broiler mortality. However, it was found in our laboratory that chicks survived the negative air pressures of 17.9 kPa and 19.4 kPa. Further preliminary trials determined that a negative air pressure of 15.3 kPa would result in 100% chick mortality. Therefore, air pressure inside the chamber was reduced from 100.12 kPa to 15.3 kPa in the LAPS treatment. The decrease in air pressure over time is shown in
Figure 3. Once the required gas concentration or negative air pressure was achieved, chicks were exposed further for a period of 5 min. Chicks were observed for any signs of recovery after the end of that 5 min holding time.
2.4. Stress Physiology
Blood samples were collected from each chick by cardiac puncture after death had been verified in all treatments, except for those belonging to the negative control group. In the negative control group, chicks were decapitated within 30 s of their removal from the experimental chamber at the end of the duration of treatment and blood samples were collected subsequently. The blood samples were allowed to clot overnight at 4 °C. The next day, serum was collected from the blood samples by centrifuging at 500× g at 4 °C. Competitive enzyme linked immunosorbent assay (ELISA) kits ADI-901-097 and ADI-900-175, were used to measure the concentration of CORT and 5-HT, respectively (Enzo Life Sciences, Farmingdale, NY, USA), following the instructions in the kit manuals. All samples were run in duplicate. Intra-assay and inter-assay variabilities were 1.9% and 7.2%, respectively, for the corticosterone assay. Intra-assay and inter-assay variabilities for the 5-HT assay were 1.6% and 5.8%, respectively. Serum CORT and 5-HT concentrations were interpolated from a standard curve using curve-fitting software (Gen 5.0, Bio-Tek Instruments, Winooski, VT, USA).
2.5. Behavioral Observations
Treatments were videotaped for evaluation of behavioral responses of the chicks using a digital video camera (Sony, New York City, NY, USA). The variables measured were latencies to loss of posture and motionlessness. Each video segment included all the events starting from application of treatment till the end of the five min holding period. The time point when 100% of the chicks demonstrated the defined behavior was noted and the differences from the start of treatment were recorded as latency measurements. Chicks in all treatments, except in the air group, demonstrated ataxia, loss of posture, convulsions and motionlessness. Behaviors such as failure to maintain body balance, swaying of body, inability to stand, and flipping were signs of ataxia. The state of loss of posture was determined once the chicks were lying with necks extended and unable to control body posture [
17,
25]. This was followed by onset of convulsions in which signs like wing flapping, muscle twitching, leg paddling, and flipping over could be seen. These behavioral signs ended with straightening of legs and cessation of convulsions. The state of motionlessness was established once any visible movements including breathing motion had stopped [
17,
25]. Latencies to loss of posture and motionlessness were recorded as time durations after which the respective behaviors were exhibited. Other behaviors such as alertness, headshaking, gasping, neck extension and vocalizations were noticed but not quantified.
2.6. Statistical Analysis
The serum CORT and 5-HT levels obtained after ELISA and the data of behavioral variables were summarized in a spreadsheet (MS-EXCEL, Microsoft, Redmond, WA, USA). Data were statistically analyzed using a one-way analysis of variance following PROC ANOVA procedures (SAS 9.4, Cary, NC, USA). Least-squares means were compared using Fisher’s Least significant difference (LSD) post hoc test in the event of significant global effects [
26]. The statistical tests were carried out at 5% significance level. Two CORT samples from the 90% CO
2 treatment and one from 100% N
2 treatment were identified as outliers by Tukey’s boxplot method and removed from study.
3. Results and Discussion
Day-of-hatch male chicks exposed to the 25% CO
2 and 50% CO
2 treatments recovered after the end of a five min holding period. All ten chicks in the 25% CO
2 group recovered, and only two out of ten chicks died in the 50% CO
2 group. Therefore, the two treatments were discontinued after the first replicate time period. Chicks exposed to a CO
2 concentration of 75% or above for a period of five min did not recover. This finding demonstrates that chicks require a higher concentration of CO
2 than adult birds. The results described are of the following five treatments: negative control (air), 75% CO
2, 90% CO
2, 100% N
2, and LAPS. In the 75% and 90% CO
2 treatments, air inside the chamber was displaced by CO
2.
Figure 1 demonstrates the wash-in curve for both CO
2 treatments. A 100% N
2 source was used to fill the chamber with this anoxic gas during N
2 treatment. As N
2 was being filled in the chamber oxygen levels continuously decreased.
Figure 2 illustrates the wash-out process of oxygen during N
2 treatment. Air pressure inside the chamber was gradually decreased during the LAPS treatment.
Figure 3 shows the time taken for the reduction in air pressure of the chamber from 100.12 kPa to 15.3 kPa. At such a reduced air pressure level, oxygen fails to diffuse from alveoli into the capillaries because of decrease in partial oxygen pressure in the inspired air. This leads to death from anoxia.
3.1. Serum Corticosterone
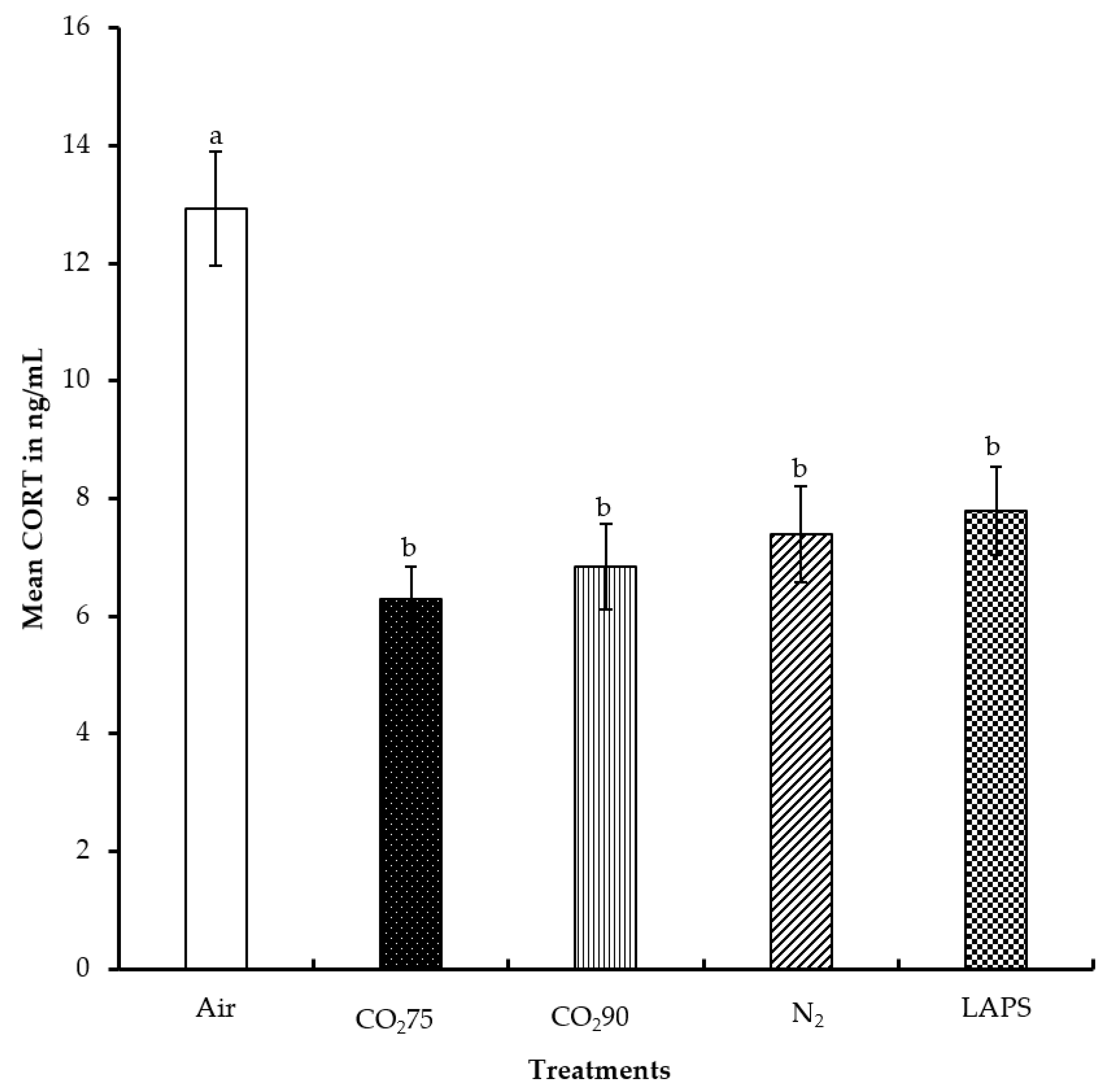
The average CORT levels of the chicks subjected to the negative control, 75% CO
2, 90% CO
2, 100% N
2 and LAPS treatments were 12.9, 6.3, 6.8, 7.4, and 7.8 ng/mL, respectively (
Figure 4). The chicks in the negative control treatment had a significantly higher serum CORT concentration than those chicks in all the rest of the treatments (
p ≤ 0.0001). However, the serum CORT concentrations of the chicks subjected to the gas inhalation methods, namely 75% CO
2, 90% CO
2, and 100% N
2, were similar to those of the LAPS treatment (
p > 0.05). The chicks in the negative control group were alive for the longest duration among all the treatments, until they were decapitated for blood collection at the end of their treatment. The chamber was a novel environment for the chicks and the handling of chicks prior to decapitation for blood collection are probable reasons for their higher CORT levels.
Carbon dioxide causes pain and discomfort to the animal due to the formation of carbonic acid in the mucous membrane [
27]. Kaye et al. [
28] reported that hypercapnia due to CO
2 stimulated the sympathetic nervous system and the hypothalamo-pituitary-adrenal axis. In the current study, the chicks belonging to 75% and 90% CO
2 treatment groups might have lost consciousness before a potentially painful CO
2 level was attained inside the chamber due to the analgesic [
29] and anesthetic effect [
30] of carbon dioxide gas. Therefore, the serum CORT levels were statistically lower as compared to those in the negative control group. Exposure to 100% N
2 did not elevate CORT levels in the chicks in comparison to those chicks in the negative control treatment group. Birds lack intrapulmonary chemoreceptors sensitive to N
2 [
12] and do not demonstrate aversive responses in the beginning. In addition, normal breathing air consists of 78% N
2 by volume. Hence, animals are continuously being exposed to higher levels of N
2. In the LAPS treatment group, the chicks experienced a reduction in air pressure and a subsequent reduction in the partial pressure of oxygen. This led to a reduction in the diffusion of oxygen into the blood stream, causing the chicks to die from anoxia. The mechanism of death from the LAPS treatment is similar to that of gas inhalation methods. The CORT data also suggests that the LAPS method is similar to CO
2 and N
2 inhalation, in terms of a stress response. In a study by Vizzier-Thaxton et al. [
14], broilers of market age and live weight ranging from 2.7 kg to 4.1 kg subjected to LAPS had significantly lower CORT levels as compared to electrical stunning. Shackling during electrical stunning might be the reason for the higher CORT concentrations.
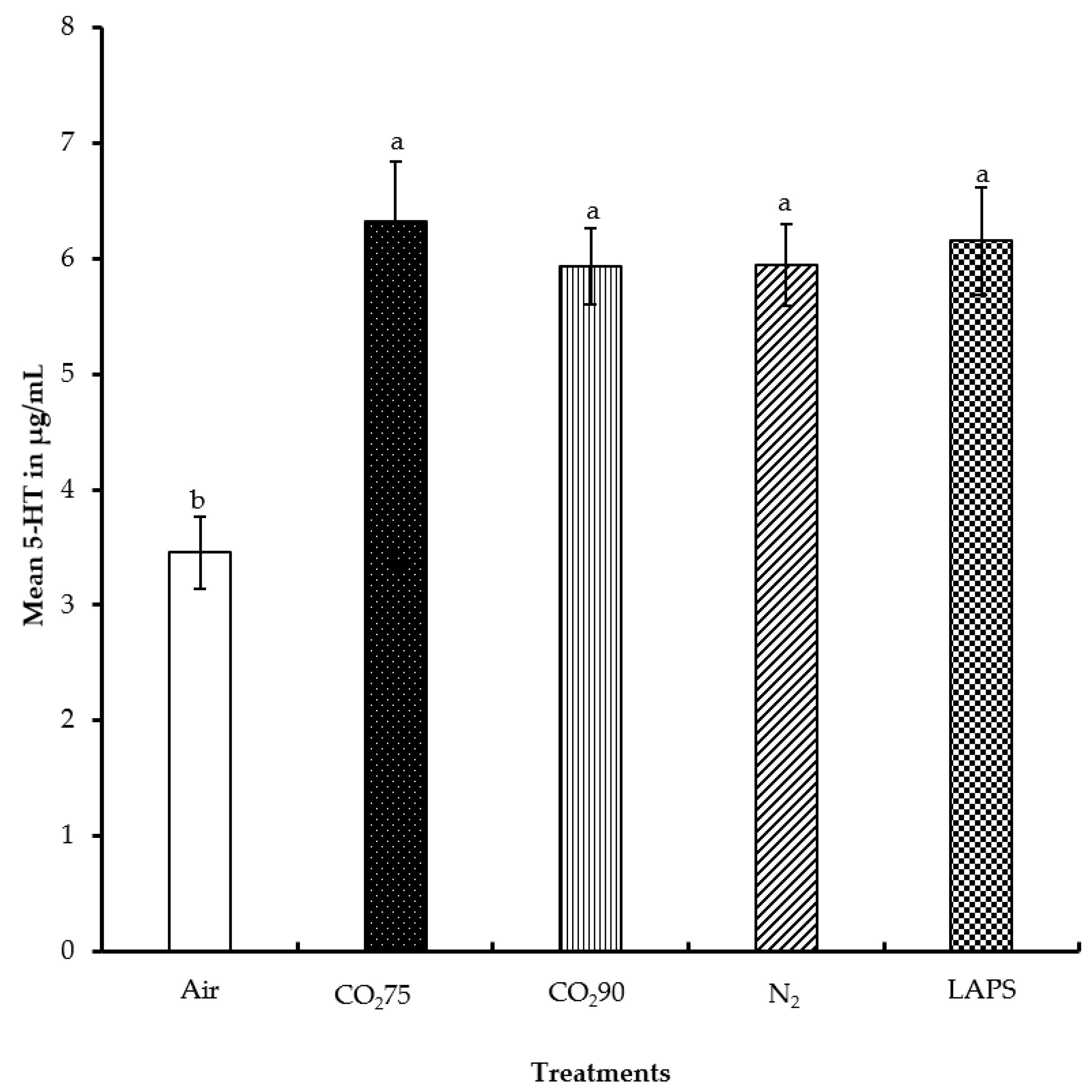
3.2. Serum Serotonin
The 5-HT concentration in the peripheral blood of male layer chicks subjected to the five treatments were evaluated and compared. The mean serum 5-HT levels of chicks in the negative control, 75% CO
2, 90% CO
2, 100% N
2, and LAPS treatments were 3.5, 6.3, 5.9, 5.9, and 6.2 µg/mL, respectively (
Figure 5). Chicks in the negative control group had a significantly lower concentration of serum 5-HT than those in the rest of the treatments (
p ≤ 0.0001). No significant differences were found in the 5-HT levels among the 75% CO
2, 90% CO
2, 100% N
2, and LAPS treatment groups.
Serotonin has multiple functions in the central nervous system and in various peripheral tissues and organ systems. The level of 5-HT in the brain affects behavioral and neuropsychological processes such as mood, perception, memory, anger, aggression, fear, stress responses, appetite, behavior, and circadian rhythm [
31]. Peripherally, 5-HT is vital in platelet aggregation, vasoconstriction, vasodilation, and intestinal motility [
32]. In humans, anxiety disorders are related to a decrease in brain 5-HT levels [
33]. Patients with depression have reduced whole blood [
34,
35] and platelet [
36] 5-HT levels. Williams et al. [
37] reported that higher blood 5-HT levels were positively associated with a better “mood”, in humans. Uitdehaag et al. [
38] reported a moderate positive correlation between whole blood 5-HT and brain 5-HT of laying hens. The study suggested that peripheral 5-HT measurement might reflect brain 5-HT functioning. Rodenburg et al. [
39] evaluated the effect of brooding and group selection for lower mortality on peripheral 5-HT serotonin and CORT levels of laying hens. The hens from randomly selected group raised without maternal care had a lower level of whole blood 5-HT as compared to birds from lower mortality group. Bolhuis et al. [
40] studied the effect of selection against mortality on whole blood 5-HT and fear-related behavioral responses in beak trimmed or non-beak trimmed laying hens. The study observed that changes in peripheral 5-HT might be a feature of laying hens likely to demonstrate feather pecking behavior. In the present study, chicks in the negative control group had significantly higher CORT levels but lower 5-HT levels. Studies conducted in our laboratory on layer hen depopulation produced similar findings. Spent hens with higher CORT levels were found to have lower 5-HT levels [
41]. A study by Inoue and Koyama [
42] reported a decrease in 5-HT levels in the hippocampus of rats in response to acute corticosterone administration. In the present study, chicks in the 75% CO
2, 90% CO
2, 100% N
2, and LAPS treatments had significantly higher 5-HT, but lower CORT levels as compared to those in the negative control group. The interesting relationship between serum CORT and 5-HT level needs further investigation.
However, exposure to CO
2 results in hypercapnia increased CO
2 tension in the blood, which is a stimulus for breathlessness [
43]. Low atmospheric pressure stunning and nitrogen inhalation treatments act by reducing oxygen tension in the chamber and subsequently decreasing the O
2 tension in blood. The ventilatory drive is increased immediately in case of CO
2 inhalation and animals exhibit breathlessness. The experience of breathlessness has various degrees of unpleasantness and hence has potential to challenge animal welfare [
43]. No assessments were made in this study among the treatments in terms of breathlessness.
3.3. Behavioral Responses
The behavioral responses of the chicks subjected to gas inhalation and LAPS treatments were video recorded. Chicks subjected to CO
2 treatments demonstrated headshaking, gasping with neck extended immediately (approximately 10–15 s) after introducing the gas into the chamber. Headshakes, gasping and neck extension were more pronounced in chicks subjected to CO
2 treatments (almost 100%) than in chicks subjected to N
2 and LAPS. These are signs of aversion and have been reported in other studies [
11,
12]. These chicks then demonstrated signs of ataxia and loss of posture. Most of the chicks were lying in a prone position with slow and heavy open mouth breathing. Chicks subjected to 100% N
2 and LAPS treatments showed a delayed response to gas introduction compared to chicks in the CO
2 treatment. The prominent signs shown by chicks prior to loss of posture were similar in the N
2 and LAPS treatments. Fewer of the chicks (1–2%) exhibited headshakes and gasping. These chicks looked drowsy, were falling on their sides and gradually shifting to sitting or prone position, and ultimately lost body posture and balance. Frequent labored or heavy breathing was seen in the chicks after losing posture. This was followed by convulsive movements such as wing flapping, leg paddling, and flipping over and was more common in chicks subjected to N
2 and LAPS treatments (almost 100%). The period of latency until all chicks exhibited loss of posture or motionlessness were determined for all treatment groups, except for the negative control group.
Loss of posture is a behavioral indicator of loss of consciousness [
44]. It is manifested by the inability to maintain neck tension and body balance [
45,
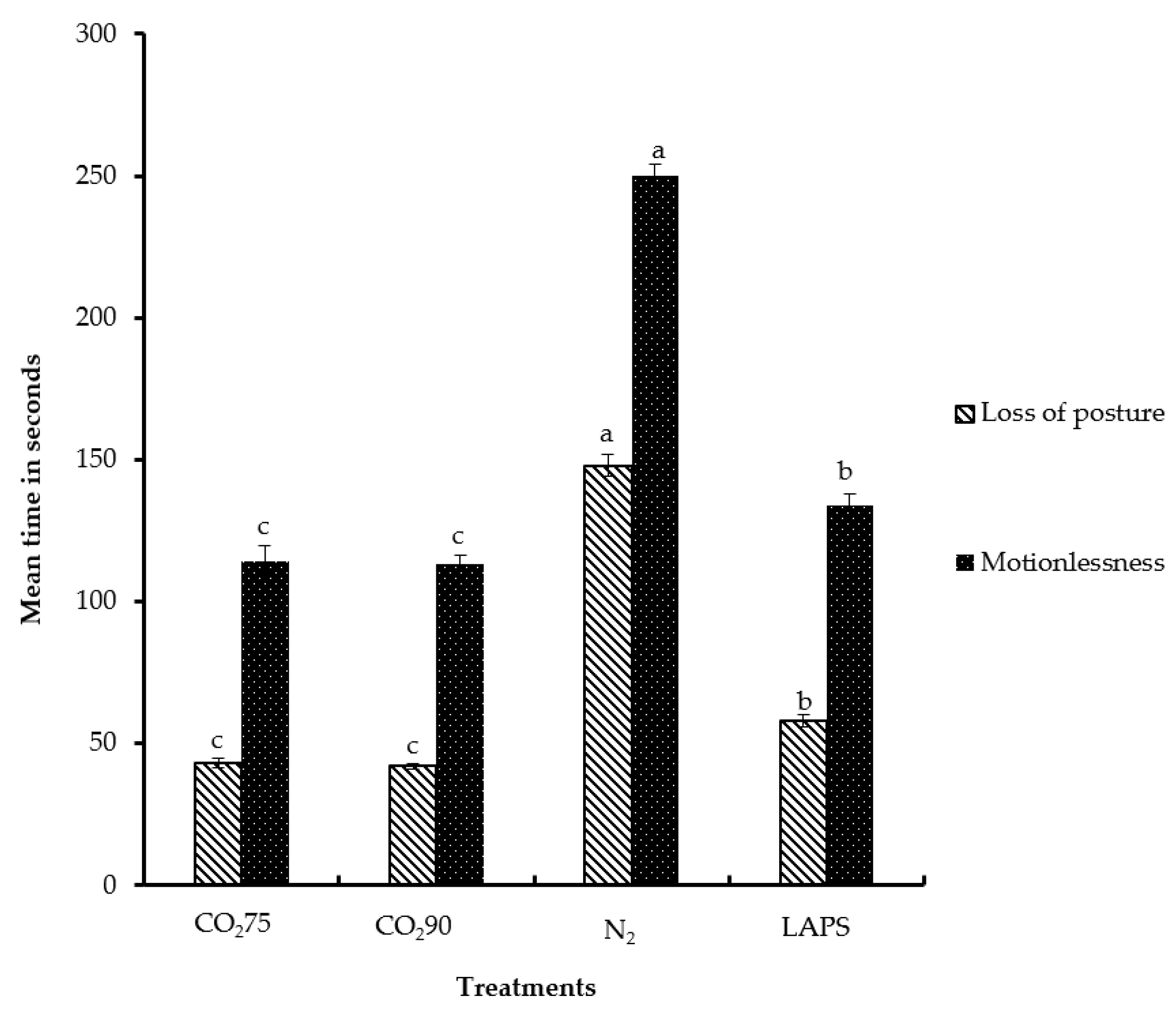
46]. In the present study, the latency periods until loss of posture in the chicks subjected to 75% CO
2, 90% CO
2, 100% N
2, and LAPS were 43, 42, 148, and 58 s, respectively (
Figure 6). Significant differences were observed in latencies to loss of posture among the four treatments. The chicks subjected to 100% N
2 took the longest time to lose body posture compared to those in the rest of the treatments (
p < 0.05). The chicks exposed to the 75% CO
2 and 90% CO
2 treatments lost body posture significantly faster than those chicks in the LAPS or 100% N
2 groups. The chicks subjected to LAPS lost posture earlier than chicks in the 100% N
2 group, but their loss of posture was later in comparison to those in the CO
2 inhalation treatments (
p < 0.05). The findings in this study also demonstrate that CO
2 is faster than N
2 in inducing loss of posture in male layer chicks. Poole and Fletcher [
46] reported that time to loss of posture in broilers exposed to CO
2 was significantly shorter than that caused by N
2. Gerritzen et al. [
47] showed that the time to loss of posture of broilers subjected to multistage CO
2 stunning ranged from 80 to 93 s. Unlike N
2, exposure to CO
2 leads to a reduction of intracellular pH [
48]. Martoft et al. [
7] reported that exposure to higher concentrations of CO
2 leads to a rapid induction of anesthesia mediated by a decrease in the intracellular pH of the brain. The anesthetic [
30] and analgesic effect of CO
2 [
29] may be the probable mechanism for rapid loss of posture. Nitrogen inhalation results in death by anoxia [
6]. Unlike CO
2 inhalation, chicks in the LAPS treatment group did not experience the anesthetic effect. Therefore, chicks in the LAPS method group took a significantly longer time to be affected than those in the CO
2 treatment groups. Mackie and McKeegan [
49] observed that mean latency to loss of posture of broilers subjected to LAPS was 80.7 s.
The cessation of visible movements, including respiratory motion, is a state of motionlessness [
25]. The time of latency to motionlessness was determined for chicks in each replicate in all treatment groups except for the negative control group (
Figure 6). In this study, chicks exposed to 75% and 90% CO
2 took 114 and 113 s to reach a state of motionlessness, respectively. The male layer chicks subjected to 100% N
2 and LAPS were motionless after 250 and 134 s, respectively. The period of latency to motionlessness of the chicks followed a similar pattern to that of latency to loss of posture. The chicks in the 100% N
2 treatment group took a significantly longer time to become motionless in comparison to those in the rest of the treatment groups. The chicks in the LAPS group took a longer time than those in the 75% CO
2 and 90% CO
2 groups, but a shorter time than those in the 100% N
2 group to become motionless (
p < 0.05). In the present study, the treatments that induced rapid loss of posture were the ones in which the chicks demonstrated motionlessness faster. Maintenance of an adequate concentration of gas was also vital in preventing any recovery and in achieving death. In the present study, the chicks subjected to the CO
2 treatments became motionless faster than did those in the 100% N
2 treatment group. A similar result was reported by Gerritzen et al. [
45]. They reported that broilers (two weeks old) exposed to 100% CO
2 become motionless significantly faster than did those in the other treatment groups, including the 50% N
2 + 50% CO
2 and 30% O
2 + 30% N
2 + 40% CO
2 gas mixture treatments. Broilers stunned by LAPS took an average of 199.4 s to become motionless, with a range of 158.2 s to 245.6 s [
49]. The latency period to motionlessness of the chicks in this study that were subjected to LAPS ranged from 115 to 151 s.