Rocks, Clays, Water, and Salts: Highly Durable, Infinitely Rechargeable, Eminently Controllable Thermal Batteries for Buildings
Abstract
:1. Introduction
2. Thermal Mass Design Strategies
2.1. Design Intents
2.2. Ideal Heat Flux Profiles
2.3. Design Approaches
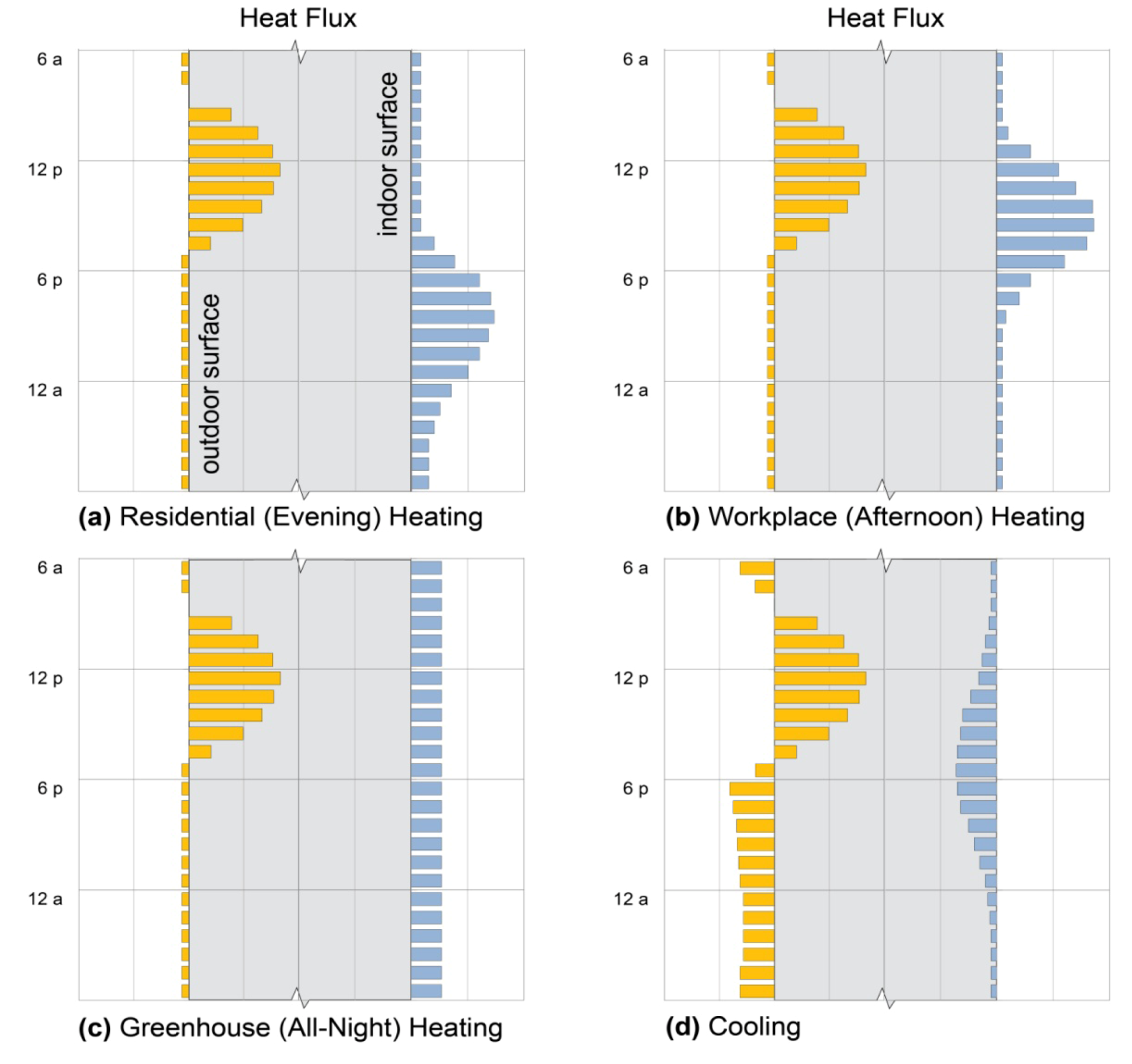

2.4. Mass Sizing
3. Material Properties
3.1. Heat Capacity
3.2. Emissivity
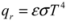
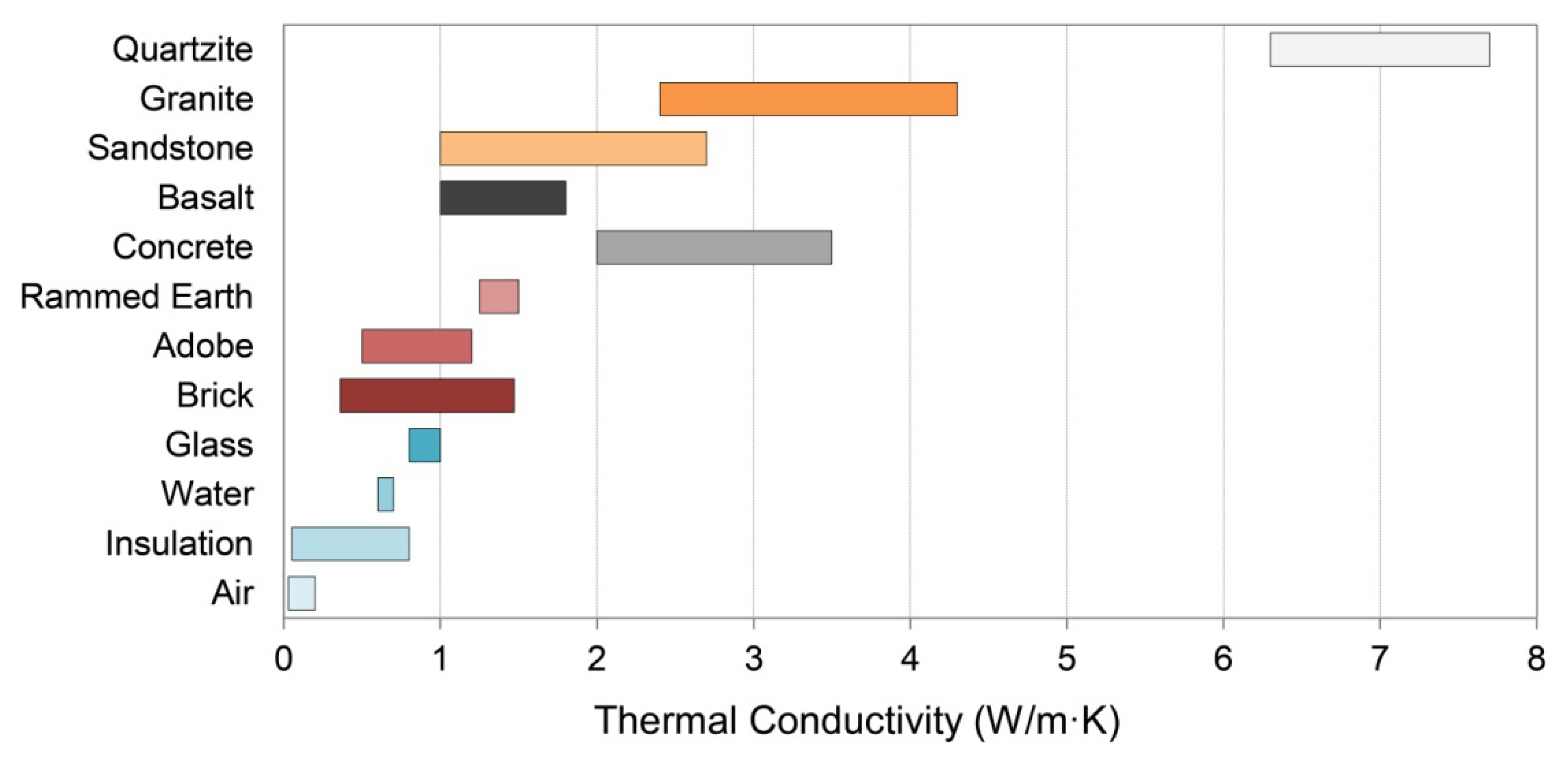
3.3. Thermal Conductivity
| Material | Thermal Conductivity [W/(m·K)] | Specific Heat Capacity [J/(kg·K)] | Density (kg/m3) | Thermal Diffusivity (mm2/s) | Thermal Effusivity [J/(K·m2 s0.5)] | Emissivity b | |
|---|---|---|---|---|---|---|---|
| Air | Air (20 °C) a | 0.025 | 1,005 | 1.2 | 20 | 5 | |
| Insulation (foam) b | 0.03 | 1,210 | 43 | 0.6 | 40 | ||
| Insulation (fiber) b | 0.05 | 960 | 19 | 2.74 | 30 | ||
| Water | Liquid, 20 °C b | 0.6 | 4,180 | 998 | 0.14 | 1,580 | |
| Glass | Clear Float b | 0.9 | 840 | 2,500 | 0.43 | 1,375 | 0.84–0.95 |
| Salts | CaCl2·6H2O c | 1.0 | 1,450 | 1,490 | 0.46 | 1,470 | |
| Na2SO4·10H2O d | 0.54 | 1,930 | 1,485 | 0.19 | 1,240 | ||
| Zn(NO3)2·6H2O e | 0.46 | 1,340 | 1,900 | 0.18 | 1,080 | ||
| Clays | Adobe f | 0.5–1.2 | 840–1,000 | 1,200–2,000 | 0.42–0.71 | 900–1,200 | 0.9 |
| Rammed Earth g | 0.7–1.25 | 870–1260 | 1540–1830 | 0.36–0.79 | 970–1,700 | 0.9 | |
| Brick b | 0.7–1.0 | 790–800 | 1,920–1,970 | 0.4–0.6 | 1,000–1,250 | 0.75–0.93 | |
| Concrete | Heavyweight b,h | 2.0–3.5 | 900–1,000 | 2,200–2,400 | 0.89–1.6 | 2,000–2,800 | 0.85–0.9 |
| Stone | Basalt i | 1.0–2.0 | 720–1040 | 2,700–3,310 | 0.52–0.71 | 1,680–1,960 | 0.72 |
| Sandstone i | 1.0–2.7 | 730–930 | 1,990–2,450 | 0.54–1.51 | 1,340–2,240 | 0.9 | |
| Granite i | 2.4–4.5 | 650–800 | 2,600–2,720 | 1.15–2.55 | 2,060–3,060 | 0.9 | |
| Quartzite i | 6.3–7.7 | 700 | 2,500–2,700 | 3.6–4.1 | 3,450–3,670 | ||
| Metals | Aluminum b | 220 | 896 | 2,740 | 90 | 23,000 | 0.05–0.09 |
| Copper b | 393 | 390 | 8,910 | 113 | 37,000 | 0.07 | |
| Iron, cast b | 48 | 500 | 7,200 | 13 | 13,000 | 0.45 | |
 (K/m), according to Fourier’s law [51]:
(K/m), according to Fourier’s law [51]:
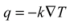
3.4. Thermal Diffusivity
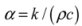
3.5. Thermal Effusivity
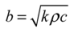
3.6. Kinematic Viscosity
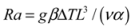
4. External Mass Walls
4.1. Adobe, Rammed Earth, and Brick
4.1.1. Adobe
4.1.2. Rammed Earth
4.1.3. Brick
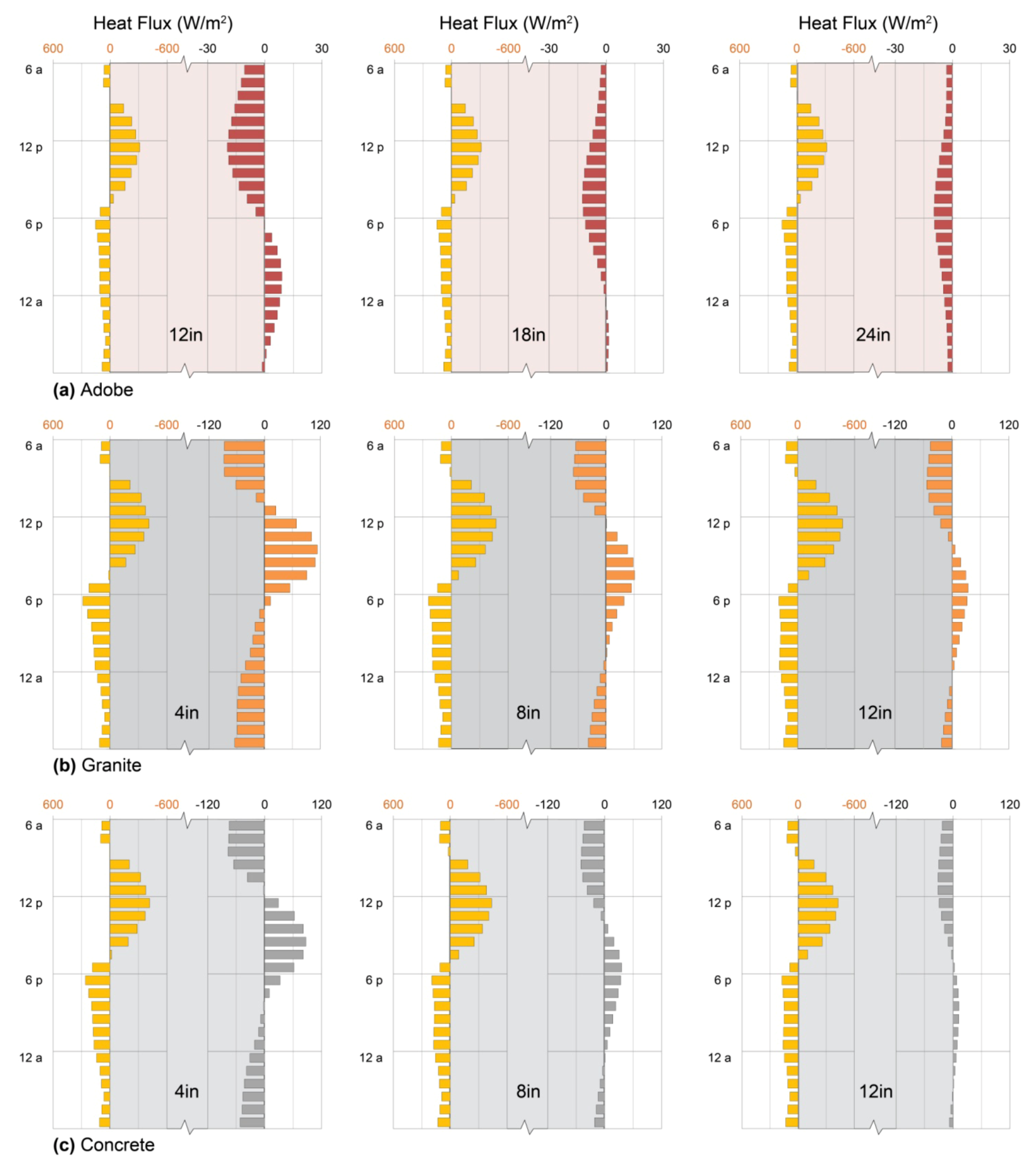
4.1.4. Performance: Exposed Earth Walls
4.1.5. Designer’s Dilemma
4.2. Stone
4.2.1. Mineral Content
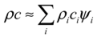
4.2.2. Mineral Packing
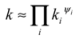
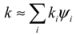
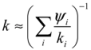
4.2.3. Porosity
4.2.4. Performance: Exposed Stone Walls
4.3. Concrete
4.4. Performance: Exposed Concrete Walls
4.5. Performance: Materials for Exposed Mass Walls
5. Trombe Walls, Water Walls, and PCM Walls
5.1. Trombe Walls
5.1.1. Selective Surfaces
5.1.2. Sizing
5.1.3. Performance: Concrete Trombe Wall
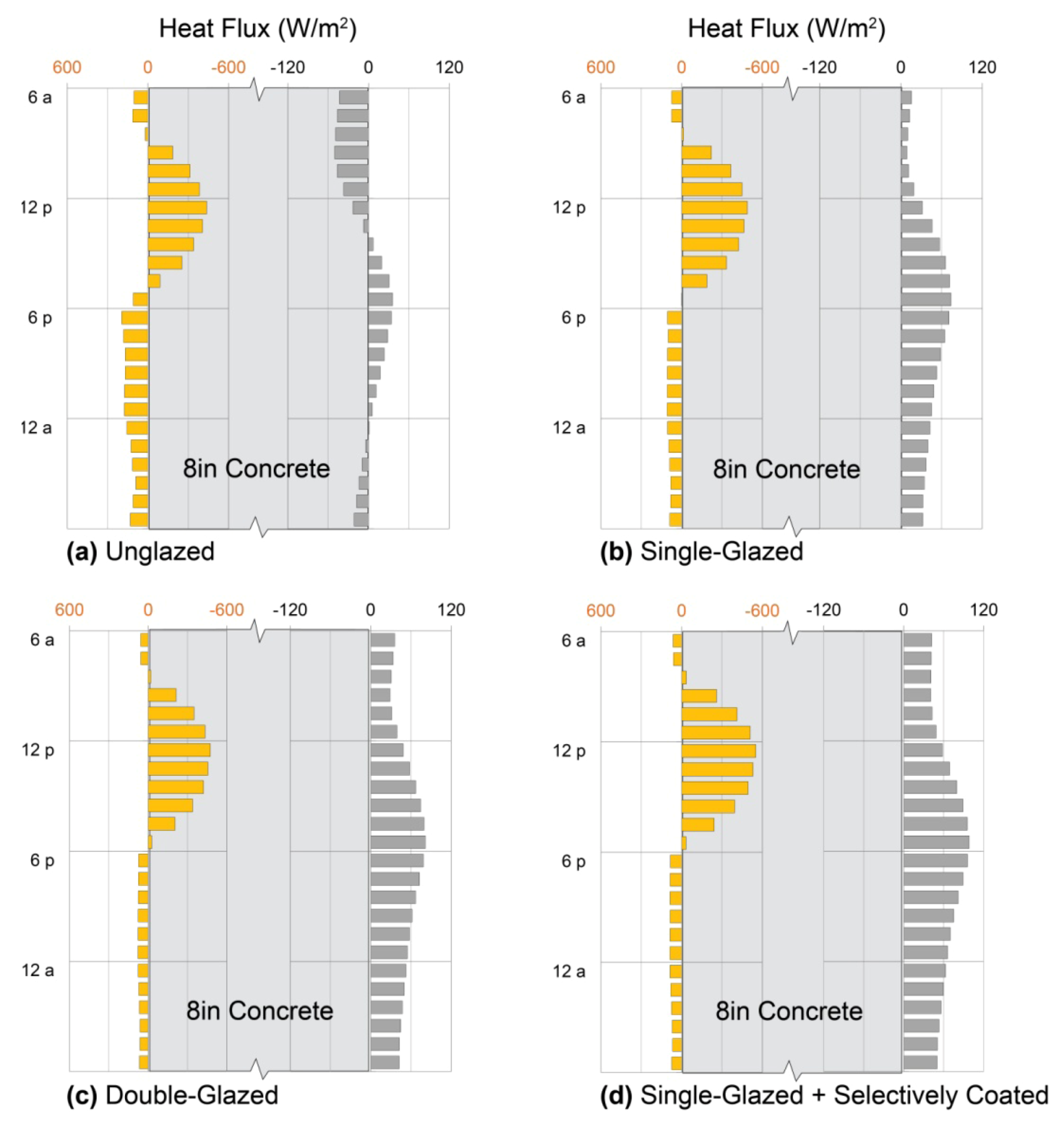
5.2. Water Walls
5.2.1. Containment and Sizing
5.2.2. Convective Heat Transfer
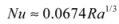
5.2.3. Performance: Water Wall
 , while the effective diffusivity increases by a factor of Nu. As a result, convective heat transport may be used to further advantage in conditioning entire spaces through cold nights, as illustrated below (Section 6).
, while the effective diffusivity increases by a factor of Nu. As a result, convective heat transport may be used to further advantage in conditioning entire spaces through cold nights, as illustrated below (Section 6).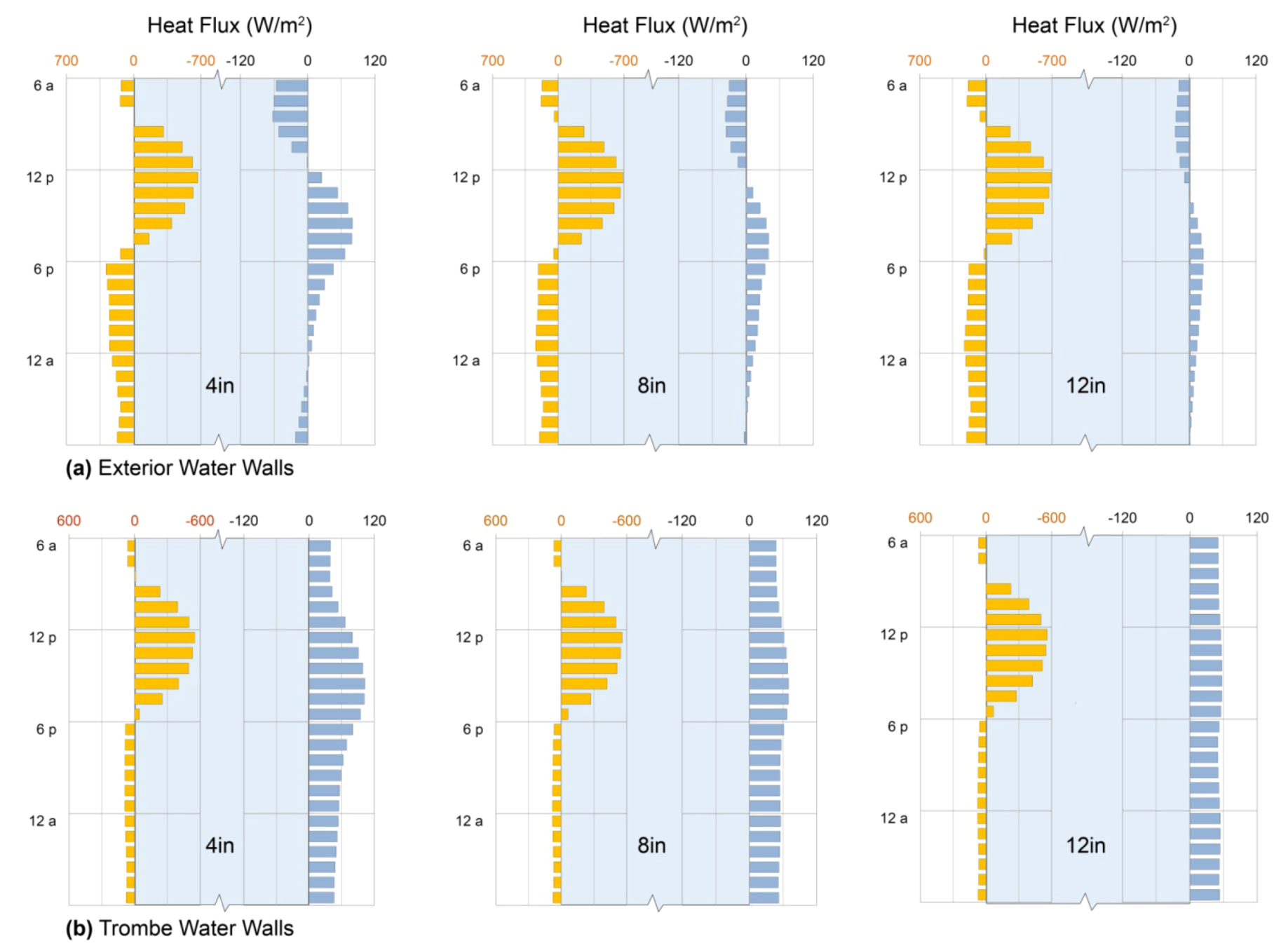
5.3. Phase-Change Material Walls
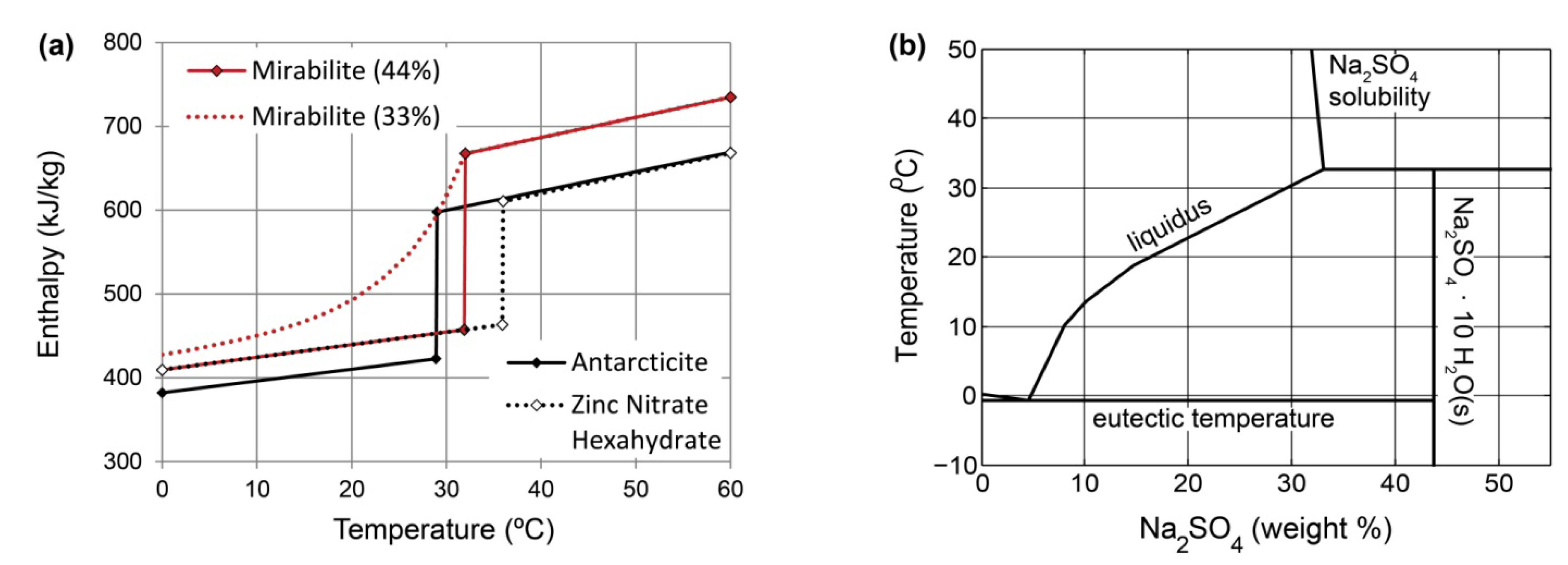
5.3.1. Candidate Materials
5.3.2. Deployment Strategies
5.3.3. Phase-Change Enthalpies
5.3.4. Solubility Mismatch
5.3.5. Performance: PCM-Trombe Wall

6. Internal Mass: Direct-Gain and Sun Spaces
6.1. Direct-Gain Systems
6.1.1. Comfort
6.1.2. Floor Mass and Sizing
6.1.3. Heat Loss to Soil
6.1.4. Performance: Direct-Gain Massive Floor

6.2. Sunspaces
6.2.1. Daylight and Plants
6.2.2. Thermal Buffering
6.2.3. Internal Mass
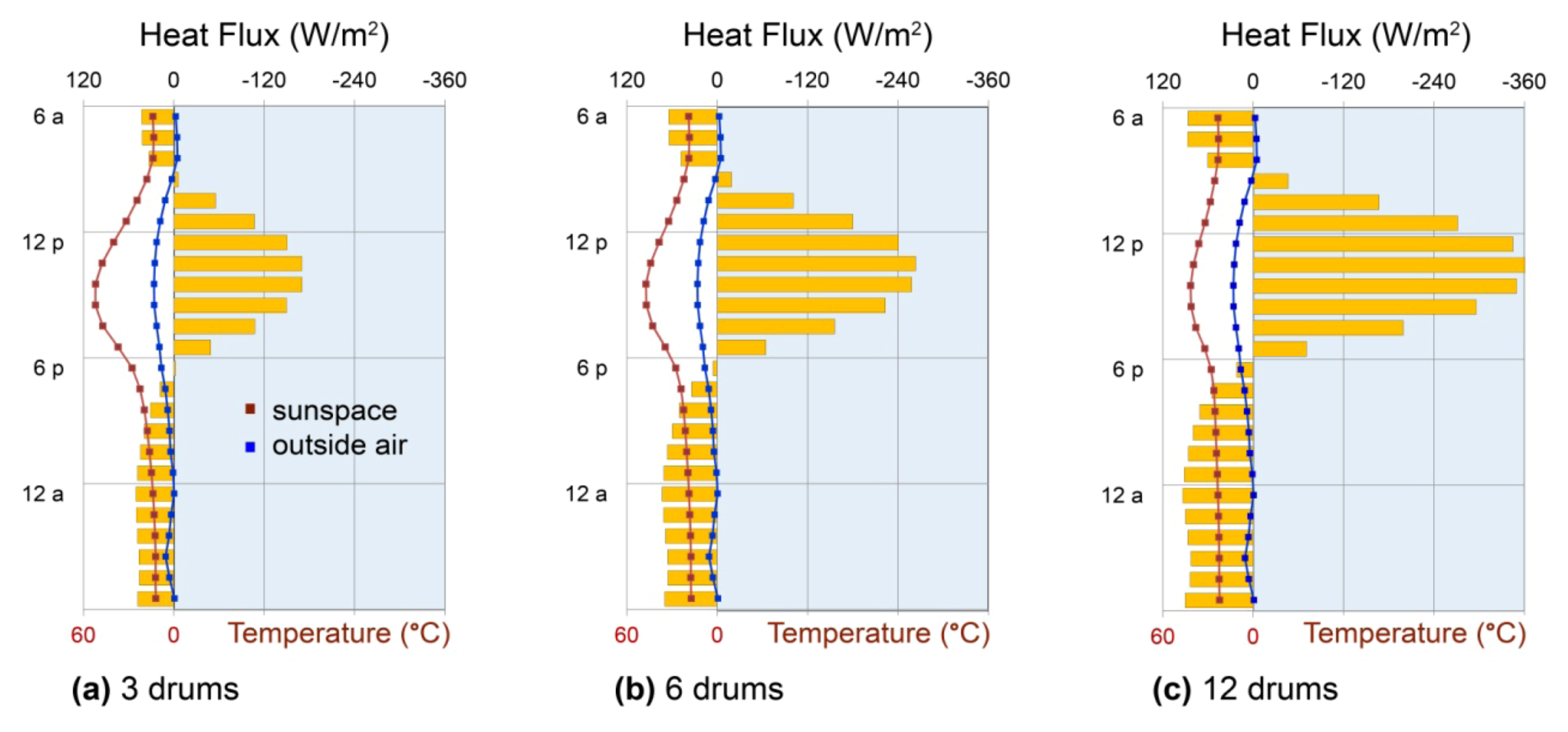
6.2.4. Performance: Sunspaces with Water Tanks
7. Building Simulation Methods
8. Conclusions
Acknowledgments
References
- Butti, K.; Perlin, J. A Golden Thread: 2500 Years of Solar Architecture and Technology; Cheshire Books: Palo Alto, CA, USA, 1980; pp. 7–27, 156–179, 235. [Google Scholar]
- McHenry, P.G. Adobe and Rammed Earth Buildings: Design and Construction; John Wiley & Sons: New York, NY, USA, 1984; pp. 1–112. [Google Scholar]
- Fardeheb, F. Examination and Review of Passive Solar Cooling Strategies in Middle Eastern and North African Vernacular Architecture. In Proceedings of the International Solar Energy Society World Congress, Beijing, China, 18–21 September 2007.
- Christenson, A.L. The microenvironment of cliff dwellings in Tsegi Canyon, Arizona. Kiva 1991, 57, 39–54. [Google Scholar]
- Hastings, S.R.; Wall, M. Sustainable Solar Housing: Strategies and Solutions; Earthscan: London, UK, 2007; pp. 1–3. [Google Scholar]
- Balcomb, J.D. Introduction. In Passive Solar Buildings; Balcomb, J.D., Ed.; MIT Press: Cambridge, MA, USA, 1992; pp. 1–38. [Google Scholar]
- Mazria, E. The Passive Solar Energy Book; Rodale Press: Emmaus, PA, USA, 1979; pp. 28–35, 51, 133–171, 181–185, 231–239, 311. [Google Scholar]
- Boyer, L.L. Earth shelter goes international. In Renewable Energy, Renewable Living: International Interests; American Solar Energy Society: Boulder, CO, USA, 1983; pp. 549–643. [Google Scholar]
- Niles, P.W.B.; Haggard, K. Passive Solar Handbook; California Energy Commission: Sacramento, CA, USA, 1980; pp. 91, 209. [Google Scholar]
- Wilson, A. Passive solar heating. Environ. Build. News 2012, 21, 1–15. [Google Scholar] [CrossRef]
- Bainbridge, D.A.; Haggard, K. Passive Solar Architecture; Chelsea Green Publishing: White River Junction, VT, USA, 2011; pp. 33, 58–74. [Google Scholar]
- Stein, B.; Reynolds, J.S.; Grondzik, W.T.; Kwok, A.G. Mechanical and Electrical Equipment for Buildings, 10th ed; John Wiley & Sons: New York, NY, USA, 1609; pp. 223–235 ,259–265, 275, 1582–1583, 1609. [Google Scholar]
- Lechner, N. Heating, Cooling, and Lighting: Design Methods for Architects, 3rd ed; John Wiley & Sons: New York, NY, USA, 2009; pp. 141–175, 477–478. [Google Scholar]
- Chiras, D.D. The Solar House: Passive Heating and Cooling; Chelsea Green Publishing: White River Junction, VT, USA, 2002; pp. 28–29, 102–105. [Google Scholar]
- Brown, G.Z.; DeKay, M. Sun, Wind & Light: Architectural Design Strategies; John Wiley & Sons: New York, NY, USA, 2001; pp. 169, 172–175, 230–231, 258–259. [Google Scholar]
- Stephen Winter Associates, The Passive Solar Design and Construction Handbook; Crosbie, M. (Ed.) John Wiley & Sons: New York, NY, USA, 1998; pp. 38–41, 113–121, 256–266.
- Salazar, A. On thermal diffusivity. Eur. J. Phys. 2003, 24, 351–358. [Google Scholar] [CrossRef]
- Clauser, C.; Huenges, E. Thermal conductivity of rocks and minerals. In Rock Physics and Phase Relations—A Handbook of Physical Constants; Ahrens, T.J., Ed.; American Geophysical Union: Washington, DC, USA, 1995; Volume 3, pp. 105–126. [Google Scholar]
- Kuznik, F.; David, D.; Johannes, K.; Roux, J.-J. A review on phase change materials integrated in building walls. Renew. Sustain. Energy Rev. 2011, 15, 379–391. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef] [Green Version]
- EnergyPlus Energy Simulation Software; Office of Energy Efficiency and Renewable Energy, U.S. Department of Energy, 2012. Available online: http://apps1.eere.energy.gov/buildings/energyplus/ (accessed on 16 October 2012).
- Niles, P.W.B. Simulation analysis. In Passive Solar Buildings; Balcomb, J.D., Ed.; MIT Press: Cambridge, MA, USA, 1992; pp. 111–180. [Google Scholar]
- Rempel, A.R.; Rempel, A.W.; Cashman, K.V.; Gates, K.N.; Page, C.J.; Shaw, B. Interpretation of passive solar field data with EnergyPlus models: Un-conventional wisdom from four sunspaces in Eugene, Oregon. Build. Env. 2013, 60, 158–172. [Google Scholar] [CrossRef]
- Sharag-Eldin, A.; Bergman, G. Using Energy-10 to Determine Thermal Comfort Conditions in Anasazi Pit Dwellings. In Proceedings of the American Solar Energy Society Annual Conference, Washington, DC, USA, 21–25 April 2001; pp. 503–510.
- Balcomb, J.D.; Barley, D.; McFarland, R.; Perry, J.; Wray, W.; Noll, S. Sensitivity analysis of thermal storage walls. In Passive Solar Design Handbook, Part Two: Passive Solar Design Analysis; Los Alamos National Laboratory: Los Alamos, NM; University of California: Berkeley, CA, USA, 1984; pp. 86–105. [Google Scholar]
- ANSI/ASHRAE/IESNA Standard 90.1-2010: Energy Standard for Buildings Except Low-Rise Residential Buildings; American Society of Heating, Refrigerating, and Air-Conditioning Engineers: Atlanta, GA, USA, 2010.
- Holtz, M.J. Commercial building integration. In Passive Solar Buildings; Balcomb, J.D., Ed.; MIT Press: Cambridge, MA, USA, 1992; pp. 364–388. [Google Scholar]
- Shapiro, A.M. Add-on Solar Greenhouses & Sunspaces; Rodale Press: Emmaus, PA, USA, 1985; pp. 56–68, 79–112. [Google Scholar]
- Braun, J.E.; Montgomery, K.W.; Chaturvedi, N. Evaluating the performance of building thermal mass control strategies. HVAC&R Res. 2001, 7, 403–428. [Google Scholar]
- Kachadorian, J. The Passive Solar House; Chelsea Green Publishing: White River Junction, VT, USA, 1997; pp. 31–33. [Google Scholar]
- Anderson, B. Thermal storage walls. In Passive Solar Design Handbook; Van Nostrand Reinhold: New York, NY, USA, 1984; pp. 22–31, 41–51. [Google Scholar]
- Balcomb, J.D.; Jones, R.W.; McFarland, R.D.; Wray, W.O. The monthly SLR method. In Passive Solar Heating Analysis; Los Alamos National Laboratory: Los Alamos, NM; American Society for Heating, Refrigerating, and Air-Conditioning Engineers: Atlanta, GA, USA, 1980; pp. 8–1. [Google Scholar]
- Balcomb, J.D.; Barley, D.; McFarland, R.; Perry, J.; Wray, W.; Noll, S. Estimating temperature swings in direct-gain buildings. In Passive Solar Design Handbook; Van Nostrand Reinhold: New York, NY, USA, 1984; pp. 106–113. [Google Scholar]
- Balcomb, J.D.; Jones, R.W.; McFarland, R.D.; Wray, W.O. The load coefficients. In Passive Solar Heating Analysis; Los Alamos National Laboratory: Los Alamos, NM; American Society for Heating, Refrigerating, and Air-Conditioning Engineers: Atlanta, GA, USA, 1980; pp. 3–1. [Google Scholar]
- Balcomb, J.D.; Jones, R.W.; McFarland, R.D.; Wray, W.O. Degree-days and base temperature. In Passive Solar Heating Analysis; Los Alamos National Laboratory: Los Alamos, NM; American Society for Heating, Refrigerating, and Air-Conditioning Engineers: Atlanta, GA, 1980; pp. 4–1. [Google Scholar]
- National Climatic Data Center. NOAA 1981–2010 Climate Normals. Available online: http://www.ncdc.noaa.gov/oa/climate/normals/usnormals.html (accessed on 1 November 2012).
- Newcomb, D. The Owner-Built Adobe House; University of New Mexico Press: Albuquerque, NM, USA, 2001; pp. 11, 35–48. [Google Scholar]
- Minke, G. Building with Earth: Design and Technology of a Sustainable Architecture, 2nd ed; Birkhauser: Boston, MA, USA, 2009; pp. 30–32. [Google Scholar]
- Revuelta-Acosta, J.D.; Garcia-Diaz, A.; Soto-Zarazua, G.M.; Rico-Garcia, E. Adobe as a sustainable material: A thermal performance. J. Appl. Sci. 2010, 10, 2211–2216. [Google Scholar] [CrossRef]
- Trombe, F.; Robert, J.F.; Cabanot, M.; Sesolis, B. Concrete walls to collect and hold heat. Sol. Age 1977, 2, 13–19. [Google Scholar]
- Wilson, A. Thermal Storage Wall Design Manual; New Mexico Solar Energy Association: Albuquerque, NM, USA, 1979. [Google Scholar]
- Quesada, G.; Rousse, D.; Dutil, Y.; Badache, M.; Halle, S. A comprehensive review of solar facades: Opaque solar facades. Renew. Sustain. Energy Rev. 2012, 16, 2820–2832. [Google Scholar] [CrossRef]
- Koyunbaba, B.K.; Yilmaz, Z. A comparison of Trombe wall systems with single glass, double glass and PV panels. Renew. Energy 2012, 45, 111–118. [Google Scholar] [CrossRef]
- Gan, G. A parametric study of Trombe walls for passive cooling of buildings. Energy Build. 1998, 27, 37–43. [Google Scholar]
- Alvarado, J.L.; Terrell, W.; Johnson, M.D. Passive cooling systems for cement-based roofs. Build. Environ. 2009, 44, 1869–1875. [Google Scholar] [CrossRef]
- Ballinger, A.; Oppenheim, D.; Ramachandran, A. National Design Handbook Prototype on Passive Solar Heating and Natural Cooling of Buildings; United Nations Centre for Human Settlements (Habitat): Nairobi, Kenya, 1990; pp. 42–43, 88–90. [Google Scholar]
- Engel, T.; Reid, P. Thermodynamics, Statistical Thermodynamics, and Kinetics, 2nd ed; Pearson: Upper Saddle River, NJ, USA, 2010; pp. 359–372. [Google Scholar]
- Kaviany, M. Principles of Heat Transfer; Wiley: New York, NY, USA, 2002; pp. 175–183, 269–275, 899–955. [Google Scholar]
- Riedi, P.C. Thermal Physics, 2nd ed; Oxford: New York, NY, USA, 1988; pp. 247–261. [Google Scholar]
- Carslaw, H.S.; Jaeger, J.C. Conduction of Heat in Solids, 2nd ed; Clarendon Press: Oxford, UK, 1959; pp. 1–49. [Google Scholar]
- Owen, M.S.; Kennedy, H.E. ASHRAE Handbook: Fundamentals; American Society of Heating, Refrigerating, and Air-Conditioning Engineers: Atlanta, GA, USA, 2005; pp. 24.1–24.16, 39.2–39.4. [Google Scholar]
- Yener, Y.; Kakac, S. Heat Conduction, 4th ed; Taylor & Francis: New York, NY, USA, 2008; pp. 20–22. [Google Scholar]
- Evers, A.C.; Medina, M.A.; Fang, Y. Evaluation of the thermal performance of frame walls enhanced with paraffin and hydrated salt phase change materials using a dynamic wall simulator. Build. Environ. 2010, 45, 1762–1768. [Google Scholar] [CrossRef]
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Lane, G.A. Low temperature heat storage with phase change materials. Int. J. Ambient Energy 1980, 1, 155–168. [Google Scholar] [CrossRef]
- Lertwattanaruk, P.; Choksiriwanna, J. The physical and thermal properties of adobe brick containing bagasse for earth construction. Built 2011, 1, 54–61. [Google Scholar]
- Allinson, D.; Hall, M. Hygrothermal analysis of a stabilised rammed earth test building in the UK. Energy Build. 2010, 42, 845–852. [Google Scholar] [CrossRef]
- Yan, Z.; Lam, J.C.; Liu, J. Experimental studies on the thermal and moisture properties of rammed earth used in adobe buildings in China. Archit. Sci. Rev. 2005, 48, 55–60. [Google Scholar] [CrossRef]
- Du, S.; Ma, J.; Wang, D. Experimental research on thermal and mechanical properties of modified rammed earth material. Adv. Mater. Res. 2012, 450–451, 773–777. [Google Scholar] [CrossRef]
- Soebarto, V. Analysis of Indoor Performance of Houses Using Rammed Earth Walls. In Building Simulation 2009, Proceedings of the 11th International IBPSA Conference, Glasgow, UK, 27–30 July 2009.
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill: New York, NY, USA, 2006; pp. 3–19, 114–120. [Google Scholar]
- Olhoeft, G.R.; Johnson, G.R. Densities of rocks and minerals. In CRC Practical Handbook of Physical Properties of Rocks and Minerals; Carmichael, R.S., Ed.; CRC Press: Boca Raton, FL, USA, 1989; pp. 141–176. [Google Scholar]
- Winkler, E.M. Stone in Architecture: Properties, Durability, 3rd ed; Springer-Verlag: New York, NY, USA, 1994; pp. 32–62. [Google Scholar]
- Kobranova, V.N. Petrophysics [Translated by Kuznetsov, V.V.]; Springer-Verlag: New York, NY, USA, 1989; pp. 193–222. [Google Scholar]
- Robertson, E.C. Thermal Properties of Rocks; Open File Report 88-441 of USGS: Reston, VA, USA, 1988. [Google Scholar]
- Mellon, M.T.; Jakosky, B.M.; Kieffer, H.H.; Christensen, P.R. High-resolution thermal inertia mapping from the Mars Global Surveyor Thermal Emission Spectrometer. Icarus 2000, 148, 437–455. [Google Scholar] [CrossRef]
- Heat, Air, and Moisture Laboratory. Impact of Thermal Diffusivity and Thermal Effusivity; Department of the Built Environment, Technische Universiteit Eindhoven: Eindhoven, NL, USA, 2010. Available online: http://archbps1.campus.tue.nl/bpswiki/images/5/5b/H1.pdf (accessed on 1 October 2012).
- Wright, J.L. A correlation to quantify convective heat transfer between vertical window glazings. ASHRAE Trans. 1996, 102, 940–946. [Google Scholar]
- Zhao, Y.; Curcija, D.; Goss, W.P. Convective heat transfer correlations for fenestration glazing cavities: A review. ASHRAE Trans. 1999, 105, 900–908. [Google Scholar]
- Dobson, S. Continuity of tradition: New earth building. In Terra 2000; Keynote Address: Torquay, UK, May 2000. [Google Scholar]
- Norton, J. Building with Earth: A Handbook, 2nd ed; Intermediate Technology Publications: London, UK, 1997; pp. 4–5, 28, 43. [Google Scholar]
- Campbell, J.W.P.; Pryce, W. Brick: A World History; Thames & Hudson: London, UK, 2003; pp. 13, 30–33, 50, 215–217. [Google Scholar]
- Tiller, T.P.; Look, D.W. Preservation of historic adobe buildings. In Preservation Briefs; No. 5; U.S. Department of the Interior: Washington, DC, USA, 1978; pp. 1–8. [Google Scholar]
- Binici, H.; Aksogan, O.; Nuri, B.M.; Akca, E.; Kapur, S. Thermal isolation and mechanical properties of fibre reinforced mud bricks as wall materials. Constr. Build. Mater. 2007, 21, 901–906. [Google Scholar] [CrossRef]
- Southwick, M. Build with Adobe; Sage Books: Chicago, IL, USA, 1966; pp. 2–3. [Google Scholar]
- Maniatidis, V.; Walker, P. A Review of Rammed Earth Construction; DTi Partners in Innovation Project “Developing Rammed Earth for UK Housing”: Bath, UK, 2003. [Google Scholar]
- Easton, D. The Rammed Earth House; Chelsea Green Publishing Company: White River Junction, VT, USA, 2007; pp. 3–37. [Google Scholar]
- Goodhew, S.; Griffiths, R. Sustainable earth walls to meet the building regulations. Energy Build. 2005, 37, 451–459. [Google Scholar] [CrossRef]
- Cultrone, G.; Sebastian, E.; Elert, K.; de la Torre, M.J.; Cazalla, O.; Rodriguez-Navarro, C. Influence of mineralogy and firing temperature on the porosity of bricks. J. Eur. Ceram. Soc. 2004, 24, 547–564. [Google Scholar] [CrossRef]
- Alden, A. The Geology of Bricks. Available online: http://geology.about.com/od/mineral_resources/a/bricks.htm (accessed on 1 November 2012).
- Brick Passive Solar Heating Systems, Part 4: Material Properties; Technical Note on Brick Construction 43D; Brick Industry Association: Reston, VA, USA, 1988; pp. 1–13.
- Beall, C. Masonry Design and Detailing: for Architects, Engineers, and Contractors, 3rd ed; McGraw-Hill: New York, NY, USA, 1993; pp. 89–105. [Google Scholar]
- Winkler, E.M. Stone: Properties, Durability in Man’s Environment, 2nd ed; Springer-Verlag: New York, NY, USA, 1975; pp. 102–168, 198–202. [Google Scholar]
- Murton, J.B.; Peterson, R.; Ozouf, J.-C. Bedrock fracture by ice segregation in cold regions. Science 2006, 314, 1127–1129. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jeon, S.-E.; Kim, J.-K.; Sungchul, Y. An experimental study on thermal conductivity of concrete. Cem. Concr. Res. 2003, 33, 363–371. [Google Scholar]
- Ellis, P.G. Development and Validation of the Unvented Trombe Wall Model in EnergyPlus.
- Ellis, P.G.; Liesen, R.J.; Pedersen, C.O. Energyplus Experimental Data Validation Work: Development and Validation of the Unvented Trombe Wall Model and Other Heat Balance Components; Final Report DACA42-01-D-0004, Task 3; U.S. Army Construction Engineering Research Laboratory: Champaign, IL, USA, 2003. [Google Scholar]
- Chan, H.-Y.; Riffat, S.B.; Zhu, J. Review of passive solar heating and cooling technologies. Renew. Sustain. Energy Rev. 2010, 14, 781–789. [Google Scholar] [CrossRef]
- Gordon, J.M. Selective coatings in passive solar heating. Sol. Energy 1982, 29, 13–17. [Google Scholar] [CrossRef]
- Goswami, D.Y.; Kreith, F.; Kreider, J.F. Passive methods for heating, cooling, and daylighting. In Principles of Solar Engineering, 2nd ed; Taylor & Francis: Philadelphia, PA, USA, 2000; pp. 297–336, 655–676. [Google Scholar]
- Shelby, J.E. Introduction to Glass Science and Technology, 2nd ed; Royal Society of Chemistry: Cambridge, UK, 2005; pp. 202–221. [Google Scholar]
- Torcellini, P.; Pless, S. Trombe Walls in Low-Energy Buildings: Practical Experiences; NREL/CP-550-36277; National Renewable Energy Laboratory: Golden, CO, USA, 2004. [Google Scholar]
- Torcellini, P.; Long, N.; Pless, S.; Judkoff, R. Evaluation of the Low-Energy Design and Energy Savings of the Zion National Park Visitors Center; NREL/TP-550-34607; National Renewable Energy Laboratory: Golden, CO, USA, 2005. [Google Scholar]
- Fernandez-Gonzalez, A. Analysis of the thermal performance and comfort conditions produced by five different passive solar heating strategies in the United States Midwest. Sol. Energy 2007, 81, 581–593. [Google Scholar] [CrossRef]
- Churchill, S.W.; Chu, H.H.S. Correlating equations for laminar and turbulent free convection from a vertical plate. Int. J. Heat Mass Transf. 1975, 18, 1323–1329. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castrell, A.; Barreneche, C.; de Gracia, A.; Fernandez, A.I. Materials used as PCM inthermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar]
- Telkes, M. Trombe Wall with Phase Change Storage Material. In Proceedings of the National Passive Solar Conference, Philadelphia, PA, USA, 16–18 March 1978; II, p. 271.
- Pasupathy, A.; Velraj, R.; Seeniraj, R.V. Phase change material-based building architecture for thermal management in residential and commercial establishments. Renew. Sustain. Energy Rev. 2008, 12, 39–64. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Buddhi, D. PCM thermal storage in buildings: A state of art. Renew. Sustain. Energy Rev. 2007, 11, 1146–1166. [Google Scholar] [CrossRef]
- Ure, Z. Phase Change Material (PCM) Based Energy Storage Materials and Global Application Examples. In Proceedings of CIBSE Technical Symposium 2011, Leicester, UK, 6–7 September 2011.
- Negi, A.S.; Anand, S.C. A Textbook of Physical Chemistry; Wiley Eastern: New Delhi, India, 1985; pp. 459–461. [Google Scholar]
- Feltham, D.H.; Untersteiner, N.; Wettlaufer, J.S.; Worster, M.G. Sea ice is a mushy layer. Geophys. Res. Lett. 2006, 33, L14501:1–L14501:4. [Google Scholar]
- Huppert, H.E.; Worster, M.G. Dynamic solidification of a binary alloy. Nature 1985, 314, 703–707. [Google Scholar]
- Feichenfeld, H.; Sarig, S. Calcium chloride hexahydrate: A phase changing material for energy storage. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 130–133. [Google Scholar] [CrossRef]
- Millard, E.B. Physical Chemistry for Colleges: A Course of Instruction Based Upon the Fundamental Laws of Chemistry; McGraw-Hill: New York, NY, USA, 1921; p. 289. [Google Scholar]
- Balcomb, J.D.; Barley, D.; McFarland, R.; Perry, J.; Wray, W.; Noll, S. Direct gain. In Passive Solar Design Handbook; Van Nostrand Reinhold: New York, NY, USA, 1984; pp. 153–173. [Google Scholar]
- Krarti, M. Effect of spatial variation of soil thermal properties on slab-on-ground heat transfer. Build. Environ. 1996, 31, 51–57. [Google Scholar] [CrossRef]
- Arya, S.P. Soil temperatures and heat transfer. In Introduction to Micrometeorology, 2nd ed; Academic Press: San Diego, CA, USA, 2001; pp. 46–61. [Google Scholar]
- Chuangchid, P.; Krarti, M. Foundation heat loss from heated concrete slab-on-grade floors. Build. Environ. 2001, 36, 637–655. [Google Scholar] [CrossRef]
- Deru, M. A Model for Ground-coupled Heat and Moisture Transfer from Buildings; Report No. NREL/TP-550-33954; National Renewable Energy Laboratory: Golden, CO, USA, 2003. [Google Scholar]
- Janssen, H.; Carmeliet, J.; Hens, H. Influence of soil moisture transfer on building heat loss via the ground. Build. Environ. 2004, 39, 825–836. [Google Scholar] [CrossRef]
- Farouki, O.T. Thermal Properties of Soils; Report No. CRREL Monograph 81-1; U.S. Army Corps of Engineers Cold Regions Research and Engineering Laboratory: Hanover, NH, USA, 1981. [Google Scholar]
- Dingman, S.L. Water in soils: Infiltration and redistribution. In Physical Hydrology, 2nd ed; Prentice-Hall: Upper Saddle River, NJ, USA, 2002; pp. 220–264. [Google Scholar]
- Bahnfleth, W.P. Three-Dimensional Modelling of Heat Loss from Slab Floors; Report No. USACERL Technical Manuscript E-89/11; U.S. Army Corps of Engineers Cold Regions Research and Engineering Laboratory: Hanover, NH, USA, 1989. [Google Scholar]
- Kusuda, T.; Bean, J.W. Simplified methods for determining seasonal heat loss from uninsulated slab-on-grade floors. ASHRAE Transactions 1984, 90, 611–632. [Google Scholar]
- National Renewable Energy Laboratory Web Page. National Solar Radiation Data Base 1991–2005 Update: Typical Meteorological Year 3. 2005. Available online: http://rredc.nrel.gov/solar/old_data/nsrdb/1991-2005/tmy3/ (accessed on 2 November 2012).
- Tarnawski, V.R.; Wagner, B. A new computerized approach to estimating the thermal properties of unfrozen soils. Can. Geotech. J. 1992, 29, 714–720. [Google Scholar] [CrossRef]
- Anderson, B.; Wells, M. Passive Solar Energy: The Homeowner’s Guide to Natural Heating and Cooling, 2nd ed; Brick House Publishing Company: Amherst, NH, USA, 1994; pp. 33–46. [Google Scholar]
- MacGregor, A.W.K. A Comparison of the Climatic Suitability of Various Locations in the European Community for Solar Space Heating. In Proceedings ofthe World Solar Forum, Brighton, UK, 23–28 August 1981; pp. 1852–1857.
- Porteous, C.; MacGregor, A.W.K. Latitude myths challenged. In Solar Architecture in Cool Climates; Porteous, C., MacGregor, A.W.K., Eds.; Earthscan Publishers: London, UK, 2005; pp. 2–5. [Google Scholar]
- Legacy OpenStudio Plug-in for Google SketchUp, version 1.0.9; Office of Energy Efficiency and Renewable Energy, U.S. Department of Energy: Washington, DC, USA, 2012. Available online: http://apps1.eere.energy.gov/buildings/energyplus/openstudio.cfm (accessed on 16 October 2012).
- WINDOW 6.3: Complex Glazing System Modeling; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2012. Available online: http://windows.lbl.gov/software/window/window.html (accessed on 16 October 2012).
- EnergyPlus 7.1 Engineering Reference; Office of Energy Efficiency and Renewable Energy, U.S. Department of Energy: Washington, DC, USA, 2012. Available online: http://apps1.eere.energy.gov/buildings/energyplus/pdfs/engineeringreference.pdf (accessed on 20 October 2012).
- Auxiliary EnergyPlus Programs; Office of Energy Efficiency and Renewable Energy, U.S. Department of Energy: Washington, DC, USA, 2012. Available online: http://apps1.eere.energy.gov/buildings/energyplus/pdfs/auxiliaryprograms.pdf (accessed on 16 October 2012).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rempel, A.R.; Rempel, A.W. Rocks, Clays, Water, and Salts: Highly Durable, Infinitely Rechargeable, Eminently Controllable Thermal Batteries for Buildings. Geosciences 2013, 3, 63-101. https://doi.org/10.3390/geosciences3010063
Rempel AR, Rempel AW. Rocks, Clays, Water, and Salts: Highly Durable, Infinitely Rechargeable, Eminently Controllable Thermal Batteries for Buildings. Geosciences. 2013; 3(1):63-101. https://doi.org/10.3390/geosciences3010063
Chicago/Turabian StyleRempel, Alexandra R., and Alan W. Rempel. 2013. "Rocks, Clays, Water, and Salts: Highly Durable, Infinitely Rechargeable, Eminently Controllable Thermal Batteries for Buildings" Geosciences 3, no. 1: 63-101. https://doi.org/10.3390/geosciences3010063




