A Two-Step Process of Nitrous Oxide before Carbon Dioxide for Humanely Euthanizing Piglets: On-Farm Trials
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.1.1. Experiment 1
2.1.2. Experiment 2
2.2. Overall Experimental Design
2.3. Procedures
2.3.1. Experiment 1
2.3.2. Experiment 2
2.4. Data Processing
2.5. Statistical Methods
3. Results
3.1. Experiment 1
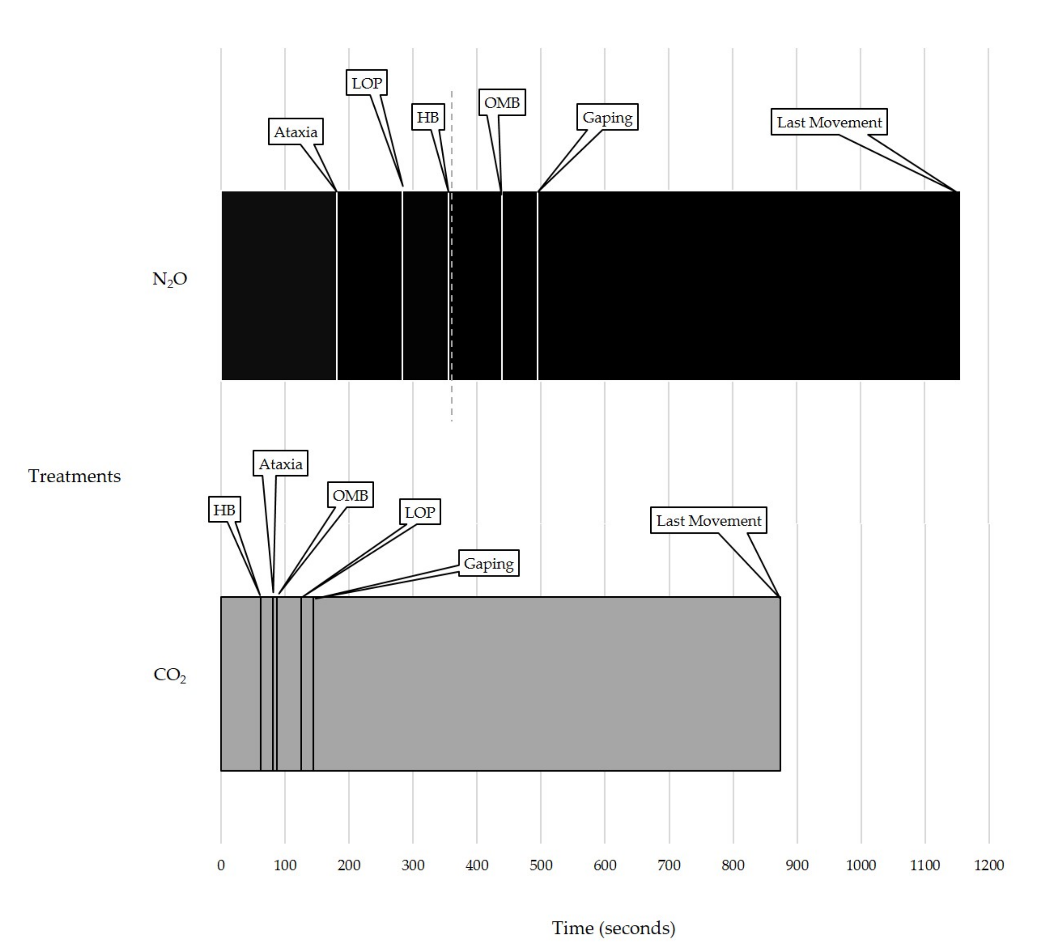
3.1.1. Durations and Latencies
3.1.2. Behavioral Events
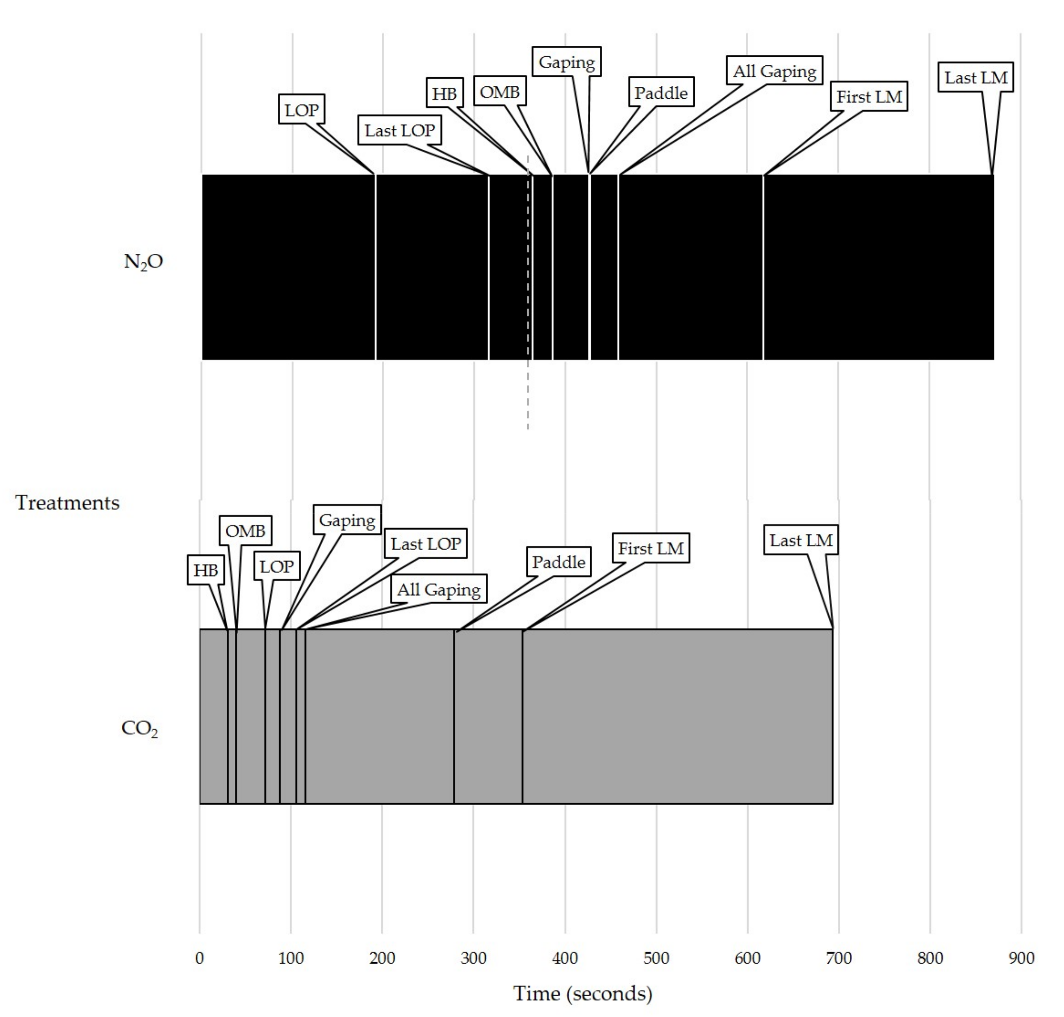
3.2. Experiment 2
Latency Data
3.3. Exposure to CO2 Post-N2Os
4. Discussion
4.1. Experiment 1
4.2. Experiment 2
4.3. Shortcomings and Future Work
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United States Department of Agriculture. Farrowing productivity and preweaning death loss. In Swine 2012 Part I: Baseline Reference of Swine Health and Management in the United States; USDA-APHIS-VS, Center for Epidemiology and Animal Health (CEAH): Fort Collins, CO, USA, 2015; pp. 45–49. [Google Scholar]
- Baxter, E.M.; Edwards, S.A. Chapter 3: Piglet mortality and morbidity: Inevitable or unacceptable. In Advances in Pig Welfare; Špinka, M., Ed.; Elsevier: Cambridge, MA, USA, 2018; ISBN 978-0-12-418670-5. [Google Scholar]
- American Veterinary Medical Association. Suckling Pigs. In AVMA Guidelines for the Euthanasia of Animals, 2013th ed.; American Veterinary Medical Association: Schaumburg, IL, USA, 2013; p. 61. ISBN 978-1-882691-21-0. [Google Scholar]
- American Association of Swine Veterinarians. National Pork Board On-Farm Euthanasia of Swine Recommendations for the Producer; America’s Pork Checkoff Program: Des Moines, IA, USA, 2008; pp. 2–15. [Google Scholar]
- Rault, J.L.; McMunn, K.A.; Marchant-Forde, J.N.; Lay, D.C. Gas alternatives to carbon dioxide for euthanasia: A piglet perspective. J. Anim. Sci. 2013, 91, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.B.M.; Gregory, N.G. Welfare implications of the gas stunning of pigs: 1. Determination of aversion to the initial inhalation of carbon dioxide or argon. Anim. Welf. 1995, 4, 273–280. [Google Scholar]
- Velarde, A.; Cruz, J.; Gispert, M.; Carrión, D.; Torre, R.J.; Diestre, A.; Manteca, X. Aversion to carbon dioxide stunning in pigs: Effect of carbon dioxide concentration and halothane genotype. Anim. Welf. 2007, 16, 513–522. [Google Scholar]
- Sadler, L.J.; Haden, C.D.; Wang, C.; Widowski, T.M.; Johnson, A.K.; Millman, S.T. Effects of flow rate and gas mixture on the welfare of weaned and neonate pigs during gas euthanasia. J. Anim. Sci. 2014, 92, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Llonch, P.; Dalmau, A.; Rodriguez, P.; Manteca, X.; Velarde, A. Aversion to nitrogen and carbon dioxide mixtures for stunning pigs. Anim. Welf. 2012, 21, 33–39. [Google Scholar] [CrossRef]
- Oka, S.; Chapman, C.R.; Kim, B.; Nakajima, I.; Shimizu, O.; Oi, Y. Pupil dilation response to noxious stimulation: Effect of varying nitrous oxide concentration. Clin. Neurophysiol. 2007, 118, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.L.; Lay, D.C.; Marchant-Forde, J.N. Castration induced pain in pigs and other livestock. Appl. Anim. Behav. Sci. 2011, 135, 214–225. [Google Scholar] [CrossRef]
- Rault, J.L.; Kells, N.; Johnson, C.; Dennis, R.; Sutherland, M.; Lay, D.C. Nitrous oxide as a humane method for piglet euthanasia: Behavior and electroencephalography (EEG). Physiol. Behav. 2015, 151, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.-L.; Lai, A.; Hemsworth, L.; Le Chevoir, M.; Bauquier, S.; Gates, R.; Lay, D.C. Wireless intracranial electroencephalography (iEEG) and corresponding behavior in piglets euthanized with nitrous oxide or carbon dioxide of unpublished work. 2018; in press. [Google Scholar]
- Verhoeven, M.T.W.; Gerritzen, M.A.; Hellebrekers, L.J.; Kemp, B. Indicators used in livestock to assess unconsciousness after stunning: A review. Animal 2015, 9, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, N.J.; Mellor, D.J. Introducing breathlessness as a significant animal welfare issue. N. Z. Vet. J. 2015, 63, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.B.M.; Gregory, N.G. Welfare implications of the gas stunning of pigs: 2. Stress of induction of anaesthesia. Anim. Welf. 1996, 5, 71–78. [Google Scholar]
- Nattie, E. CO2, Brainstem chemoreceptors and breathing. Prog. Neurobiol. 1999, 59, 299–331. [Google Scholar] [CrossRef]
- Fiedler, K.J.; Parsons, R.L.; Sadler, L.J.; Millman, S.T. Effects of stocking rate on measures of efficacy and welfare during carbon dioxide gas euthanasia of young pigs. Anim. Welf. 2014, 23, 309–321. [Google Scholar] [CrossRef]
- Kanitz, E.; Puppe, B.; Tuchscherer, M.; Heberer, M.; Viergutz, T.; Tuchscherer, A. A single exposure to social isolation in domestic piglets activates behavioural arousal, neuroendocrine stress hormones, and stress-related gene expression in the brain. Physiol. Behav. 2009, 98, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Reimert, I.; Bolhuis, J.E.; Kemp, B.; Rodenburg, T.B. Indicators of positive and negative emotions and emotional contagion in pigs. Physiol. Behav. 2013, 109, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.B.M. Behavior of pigs exposed to mixtures of gases and the time required to stun and kill them: Welfare implications. Vet. Rec. 1999, 144, 165–168. [Google Scholar] [CrossRef] [PubMed]


| Category | Behavior | Description |
|---|---|---|
| Duration | Stand | Up on four legs. |
| Lateral Lying | Lying down with side in contact with the floor. | |
| Ventral Lying | Lying down with sternum and belly in contact with the floor. | |
| Sit | One or two hind legs folded underneath the body and supporting weight on two front legs. “Sitting like a dog“. | |
| Kneel | One or two front legs folded underneath the body with hind legs straight. | |
| Locomotion | Any movement more than two steps; walk or run. | |
| Inactive | Immobile, not doing any particular behavior. | |
| Latency | Ataxic | Lack of muscle coordination in basic movements, loss of balance on one or more feet. |
| Loss of Posture 2 | Piglet lies on the ground and does not get back up. | |
| Heavy Breathing 2 | Forceful and quick repetition of flank movements, mouth closed. | |
| Open-Mouth Breathing 2 | Mouth open to breath from. | |
| Gaping 2 | Deep forceful, rhythmic movements of the chest with mouth open, a rudimentary brain stem reflex, deep state of unconsciousness. | |
| Last Movement 2 | Piglet stops gaping and does not move at all, is clinically dead. End of experiment time. | |
| Paddle Bout 1,2 | While lying laterally the piglet’s legs paddle/run. | |
| Events | Rooting Bout 1 | Snout in contact with the floor touching, sniffing, rubbing, or chewing. |
| Escape Attempt 1,2 | Rear on hind legs, jump (all limbs lose contact with the floor), or scratch with front legs against walls. | |
| Neck Stretch 1 | Extend neck as much as possible, head up. | |
| Foot Slips 1 | Piglet scrambles/loses balance while standing. | |
| Righting Response 1,2 | Unsuccessful effort to right up onto four legs. | |
| Squeal 1,2 | High-pitched vocalization; extended sound of high amplitude and frequency. | |
| Grunt 1 | Low-pitched vocalization; sound of low to medium amplitude. | |
| Intermediate 1 | Vocalization that starts out low-pitched and ends high-pitched. Neither a grunt or squeal but some combination. |
| Variable 1 | CO2 | N2O | p-Value |
|---|---|---|---|
| Day of Age | 5.75 ± 0.37 | 4.00 ± 0.62 | 0.034 * |
| Body Temperature (°C) | 37.85 ± 17.52 | 37.67 ± 17.60 | 0.562 |
| Weight (kg) | 0.96 ± 0.07 | 1.04 ± 0.08 | 0.412 |
| CO2 Total Time (min) | 14.88 ± 2.43 | 13.78 ± 2.58 | 0.410 |
| Procedure Total Time (min) | 14.57 ± 2.43 | 19.29 ± 0.75 | 0.457 |
| Latency to no Palpebral Reflex (s) | 313.38 ± 43.70 | 560.22 ± 26.63 2 | <0.001 * |
| Category | Variable (s) 1 | CO2 | N2O | p-Value |
|---|---|---|---|---|
| Duration | Standing | 99.81 ± 9.15 | 202.69 ± 51.45 | 0.946 |
| Lateral Lying | 656.32 ± 149.02 | 766.13 ± 175.47 2 | 0.630 | |
| Ventral Lying | 94.55 ± 40.09 | 164.06 ± 41.37 2 | 0.491 | |
| Sitting | 26.47 ± 4.77 | 29.37 ± 18.51 | 0.123 | |
| Kneeling | 0.00 ± 0.00 | 3.38 ± 2.92 | 0.169 | |
| Locomotion | 19.99 ± 5.01 | 52.76 ± 18.84 | 0.336 | |
| Inactivity | 226.62 ± 105.24 | 231.60 ± 36.93 2 | 0.149 | |
| Latency | Ataxia | 81.43 ± 6.03 | 181.43 ± 36.25 | 0.021 * |
| Heavy Breathing | 61.61 ± 10.93 | 356.79 ± 31.29 2 | <0.001 * | |
| Open-Mouth Breathing | 86.71 ± 9.82 | 439.87 ± 9.99 2 | 0.001 * | |
| Gaping | 143.57 ± 9.53 | 495.43 ± 13.95 2 | 0.001 * | |
| Loss of Posture | 125.16 ± 6.48 | 284.36 ± 43.29 | 0.004 * | |
| First Paddle Bout | 616.88 ± 180.62 | 960.82 ± 197.94 2 | 0.370 |
| Variable (Frequency) | CO2 | N2O | p-Value |
|---|---|---|---|
| Escape Attempts | 1.38 ± 0.60 | 2.11 ± 1.51 | 0.499 |
| Neck Stretches | 4.38 ± 1.02 | 3.89 ± 2.30 | 0.143 |
| Righting Responses | 0.88 ± 0.40 | 2.33 ± 0.90 1 | 0.249 |
| Paddle Bouts | 0.50 ± 0.27 | 0.67 ± 0.33 1 | 0.784 |
| Foot Slips | 0.75 ± 0.49 | 4.33 ± 2.22 | 0.482 |
| Rooting Bouts | 1.25 ± 0.59 | 5.00 ± 1.91 | 0.095 † |
| Squeals/Minute | 0.12 ± 0.06 | 0.80 ± 0.46 | 0.587 |
| Intermediates/Minute | 1.02 ± 0.67 | 2.18 ± 1.11 | 0.381 |
| Grunts/Minute | 1.87 ± 0.40 | 4.89 ± 1.37 | 0.175 |
| Variable 1 | CO2 | N2O | p-Value |
|---|---|---|---|
| Average Day of Age | 2.28 ± 0.64 | 1.71 ± 0.45 | 0.480 |
| Average Weight (kg) | 0.67 ± 0.07 | 0.61 ± 0.03 | 0.825 |
| Squeals/Minute | 0.48 ± 0.25 | 4.90 ± 1.41 | 0.004 * |
| Escape Attempts/Piglet | 0.17 ± 0.12 | 0.79 ± 0.32 | 0.021 * |
| Righting Responses/Piglet | 0.36 ± 0.06 | 1.41 ± 0.39 | 0.084 † |
| CO2 Total Time (min) | 8.56 ± 0.18 | 6.56 ± 0.34 | <0.001 * |
| Procedure Total Time (min) | 11.56 ± 0.59 | 14.54 ± 0.50 | 0.001 * |
| Latency Variable (s) 1 | CO2 | N2O | p-Value |
|---|---|---|---|
| First Piglet Heavy Breathing | 30.94 ± 2.15 | 364.46 ± 5.35 2 | <0.001 * |
| First Piglet Open-Mouth Breathing | 39.94 ± 2.58 | 386.22 ± 4.44 2 | <0.001 * |
| First Piglet Gaping | 88.13 ± 6.43 | 425.88 ± 4.63 2 | <0.001 * |
| All Piglets Gaping | 115.73 ± 5.24 | 458.38 ± 5.76 2 | <0.001 * |
| First Piglet Loss of Posture | 71.80 ± 5.13 | 191.50 ± 11.61 | <0.001 * |
| Last Piglet Loss of Posture | 105.65 ± 1.96 | 315.90 ± 11.61 | <0.001 * |
| First Piglet’s Paddle Bout | 278.26 ± 65.66 | 427.43 ± 17.09 2 | 0.043 * |
| First Piglet’s Last Movement | 353.05 ± 13.03 | 617.27 ± 13.36 2 | <0.001 * |
| Latency Variable (s) 1 | CO2 | Post-N2O | p-Value |
|---|---|---|---|
| Ataxia | 81.43 ± 6.03 | −178.96 ± 36.25 2 | 0.001 * |
| Heavy Breathing | 61.61 ± 10.93 | −3.21 ± 31.29 2 | 0.102 |
| Open-Mouth Breathing | 86.71 ± 9.82 | 79.87 ± 9.99 | 0.401 |
| Gaping | 143.57 ± 9.53 | 135.43 ± 13.95 | 0.826 |
| Loss of Posture | 125.16 ± 6.48 | −75.64 ± 43.29 2 | 0.002 * |
| First Paddle Bout | 616.88 ± 180.62 | 600.82 ± 197.94 | 0.564 |
| Latency Variable (s) 1 | CO2 | Post-N2O | p-Value |
|---|---|---|---|
| 1st Piglet Heavy Breathing | 30.94 ± 2.15 | 4.46 ± 5.35 | <0.001 * |
| 1st Piglet Open-Mouth Breathing | 39.94 ± 2.58 | 26.22 ± 4.44 | 0.017 * |
| 1st Piglet Gaping | 88.13 ± 6.43 | 65.88 ± 4.63 | 0.013 * |
| All Piglets Gaping | 115.73 ± 5.24 | 98.38 ± 5.76 | 0.041 * |
| 1st Piglet‘s Loss of Posture | 71.80 ± 5.13 | −168.50 ± 11.61 2 | <0.001 * |
| Last Piglet‘s Loss of Posture | 105.65 ± 1.96 | −44.10 ± 11.61 2 | <0.001 * |
| 1st Piglet’s Paddle Bout | 278.26 ± 65.66 | 67.43 ± 17.09 | 0.001 * |
| 1st Piglet’s Last Movement | 353.05 ± 13.03 | 257.27 ± 13.36 | <0.001 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, R.K.; Rault, J.-L.; Gates, R.S.; Lay, D.C., Jr. A Two-Step Process of Nitrous Oxide before Carbon Dioxide for Humanely Euthanizing Piglets: On-Farm Trials. Animals 2018, 8, 52. https://doi.org/10.3390/ani8040052
Smith RK, Rault J-L, Gates RS, Lay DC Jr. A Two-Step Process of Nitrous Oxide before Carbon Dioxide for Humanely Euthanizing Piglets: On-Farm Trials. Animals. 2018; 8(4):52. https://doi.org/10.3390/ani8040052
Chicago/Turabian StyleSmith, Rebecca K., Jean-Loup Rault, Richard S. Gates, and Donald C. Lay, Jr. 2018. "A Two-Step Process of Nitrous Oxide before Carbon Dioxide for Humanely Euthanizing Piglets: On-Farm Trials" Animals 8, no. 4: 52. https://doi.org/10.3390/ani8040052





