A Review of Success Factors for Piglet Fostering in Lactation
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Importance of Colostrum
2.1. What Is Colostrum?
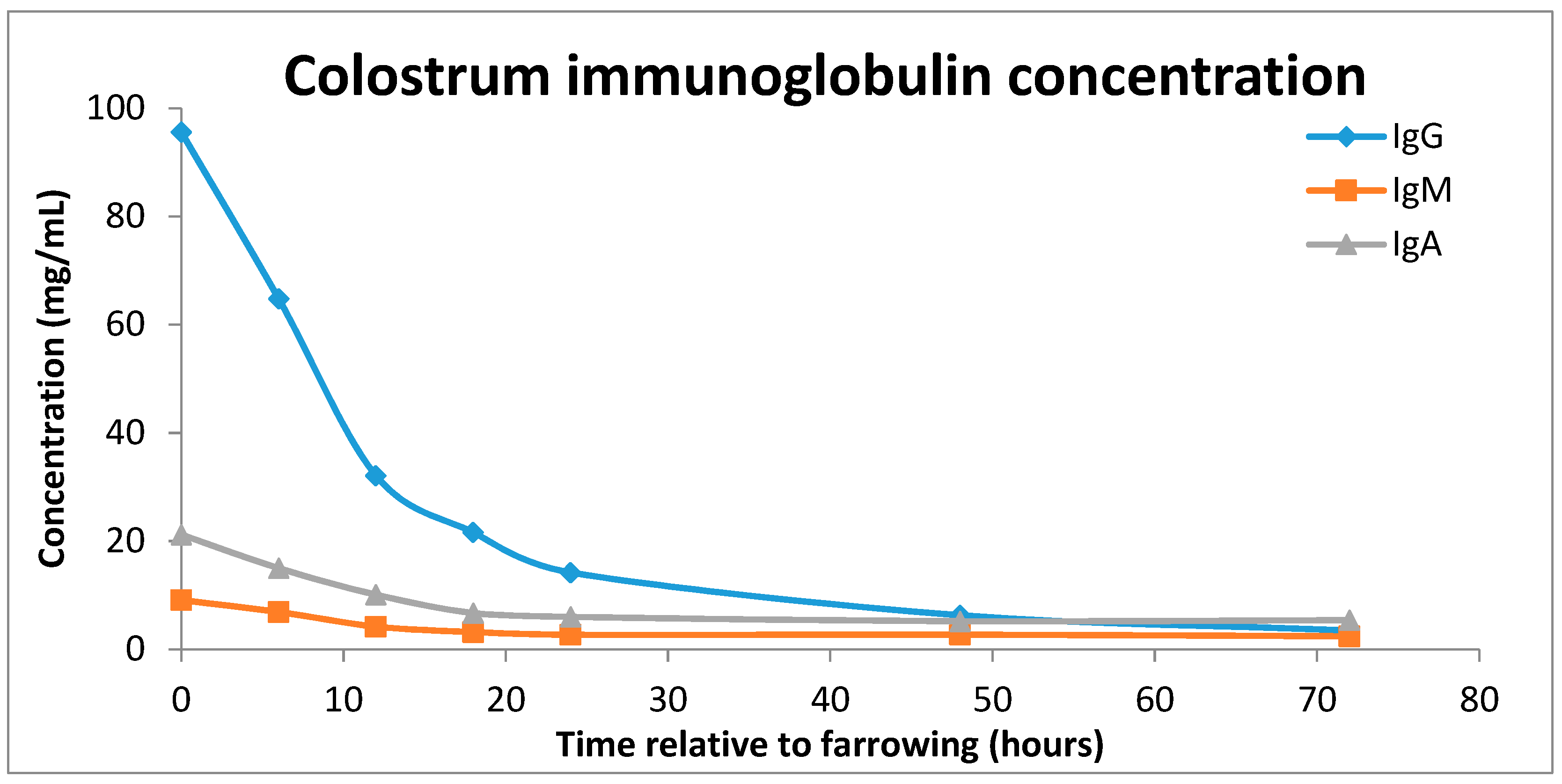
2.2. Impacts of Colostrum Intake
2.3. Sow Colostrum Production
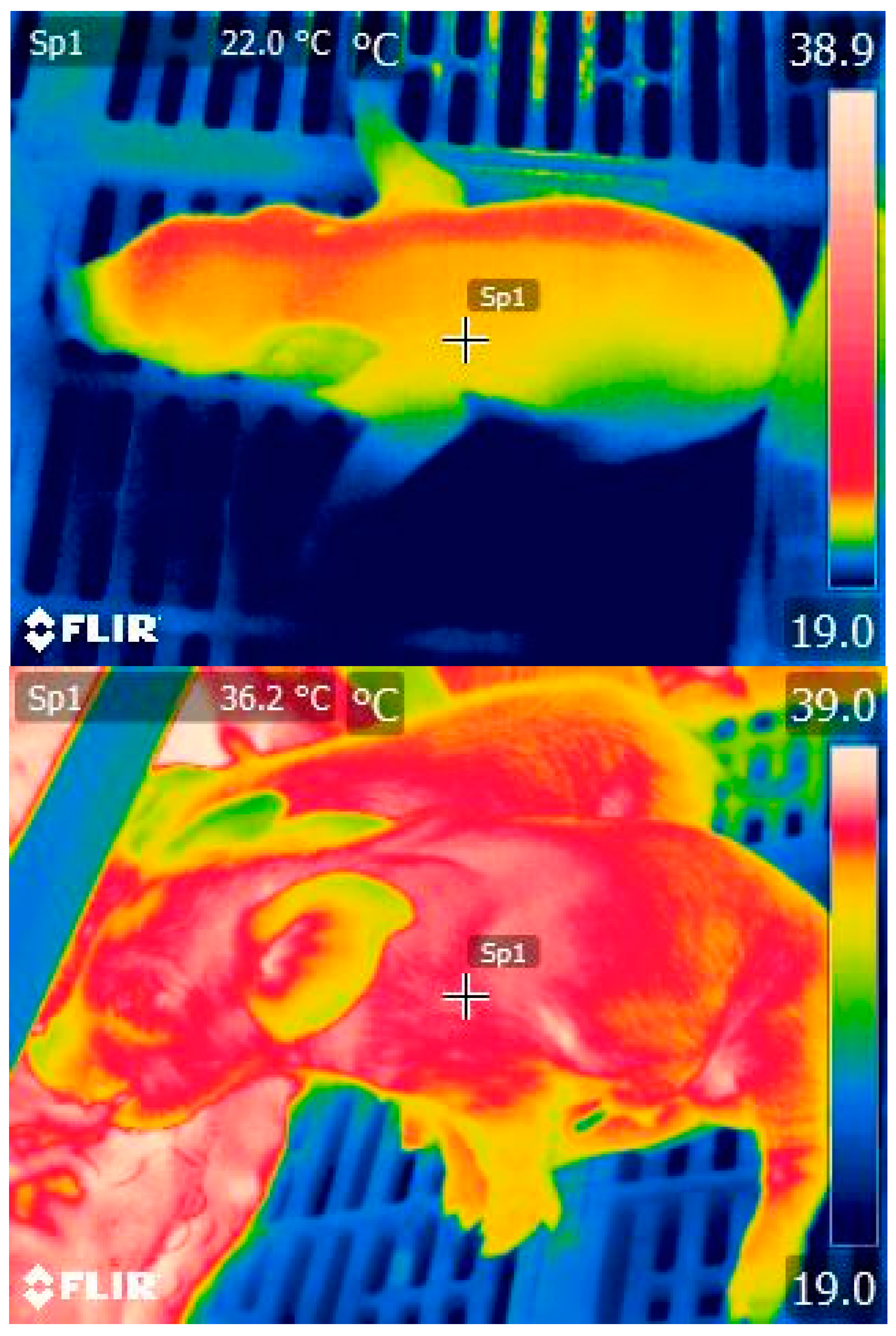
2.4. Piglet Colostrum Ingestion
2.5. Ensuring Colostrum Absorption Prior to Fostering
3. Assessment of Sow Rearing Ability Prior to Farrowing
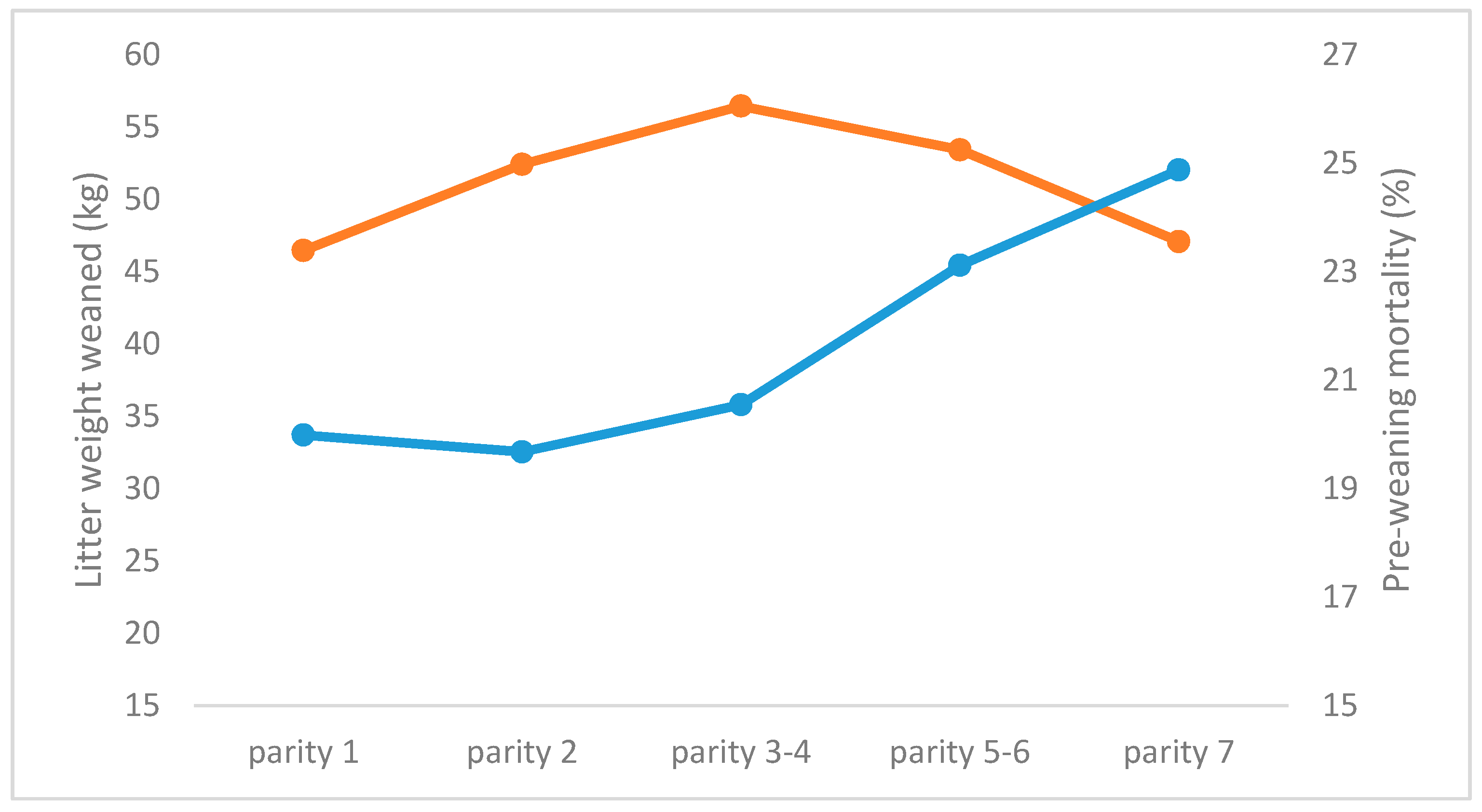
3.1. The First Lactation
3.2. Repeatability of Number of Piglets Weaned, and Weaning Weight
4. Assessment of Udders
4.1. Characteristics of a Functional Teat
4.2. What Are the Benefits of Counting Functional Teats
4.3. Other Udder Morphology of Importance
4.4. Making Progress in Udder Characteristics
5. Piglet Fostering
5.1. The Impact of Early Piglet Movement on Survival and Growth
5.2. Factors that Influence Growth and Survival of Fostered Pigs
6. Disease Risk
6.1. Piglet Movement after 24 h
6.2. Strategies to Relocate Piglets after 24 h
6.3. Conclusions and Recommendations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Theil, P.K.; Lauridsen, C.; Quesnel, H. Neonatal piglet survival: Impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal 2014, 8, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Le Dividich, J.; Rooke, J.A.; Herpin, P. Nutritional and immunological importance of colostrum for the new-born pig. J. Agric. Sci. 2005, 143, 469–485. [Google Scholar] [CrossRef]
- Vasdal, G.; Østensen, I.; Melišová, M.; Bozděchová, B.; Illmann, G.; Andersen, I.L. Management routines at the time of farrowing—Effects on teat success and postnatal piglet mortality from loose housed sows. Livest. Sci. 2011, 136, 225–231. [Google Scholar] [CrossRef]
- Le Dividich, J.; Noblet, J. Colostrum intake and thermoregulation in the neonatal pig in relation to environmental temperature. Neonatology 1981, 40, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Rooke, J.A.; Bland, I.M. The acquisition of passive immunity in the new-born piglet. Livest. Prod. Sci. 2002, 78, 13–23. [Google Scholar] [CrossRef]
- Bartol, F.F.; Wiley, A.A.; Miller, D.J.; Silva, A.J.; Roberts, K.E.; Davolt, M.L.P.; Chen, J.C.; Frankshun, A.L.; Camp, M.E.; Rahman, K.M.; et al. Lactation biology symposium: Lactocrine signaling and developmental programming. J. Anim. Sci. 2013, 91, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Klobasa, F.; Werhahn, E.; Butler, J.E. Regulation of humoral immunity in the piglet by immunoglobulin of maternal origin. Res. Vet. Sci. 1981, 32, 195–206. [Google Scholar]
- Tuboly, S.; Bernath, S.; Glavits, R.K.; Medveczky, I. Intestinal absorption of colostral lymphoid cells in newborn pigs. Vet. Immunol. Immunopathol. 1988, 20, 75–85. [Google Scholar] [CrossRef]
- Bandrick, M.; Pieters, M.; Pijoan, C.; Baidoo, S.K.; Molitor, T.W. Effect of cross-fostering on transfer of maternal immunity to mycoplasma hyopneumoniae to piglets. Vet. Rec. 2011, 168, 100. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.A. Perinatal mortality in the pig: Environmental or physiological solutions? Livest. Prod. Sci. 2002, 78, 3–12. [Google Scholar] [CrossRef]
- Quesnel, H.; Farmer, C.; Devillers, N. Colostrum intake: Influence on piglet performance and factors of variation. Livest. Sci. 2012, 146, 105–114. [Google Scholar] [CrossRef]
- Varley, M.A.; Maitland, A.; Towle, A. Artificial rearing of piglets: The administration of two sources of immunoglobulins after birth. Anim. Prod. 1986, 43, 121–126. [Google Scholar] [CrossRef]
- Ferrari, C.V.; Sbardella, P.E.; Bernardi, M.L.; Coutinho, M.L.; Vaz, I.S., Jr.; Wentz, I.; Bortolozzo, F.P. Effect of birth weight and colostrum intake on mortality and performance of piglets after cross-fostering in sows of different parities. Prev. Vet. Med. 2014, 114, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Amdi, C.; Jensen, L.L.; Oksbjerg, N.; Hansen, C.F. Supplementing newborn intrauterine growth restricted piglets with a bolus of porcine colostrum raises rectal temperatures one degree celsius. J. Anim. Sci. 2017, 95, 2968–2976. [Google Scholar] [CrossRef] [PubMed]
- Devillers, N.; Le Dividich, J.; Prunier, A. Lnfluence of colostrum intake on piglet survival and immunity. Animal 2011, 5, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.J.; Wang, F.; Zhang, S.H. Postnatal adaptation of the gastrointestinal tract in neonatal pigs: A possible role of milk-borne growth factors. Livest. Prod. Sci. 2000, 66, 95–107. [Google Scholar] [CrossRef]
- Burrin, D.G.; Davis, T.A.; Ebner, S.; Schoknecht, P.A.; Fiorotto, M.L.; Reeds, P.J.; McAvoy, S. Nutrient-independent and nutrient-dependent factors stimulate protein-synthesis in colostrum-fed newborn piglets. Pediatr. Res. 1995, 37, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Van Barneveld, R.J.; Dunshea, F.R. Colostrum protein isolate increases gut and whole body growth and plasma IGF-I in neonatal pigs. Asian-Australas. J. Anim. Sci. 2011, 24, 670–677. [Google Scholar] [CrossRef]
- Lallès, J.P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.M.; McNeilly, F.; Ellis, J.; Krakowka, S.; Botner, A.; McCullough, K.; Nauwynck, H.; Kennedy, S.; Meehan, B.; Charreyre, C. PMWS: Experimental model and co-infections. Vet. Microbiol. 2004, 98, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Elbers, A.R.W.; de Jong, M.F.; Wellenberg, G.J. Industrial risk factors of pigs with clinical occurrences of PNWS or PDNS in the Netherlands: A case-controlled inspection. Tijdschr. Diergeneeskd. 2006, 131, 318–325. [Google Scholar] [PubMed]
- Vallet, J.L.; Miles, J.R.; Rempel, L.A.; Nonneman, D.J.; Lents, C.A. Relationships between day one piglet serum immunoglobulin immunocrit and subsequent growth, puberty attainment, litter size, and lactation performance. J. Anim. Sci. 2015, 93, 2722–2729. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Quesnel, H. Nutritional, hormonal, and environmental effects on colostrum in sows. J. Anim. Sci. 2009, 87, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Loisel, F.; Farmer, C.; Ramaekers, P.; Quesnel, H. Colostrum yield and piglet growth during lactation are related to gilt metabolic and hepatic status prepartum. J. Anim. Sci. 2014, 92, 2931–2941. [Google Scholar] [CrossRef] [PubMed]
- King’Ori, A.M. The pre-weaning piglet: Colostrum and milk intake: A review. J. Anim. Prod. Adv. 2012, 2, 277–283. [Google Scholar]
- Fraser, D. The role of behaviour in swine production—A review of research. Appl. Anim. Ethol. 1984, 11, 317–339. [Google Scholar] [CrossRef]
- Fraser, D.; Rushen, J. Colostrum intake by newborn piglets. Can. J. Anim. Sci. 1992, 72, 1–13. [Google Scholar] [CrossRef]
- Devillers, N.; Farmer, C.; Le Dividich, J.; Prunier, A. Variability of colostrum yield and colostrum intake in pigs. Animal 2007, 1, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, R.A.; Lin, X.; Campbell, J.M.; Moeser, A.J.; Odle, J. Influence of birth order, birth weight, colostrum and serum immunoglobulin g on neonatal piglet survival. J. Anim. Sci. Biotechnol. 2012, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Quesnel, H. Colostrum production by sows: Variability of colostrum yield and immunoglobulin G concentrations. Animal 2011, 5, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Lines, D.S. Split Suckling to Improve Colostrum Ingestion, Survival and Performance of Gilt Progeny; APL Project 2014/490; Australian Pork Limited: Barton, Australia, 2015. [Google Scholar]
- Devillers, N.; van Milgen, J.; Prunier, A.; Le Dividich, J. Estimation of colostrum intake in the neonatal pig. Anim. Sci. 2004, 78, 305–313. [Google Scholar]
- Deen, M.G.H.; Bilkei, G. Cross fostering of low-birthweight piglets. Livest. Prod. Sci. 2004, 90, 279–284. [Google Scholar] [CrossRef]
- Depassille, A.M.B.; Rushen, J. Suckling and teat disputes by neonatal piglets. Appl. Anim. Behav. Sci. 1989, 22, 23–38. [Google Scholar] [CrossRef]
- Svendsen, L.S.; Weström, B.R.; Svendsen, J.; Ohlsson, B.G.; Ekman, R.; Karlsson, B.W. Insulin involvement in intestinal macromolecular transmission and closure in neonatal pigs. J. Pediatr. Gastroenterol. Nutr. 1986, 5, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Donovan, T.S.; Dritz, S.S. Effect of split nursing on variation in pig growth from birth to weaning. J. Am. Vet. Med. Assoc. 2000, 217, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Tokach, M. Dealing with variation in market weight. Adv. Pork Prod. 2004, 15, 281–290. [Google Scholar]
- Kirkden, R.D.; Broom, D.M.; Andersen, I.L. Invited review: Piglet mortality: Management solutions. J. Anim. Sci. 2013, 91, 3361–3389. [Google Scholar] [CrossRef] [PubMed]
- Kyriazakis, I.; Edwards, S.A. The effect of “split-suckling” on behaviour and performance of piglets. Appl. Anim. Behav. Sci. 1986, 16, 92. [Google Scholar] [CrossRef]
- Vallet, J.L. In Use of the immunocrit to monitor a split-suckle program in commercial production. In Proceedings of the International Conference on Pig Reproduction, Olsztyn, Poland, 9–12 June 2013; pp. 225–226. [Google Scholar]
- Huser, J.S.; Plush, K.J.; Pitchford, W.S.; Kennett, T.E.; Lines, D.S. Neonatal split suckling improves survival of small piglets. Anim. Prod. Sci. 2015, 55, 1477. [Google Scholar]
- Hansen, C.F.; Muller, R.; Kanitz, E.; Tuchscherer, M.; Thorup, F. In Porcine colostrum supplementation increases serum immunoglobulin concentration of light piglets. In Manipulating Pig Production XIV, Proceedings of the Fourteenth Biennial Conference of the Australasian Pig Science Association, Melbourne, Australia, 24–27 November 2013; Australasian Pig Science Association: Werribee, Australia, 2013; p. 203. [Google Scholar]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Effect of oral supplementation with different energy boosters in newborn piglets on pre-weaning mortality, growth and serological levels of IGF-I and IGG1. J. Anim. Sci. 2017, 95, 353–360. [Google Scholar] [PubMed]
- Farmer, C.; Palin, M.-F.; Theil, P.K.; Sorensen, M.T.; Devillers, N. Milk production in sows from a teat in second parity is influenced by whether it was suckled in first parity. J. Anim. Sci. 2012, 90, 3743–3751. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Amezcua, M.; Bruckmaier, R.; Wellnitz, O.; Friendship, R. Does duration of teat use in first parity affect milk yield and mammary gene expression in second parity? J. Anim. Sci. 2017, 95, 681–687. [Google Scholar] [PubMed]
- Bierhals, T.; Magnabosco, D.; Ribeiro, R.R.; Perin, J.; da Cruz, R.A.; Bernardi, M.L.; Wentz, I.; Bortolozzo, F.P. Influence of pig weight classification at cross-fostering on the performance of the primiparous sow and the adopted litter. Livest. Sci. 2012, 146, 115–122. [Google Scholar] [CrossRef]
- Thaker, M.Y.C.; Bilkei, G. Lactation weight loss influences subsequent reproductive performance of sows. Anim. Reprod. Sci. 2005, 88, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, L.; Shi, H.; Hua, Y.; Zhang, L.; Xin, L.; Lei, P.; Liang, J.; Zhang, Y.; Zhao, K. Heritabilities and genetic and phenotypic correlations of litter uniformity and litter size in large white sows. J. Integr. Agric. 2016, 15, 848–854. [Google Scholar] [CrossRef]
- Tummaruk, P.; Sang-Gassanee, K. Effect of farrowing duration, parity number and the type of anti-inflammatory drug on postparturient disorders in sows: A clinical study. Trop. Anim. Health Prod. 2013, 45, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Sauber, T.E.; Stahly, T.S.; Nonnecke, B.J. Effect of level of chronic immune system activation on the lactational performance of sows. J. Anim. Sci. 1999, 77, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Baer, C.; Bilkei, G. Ultrasonographic and gross pathological findings in the mammary glands of weaned sows having suffered recidiving mastitis metritis agalactia. Reprod. Domest. Anim. 2005, 40, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Andersen, I.L.; Nævdal, E.; Bøe, K. Maternal investment, sibling competition, and offspring survival with increasing litter size and parity in pigs (Sus scrofa). Behav. Ecol. Sociobiol. 2011, 65, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Gonyou, H.W. Comparison of management options for sows kept in pens with electronic feeding stations. Can. J. Anim. Sci. 2013, 93, 445–452. [Google Scholar]
- Anil, S.S.; Anil, L.; Deen, J. Effect of lameness on sow longevity. J. Am. Vet. Med. Assoc. 2009, 235, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Pluym, L.M.; Van Nuffel, A.; Van Weyenberg, S.; Maes, D. Prevalence of lameness and claw lesions during different stages in the reproductive cycle of sows and the impact on reproduction results. Animal 2013, 7, 1174–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldham, J.G. Clinical measurement of pain, distress and discomfort in pigs. In The Detection and Relief of Pain in Animals; Gibson, T.E., Ed.; BVA Animal Welfare Foundation: London, UK, 1985; pp. 89–91. [Google Scholar]
- Johnson, R.W. Inhibition of growth by pro-inflammatory cytokines: An integrated view. J. Anim. Sci. 1997, 75, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Dinarés, M.; Devant, M.; Carré, X. Associations between lameness and production, feeding and milking attendance of holstein cows milked with an automatic milking system. J. Dairy Res. 2007, 74, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.; D’Eath, R.B.; Fujita, K. Consistency of piglet crushing by sows. Anim. Welf. 2005, 14, 43–51. [Google Scholar]
- Rodriguez, C.; Rodriganez, J.; Silio, L. Genetic-analysis of maternal ability in Iberian pigs. J. Anim. Breed. Genet. 1994, 111, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Jonas, E.; Schreinemachers, H.J.; Kleinwächter, T.; Ün, C.; Oltmanns, I.; Tetzlaff, S.; Jennen, D.; Tesfaye, D.; Ponsuksili, S.; Murani, E. QTL for the heritable inverted teat defect in pigs. Mamm. Genome 2008, 19, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, H.; Rydhmer, L.; Lundeheim, N. Genetic analysis of functional and non-functional teats in a population of Yorkshire pigs. Livest. Sci. 2013, 152, 127–134. [Google Scholar] [CrossRef]
- Guide for Defining Whether a Teat Is Functional or Non-Functional on Visual Appearance. Available online: http://www.thepigsite.com/pighealth/article/222/udder/ (accessed on 8 March 2018).
- Vasdal, G.; Andersen, I.L. A note on teat accessibility and sow parity; consequences for newborn piglets. Livest. Sci. 2012, 146, 91–94. [Google Scholar] [CrossRef]
- Nielsen, O.L.; Pedersen, A.R.; Sørensen, M.T. Relationships between piglet growth rate and mammary gland size of the sow. Livest. Prod. Sci. 2001, 67, 273–279. [Google Scholar] [CrossRef]
- Balzani, A.; Cordell, H.J.; Edwards, S.A. Relationship of sow udder morphology with piglet suckling behavior and teat access. Theriogenology 2016, 86, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Ocepek, M.; Andersen-Ranberg, I.; Edwards, S.A.; Andersen, I.L. Udder characteristics of importance for teat use in purebred and crossbred pigs. J. Anim. Sci. 2016, 94, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Balzani, A.; Cordell, H.J.; Sutcliffe, E.; Edwards, S.A. Heritability of udder morphology and colostrum quality traits in swine. J. Anim. Sci. 2016, 94, 3636–3644. [Google Scholar] [CrossRef] [PubMed]
- Neal, S.M.; Irvin, K.M. The effects of crossfostering pigs on survival and growth. J. Anim. Sci. 1991, 69, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Heim, G.; Mellagi, A.P.G.; Bierhals, T.; de Souza, L.P.; de Fries, H.C.C.; Piuco, P.; Seidel, E.; Bernardi, M.L.; Wentz, I.; Bortolozzo, F.P. Effects of cross-fostering within 24 h after birth on pre-weaning behaviour, growth performance and survival rate of biological and adopted piglets. Livest. Sci. 2012, 150, 121–127. [Google Scholar] [CrossRef]
- Baxter, E.M.; Rutherford, K.M.D.; D’Eath, R.B.; Arnott, G.; Turner, S.P.; Sandoe, P.; Moustsen, V.A.; Thorup, F.; Edwards, S.A.; Lawrence, A.B. The welfare implications of large litter size in the domestic pig II: Management factors. Anim. Welf. 2013, 22, 219–238. [Google Scholar] [CrossRef]
- Quesnel, H.; Brossard, L.; Valancogne, A.; Quiniou, N. Influence of some sow characteristics on within-litter variation of piglet birth weight. Animal 2008, 2, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Skok, J.; Skorjanc, D. Group suckling cohesion as a prelude to the formation of teat order in piglets. Appl. Anim. Behav. Sci. 2014, 154, 15–21. [Google Scholar] [CrossRef]
- Li, Y.Z.; Anderson, J.E.; Johnston, L.J. Animal-related factors associated with piglet mortality in a bedded, group-farrowing system. Can. J. Anim. Sci. 2012, 92, 11–20. [Google Scholar] [CrossRef]
- Milligan, B.N.; Fraser, D.; Kramer, D.L. Birth weight variation in the domestic pig: Effects on offspring survival, weight gain and suckling behaviour. Appl. Anim. Behav. Sci. 2001, 73, 179–191. [Google Scholar] [CrossRef]
- Huting, A.M.S.; Almond, K.; Wellock, I.; Kyriazakis, I. What is good for small piglets might not be good for big piglets: The consequences of cross-fostering and creep feed provision on performance to slaughter. J. Anim. Sci. 2017, 95, 4926–4944. [Google Scholar] [CrossRef] [PubMed]
- Muns, R.; Silva, C.; Manteca, X.; Gasa, J. Effect of cross-fostering and oral supplementation with colostrums on performance of newborn piglets. J. Anim. Sci. 2014, 92, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.P.; Fries, H.C.C.; Heim, G.; Faccin, J.E.; Hernig, L.F.; Marimon, B.T.; Bernardi, M.L.; Bortolozzo, F.P.; Wentz, I. Behaviour and growth performance of low-birth-weight piglets cross-fostered in multiparous sows with piglets of higher birth weights. Arq. Bras. Med. Vet. Zootec. 2014, 66, 510–518. [Google Scholar] [CrossRef]
- Marcatti, N.A. Effect of cross fostering on piglets preweaning performance. Arq. Bras. Med. Vet. Zootec. 1986, 38, 413–417. [Google Scholar]
- Cutler, R.S.; Fahy, V.A.; Spicer, E.M.; Cronin, G.M. Preweaning mortality. Dis. Swine 1992, 7, 847–860. [Google Scholar]
- Widmar, D.A.; Olynk, N.J.; Richert, B.T.; Schinckel, A.P.; Foster, K.A. In Integrated on-farm decision making: Economic implications of increased variation in litter size. In Proceedings of the 2011 Annual Meeting, Corpus Christi, TX, USA, 5–8 February 2011. [Google Scholar]
- Baxter, E.M.; Jarvis, S.; Palarea-Albaladejo, J.; Edwards, S.A. The weaker sex? The propensity for male-biased piglet mortality. Public Libr. Sci. 2012, 7, e30318. [Google Scholar] [CrossRef] [PubMed]
- Bereskin, B.; Shelby, C.E.; Cox, D.F. Some factors affecting pig survival. J. Anim. Sci. 1973, 36, 821–827. [Google Scholar] [CrossRef] [PubMed]
- McGlone, J.J.; Nicholson, R.I.; Hellman, J.M.; Herzog, D.N. The development of pain in young pigs associated with castration and attempts to prevent castration-induced behavioral changes. J. Anim. Sci. 1993, 71, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Dunshea, F.R. Sexual dimorphism in growth of sucking and growing pigs. Australas. J. Anim. Sci. 2001, 14, 1610–1615. [Google Scholar] [CrossRef]
- McCaw, M.B. Effect of reducing crossfostering at birth on piglet mortality and performance during an acute outbreak of porcine reproductive and respiratory syndrome. J. Swine Health Prod. 2000, 8, 15–21. [Google Scholar]
- Price, E.O.; Hutson, G.D.; Price, M.I.; Borgwardt, R. Fostering in swine as affected by age of offspring. J. Anim. Sci. 1994, 72, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Giroux, S.; Robert, S.; Martineau, G.P. The effects of cross-fostering on growth rate and post-weaning behavior of segregated early-weaned piglets. Can. J. Anim. Sci. 2000, 80, 533–538. [Google Scholar] [CrossRef]
- Robert, S.; Martineau, G.P. Effects of repeated cross-fosterings on preweaning behavior and growth performance of piglets and on maternal behavior of sows. J. Anim. Sci. 2001, 79, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Horrell, I.; Bennett, J. Disruption of teat preferences and retardation of growth following cross-fostering of 1-week old pigs. Anim. Prod. 1981, 33, 99–106. [Google Scholar] [CrossRef]
- Straw, B.E.; Dewey, C.E.; Bürgi, E.J. Patterns of crossfostering and piglet mortality on commercial US and Canadian swine farms. Prev. Vet. Med. 1998, 33, 83–89. [Google Scholar] [CrossRef]
- Wattanaphansak, S.; Larriestra, L.; Deen, J. In The effect of cross-fostering on the risk of light weaning weight. In Proceedings of the 17th International Pig Veterinary Society Congress, Ames, IA, USA, 2–5 June 2002; pp. 2–5. [Google Scholar]
- Wattanaphansak, S.; Luengyosluechakul, S.; Larriestra, A.; Deen, J. The impact of cross-fostering on swine production. Thai J. Vet. Med. 2002, 32, 101–106. [Google Scholar]
- Straw, B.E. Veterinary practice: Art, science and politics. In Proceedings of the American Association of Swine Practitioners, Montreal, QC, Canada, 7–10 March 1997; pp. 1–31. [Google Scholar]
- Reese, D.; Straw, B. The Case against Evening-Up Litters until Weaning; University of Nebraska: Lincoln, NE, USA, 2006; pp. 7–10. [Google Scholar]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- Sorensen, J.T.; Rousing, T.; Kudahl, A.B.; Hansted, H.J.; Pedersen, L.J. Do nurse sows and foster litters have impaired animal welfare? Results from a cross-sectional study in sow herds. Animal 2016, 10, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, J.; Lines, D.S.; Plush, K.J. Nurse sows display altered reproduction in the next gestation. Anim. Prod. Sci. 2017, 57, 2445. [Google Scholar] [CrossRef]
- Amdi, C.; Moustsen, V.A.; Oxholm, L.C.; Baxter, E.M.; Sørensen, G.; Eriksson, K.B.; Diness, L.H.; Nielsen, M.F.; Hansen, C.F. Comparable cortisol, heart rate and milk let-down in nurse sows and non-nurse sows. Livest. Sci. 2017, 198, 174–181. [Google Scholar] [CrossRef]
- Bruun, T.S.; Amdi, C.; Vinther, J.; Schop, M.; Strathe, A.B.; Hansen, C.F. Reproductive performance of “nurse sows” in Danish piggeries. Theriogenology 2016, 86, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Houben, M.A.M.; Tobias, T.J.; Holstege, M.M.C. The effect of double nursing, an alternative nursing strategy for the hyper-prolific sow herd, on herd performance. Porcine Health Manag. 2017, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Prims, S.; Tambuyzer, B.; Vergauwen, H.; Huygelen, V.; van Cruchten, S.; van Ginneken, C.; Casteleyn, C. Intestinal immune cell quantification and gram type classification of the adherent microbiota in conventionally and artificially reared, normal and low birth weight piglets. Livest. Sci. 2016, 185, 1–7. [Google Scholar] [CrossRef]
- Rzezniczek, M.; Gygax, L.; Wechsler, B.; Weber, R. Comparison of the behaviour of piglets raised in an artificial rearing system or reared by the sow. Appl. Anim. Behav. Sci. 2015, 165, 57–65. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexopoulos, J.G.; Lines, D.S.; Hallett, S.; Plush, K.J. A Review of Success Factors for Piglet Fostering in Lactation. Animals 2018, 8, 38. https://doi.org/10.3390/ani8030038
Alexopoulos JG, Lines DS, Hallett S, Plush KJ. A Review of Success Factors for Piglet Fostering in Lactation. Animals. 2018; 8(3):38. https://doi.org/10.3390/ani8030038
Chicago/Turabian StyleAlexopoulos, Jena G., David S. Lines, Suzanne Hallett, and Kate J. Plush. 2018. "A Review of Success Factors for Piglet Fostering in Lactation" Animals 8, no. 3: 38. https://doi.org/10.3390/ani8030038



