Characteristics of Microbial Coalbed Gas during Production; Example from Pennsylvanian Coals in Indiana, USA
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
4. Conclusions
- (1)
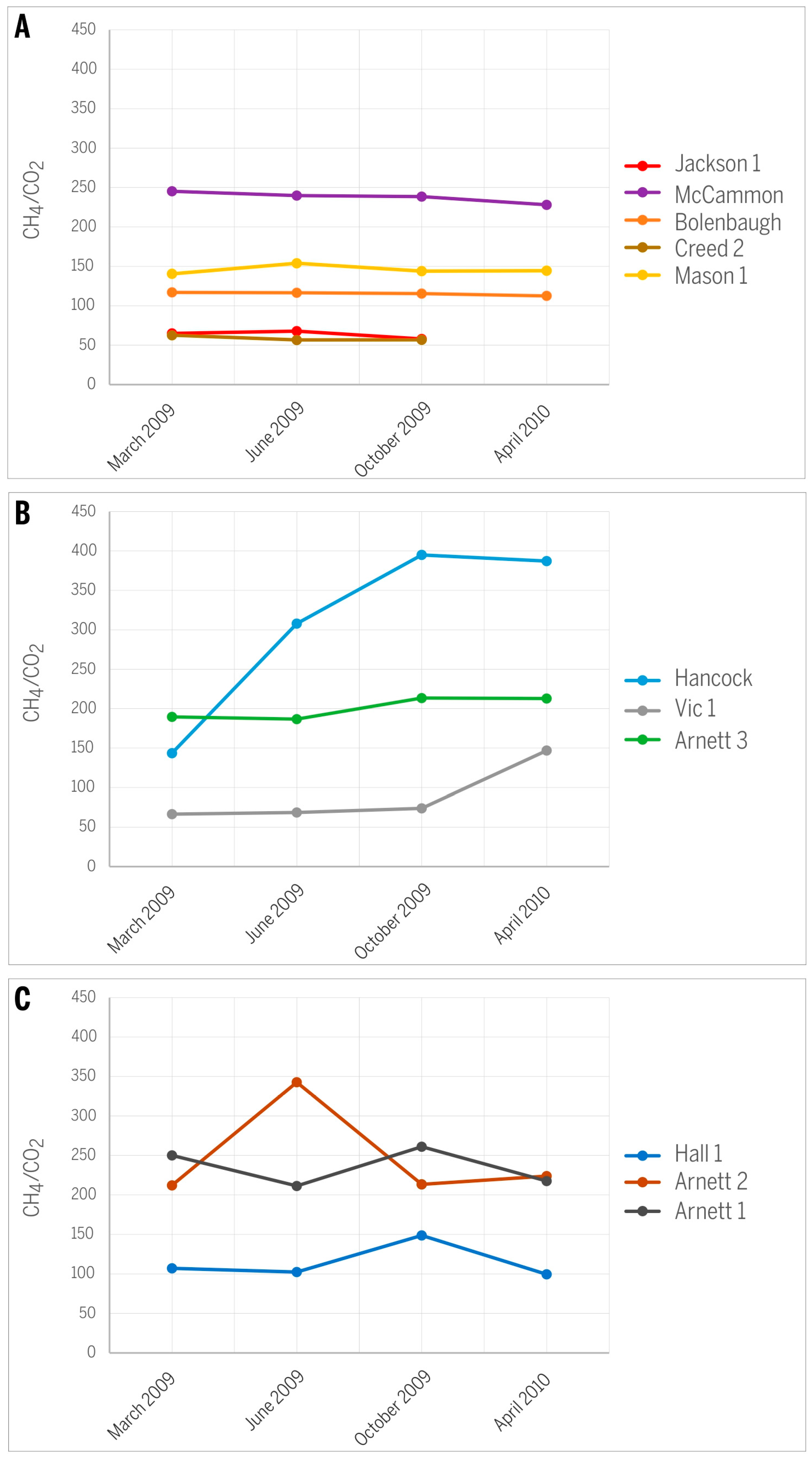
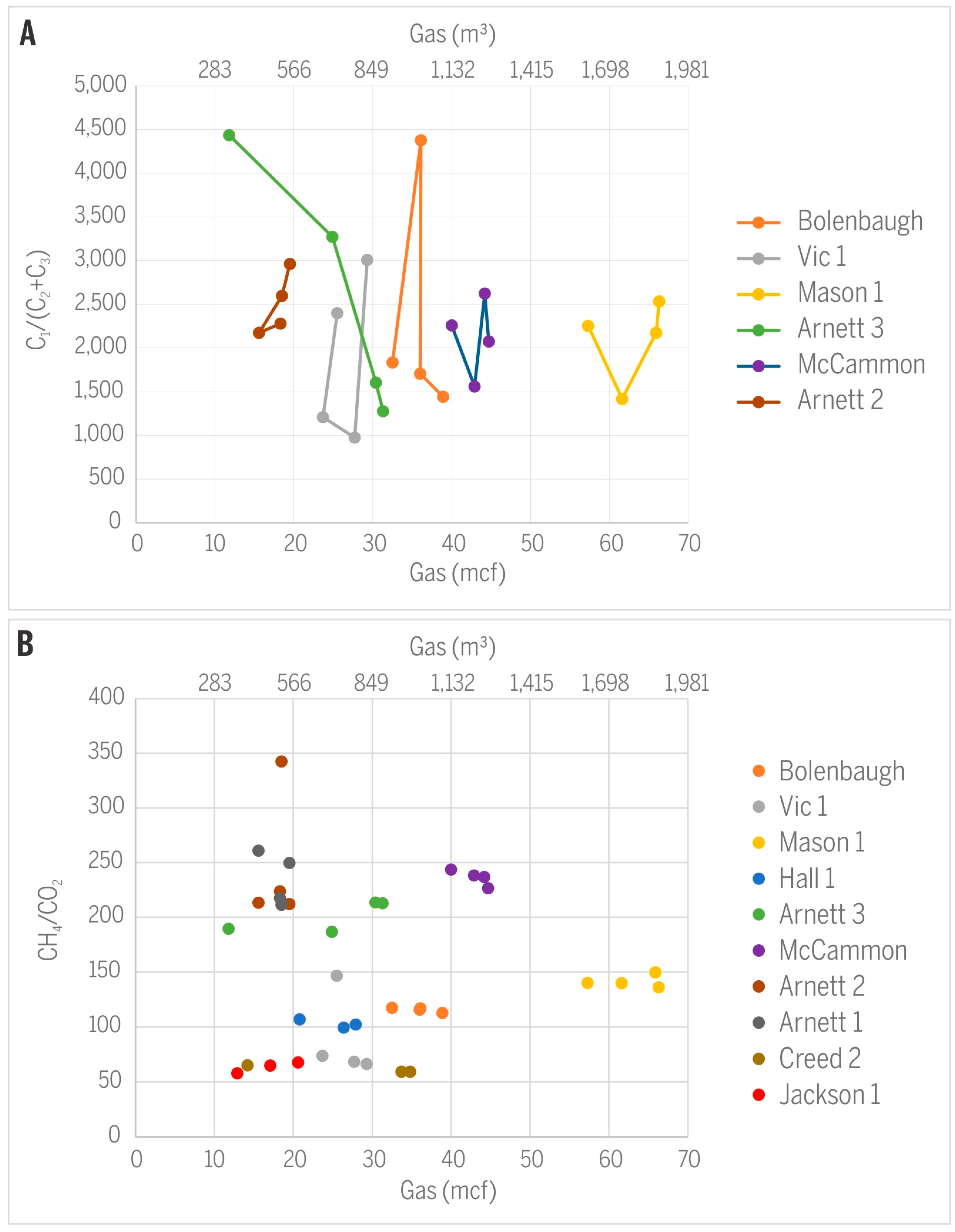
- Trends in the CH4/CO2 ratio can vary over time and can follow different directions depending on the dominance of gas adsorption versus gas diffusion, conditions related largely to permeability and pressure changes in the reservoir. Both decreasing and increasing CH4/CO2 ratios were observed in the study area, in addition to production wells expressing irregular changes, the latter likely reflecting additional local factors unrelated to gas production.
- (2)
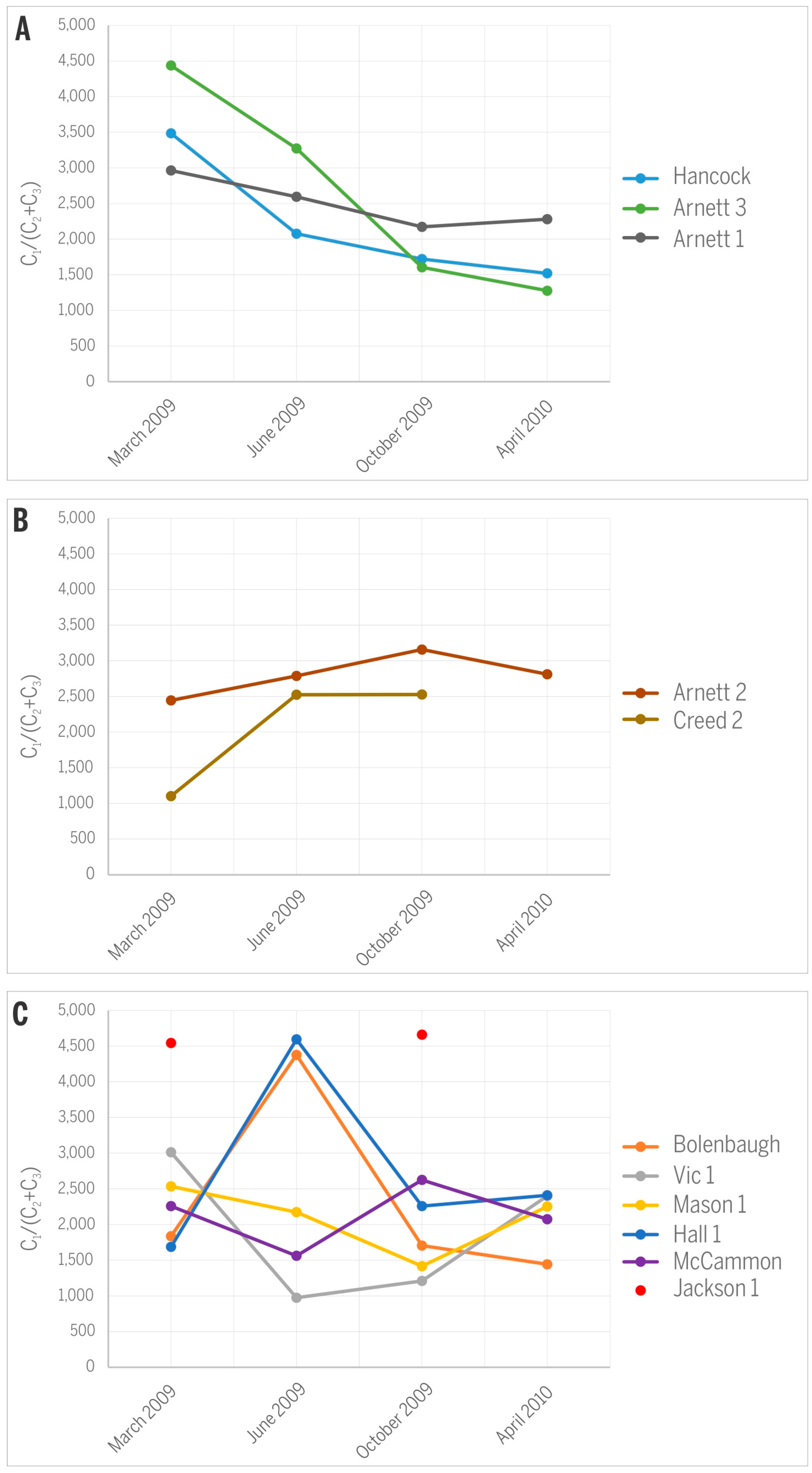
- Similar to the CH4/CO2 ratio, variations in C1/(C2 + C3) are site-specific. The decrease in CH4/CO2 over time for gases from Hancock, Arnett 1, and Arnett 3 wells can be explained by higher adsorption affinity and smaller mobility of C2+ gases compared to methane. In contrast, the opposite trend or irregular changes in gases from other wells suggest additional factors that are probably of a more local nature and not strictly related to gas production.
- (3)
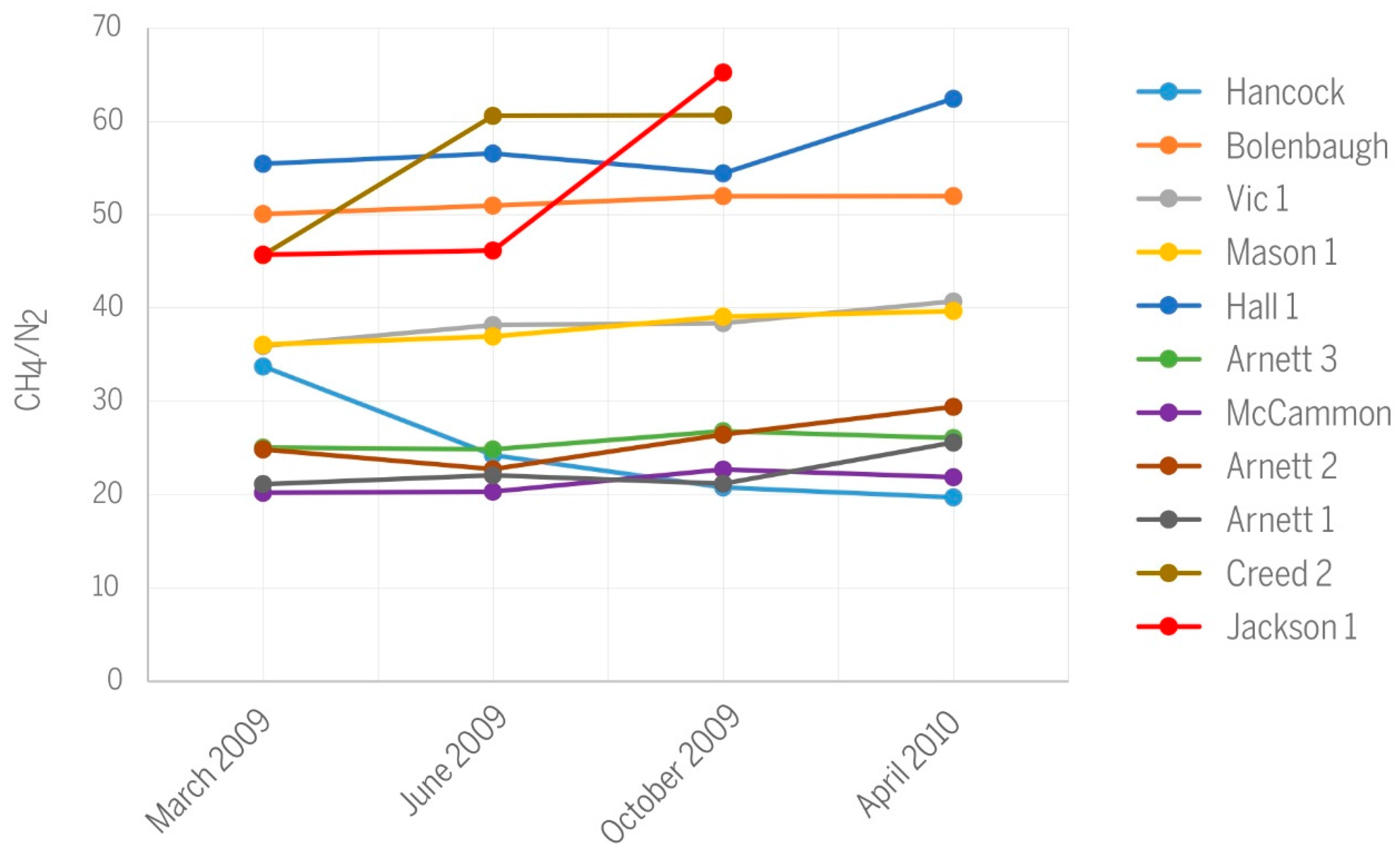
- No trends over time were observed for the CH4/N2 ratio. Values of this ratio mainly reflect the nitrogen content of gas samples. The relatively large N2 content ranging from 1.56 to 4.81 vol % likely results from the nitrogen content of the parental coal, some contribution from atmospheric nitrogen dissolved in groundwater, and possibly minor air contamination introduced during gas sampling.
- (4)
- Methane δ13C values ranging from −55.3‰ to −61.1‰ and δ2H values of −201.2‰ to −218.5‰ indicate a microbial methanogenic origin via CO2 reduction. Changes in isotopic values over a three-month period are small and do not change the assessment of the gas origin. Isotopic data give no evidence for microbial methanotrophy, perhaps with the exception of gas from mine void well Creed 2 expressing a small shift towards less negative δ13C values.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Damberger, H.H. Coalification pattern of the Illinois Basin. Econ. Geol. 1971, 66, 488–494. [Google Scholar] [CrossRef]
- Mastalerz, M.; Drobniak, A.; Rupp, J.; Shaffer, N. Characterization of Indiana’s Coal Resource: Availability of the Reserves, Physical and Chemical Properties of the Coal, and Present and Potential Uses; Indiana Geological Survey: Bloomington, IN, USA, 2009. [Google Scholar]
- Macke, D.L. Illinois basin province. In National Assessment of United States Oil and Gas Resources—Results, Methodology, and Supporting Data; Gautier, D.L., Dolton, G.L., Takahashi, K.I., Varnes, K.L., Eds.; U.S. Geological Survey Digital Data Series 30; USGS: Reston, VA, USA, 1995. [Google Scholar]
- Strąpoć, D.; Mastalerz, M.; Eble, C.; Schimmelmann, A. Characterization of the origin of coalbed gases in the southwestern Illinois Basin by compound-specific carbon and hydrogen stable isotope ratios. Organ. Geochem. 2007, 38, 267–287. [Google Scholar] [CrossRef]
- Strąpoć, D.; Mastalerz, M.; Schimmelmann, A.; Drobniak, A.; Hedges, S. Variability of geochemical properties in a microbially dominated coalbed gas system from the eastern margin of the Illinois Basin, USA. Int. J. Coal Geol. 2008, 76, 98–110. [Google Scholar] [CrossRef]
- Strąpoć, D.; Picardal, F.; Turich, C.; Schaperdott, I.; Macalady, J.L.; Lipp, J.S.; Lin, Y.-S.; Ertefai, T.F.; Schubotz, F.; Hinrichs, K.-U.; et al. Methane-producing microbial community in a coal bed of the Illinois Basin. J. Appl. Environ. Microbiol. 2008, 74, 2424–2432. [Google Scholar]
- McIntosh, J.C.; Walter, L.M.; Martini, A.M. Pleistocene recharge to midcontinent basins: Effects on salinity structure and microbial gas generation. Geochim. Cosmochim. Acta 2002, 66, 1681–1700. [Google Scholar] [CrossRef]
- Harper, D. Coalbed Methane in Indiana: Indiana Geological Survey Occasional Paper; Indiana Geological Survey: Bloomington, IN, USA, 1991; Volume 56, p. 18. [Google Scholar]
- Drobniak, A.; Mastalerz, M.; Rupp, J.; Eaton, N. Coalbed gas potential of the Seelyville Coal in Indiana. Int. J. Coal Geol. 2004, 57, 265–282. [Google Scholar] [CrossRef]
- Karacan, C.O.; Drobniak, A.; Mastalerz, M. Coal bed reservoir simulation with geostatistical property realizations for simultaneous multi-well production history matching: A case study from Illinois Basin, Indiana, USA. J. Coal Geol. 2014, 131, 71–89. [Google Scholar] [CrossRef]
- Whiticar, M.J.; Faber, E.; Schoell, M. Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation—Isotopic evidence. Geochim. Cosmochim. Acta 1986, 50, 693–709. [Google Scholar] [CrossRef]
- Vinson, D.S.; Blair, N.E.; Martini, A.M.; Larter, S.; Orem, W.H.; McIntosh, J.C. Microbial methane from in situ biodegradation of coal and shale: A review and reevaluation of hydrogen and carbon isotope signatures. Chem. Geol. 2017, 453, 128–145. [Google Scholar] [CrossRef]
- Reeves, S.R.; Decker, A.D. A reservoir simulation investigation into the interaction of in-situ stress, pore pressure, and coal rank on coalbed methane exploration strategy. In Proceedings of the 1991 SPE Gas Technology Symposium, Houston, TX, USA, 23–25 January 1991. SPE Paper 21490. [Google Scholar]
- Kaiser, W.R.; Hamilton, D.S.; Scott, A.R.; Tyler, R.; Finley, R.J. Geological and hydrological controls on the producibility of coalbed methane. J. Geol. Soc. Lond. 1994, 151, 417. [Google Scholar] [CrossRef]
- Roadifer, R.D.; Moore, T.R.; Raterman, K.T.; Farnan, R.A.; Crabtree, B.J. Coalbed methane parametric study: What is really important to production and when? In Proceedings of the 2003 SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 5–8 October 2003. SPE Paper 84425. [Google Scholar]
- Arri, L.E.; Yee, D.; Morgan, W.D.; Jeansonne, M.W. Modeling coalbed methane production with binary gas sorption. In Proceedings of the SPE Rocky Mountain Regional Meeting, Casper, Wyoming, 18–21 May 1992. SPE 24363-MS. [Google Scholar]
- Scott, A.R.; Kaiser, W.R.; Ayers, W.B., Jr. Thermogenic and secondary biogenic gases, San Juan Basin, Colorado and New Mexico–Implications for coalbed gas producibility. AAPG Bull. 1994, 78, 1186–1209. [Google Scholar]
- Strąpoć, D.; Schimmelmann, A.; Mastalerz, M. Carbon isotopic fractionation of CH4 and CO2 during canister desorption of coal. Organ. Geochem. 2006, 37, 152–164. [Google Scholar] [CrossRef]
- Cui, X.; Bustin, R.M. Controls of coal fabric on coalbed gas production and compositional shift in both field production and canister desorption tests. Soc. Petrol. Eng. J. 2006, 11, 111–119. [Google Scholar] [CrossRef]
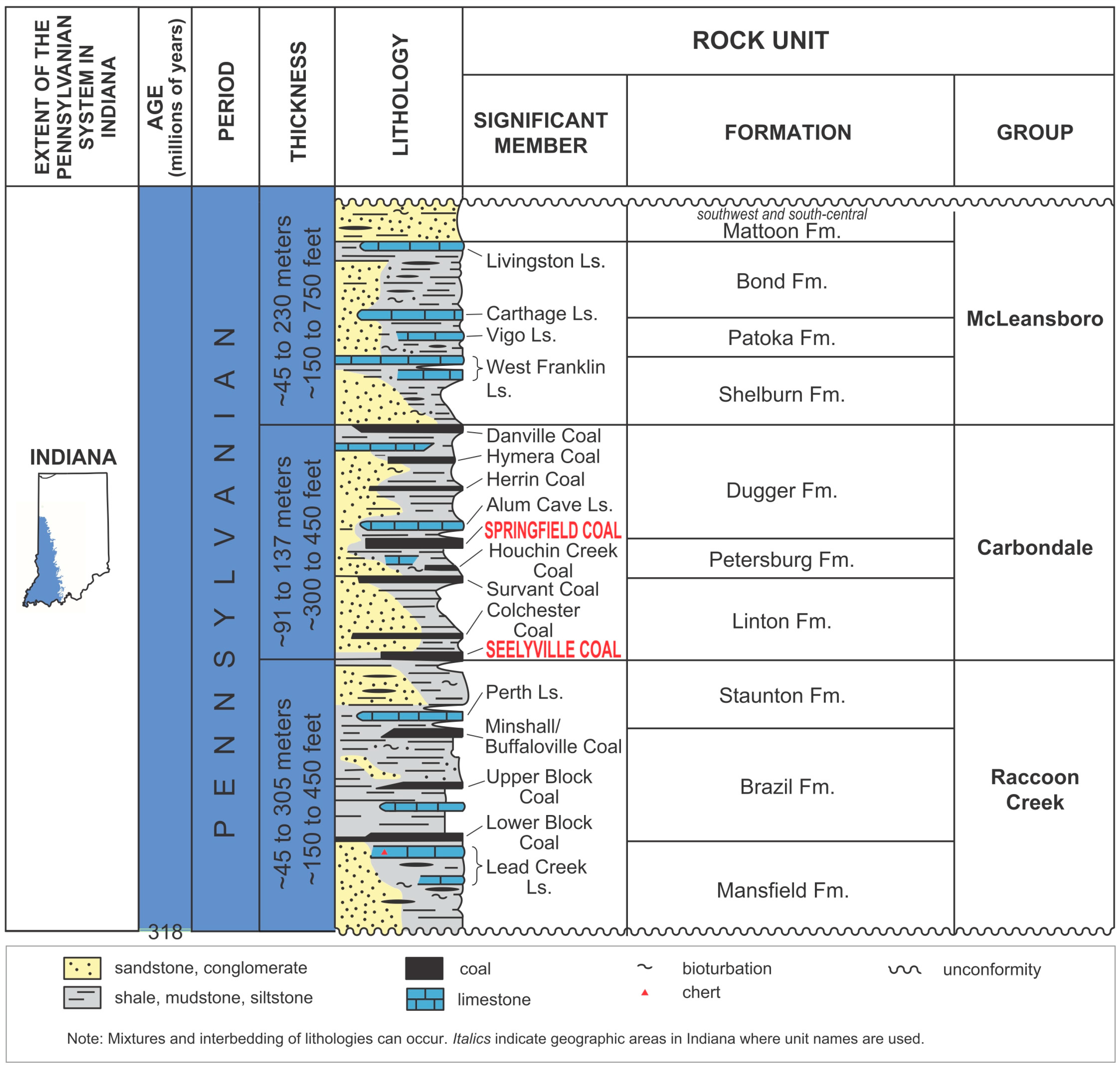
- Thompson, T.A.; Sowder, K.H.; Johnson, M. Generalized Stratigraphic Column of Indiana Bedrock: Indiana Geological Survey Poster; Indiana Geological Survey: Bloomington, IN, USA, 2013; Volume 6. [Google Scholar]
- Jin, H.; Schimmelmann, A.; Mastalerz, M.; Pope, J.; Moore, T.A. Coalbed gas desorption in canisters: Consumption of trapped atmospheric oxygen and implications for measured gas quality. J. Coal Geol. 2010, 81, 64–72. [Google Scholar] [CrossRef]
- Drobniak, A.; Mastalerz, M. The Indiana Geological Survey Coal Stratigraphic Database: The Database and Interactive Map: Indiana Geological Survey Report of Progress 39, CD-ROM; Indiana Geological Survey: Bloomington, IN, USA, 2012. [Google Scholar]
- Cui, X.; Bustin, R.M.; Dipple, G. Selective transport of CO2, CH4, and N2 in coals: Insights from modeling of experimental gas adsorption data. Fuel 2004, 83, 293–303. [Google Scholar] [CrossRef]
- Strąpoć, D.; Mastalerz, M.; Dawson, K.; Macalady, J.; Callaghan, A.V.; Wawrik, B.; Turich, C.; Ashby, M. Biogeochemistry of coal-bed methane. Annu. Earth Planet. Rev. 2011, 39, 617–656. [Google Scholar] [CrossRef]
- Gao, L.; Brassell, S.C.; Mastalerz, M.; Schimmelmann, A. Microbial degradation of sedimentary organic matter associated with shale gas and coalbed methane in Eastern Illinois Basin (Indiana), USA. J. Coal Geol. 2013, 107, 152–164. [Google Scholar] [CrossRef]
- Hale, B.H.; Firth, C.H. Production history of the San Juan 32–7 Unit No. 6 well, Northern San Juan Basin, New Mexico; Geology and Coal-Bed Methane Resources of the Northern San Juan Basin and New Mexico, 2009 Rocky Mountain Association of Geologists: Denver, CO, USA, 1988; pp. 199–204. [Google Scholar]
- Kirk, M.F.; Martini, A.M.; Breecker, D.O.; Colman, D.R.; Takacs-Vesbach, C.; Petsch, S.T. Impact of commercial natural gas production on geochemistry and microbiology in a shale-gas reservoir. Chem. Geol. 2012, 332, 15–25. [Google Scholar] [CrossRef]
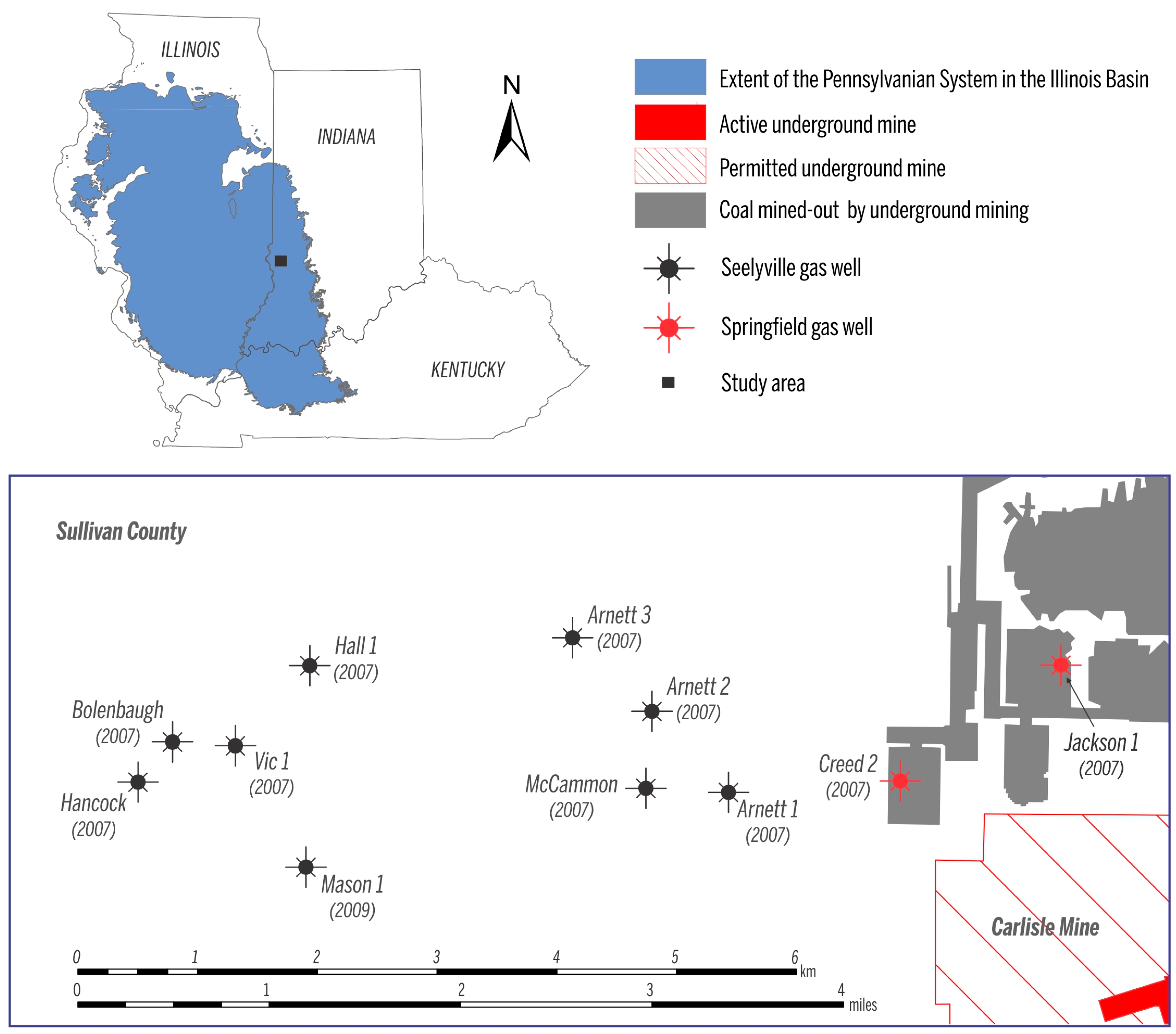
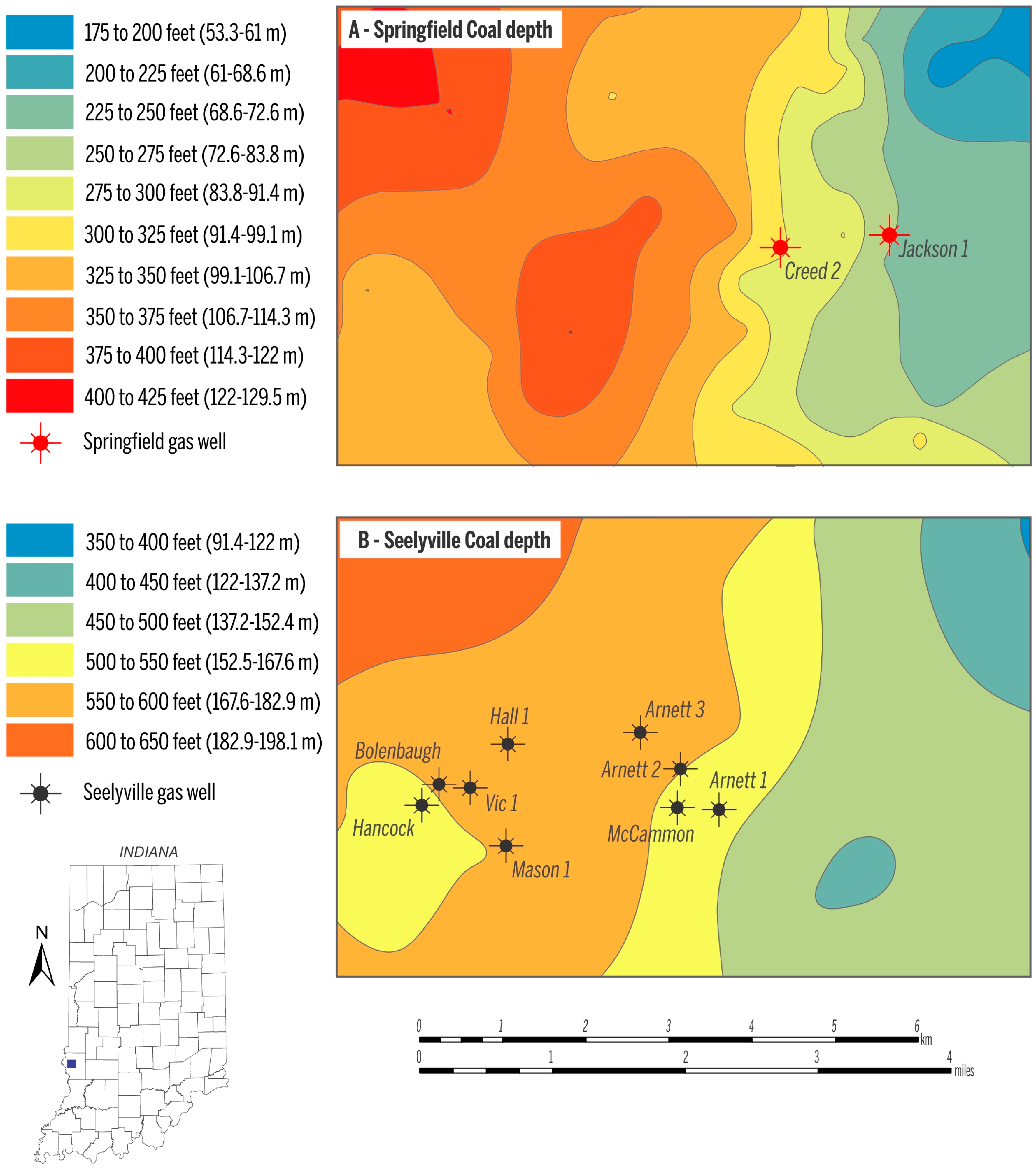
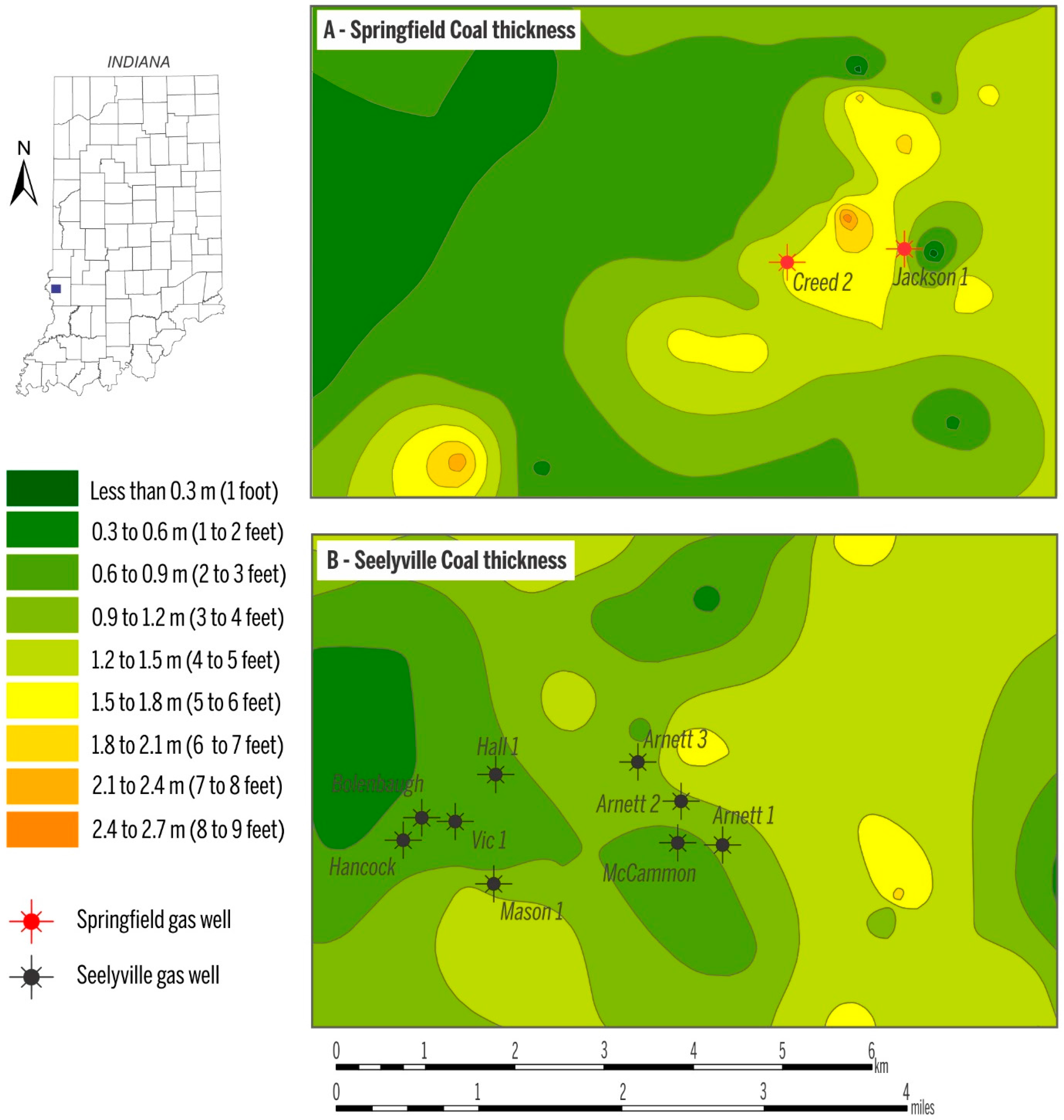
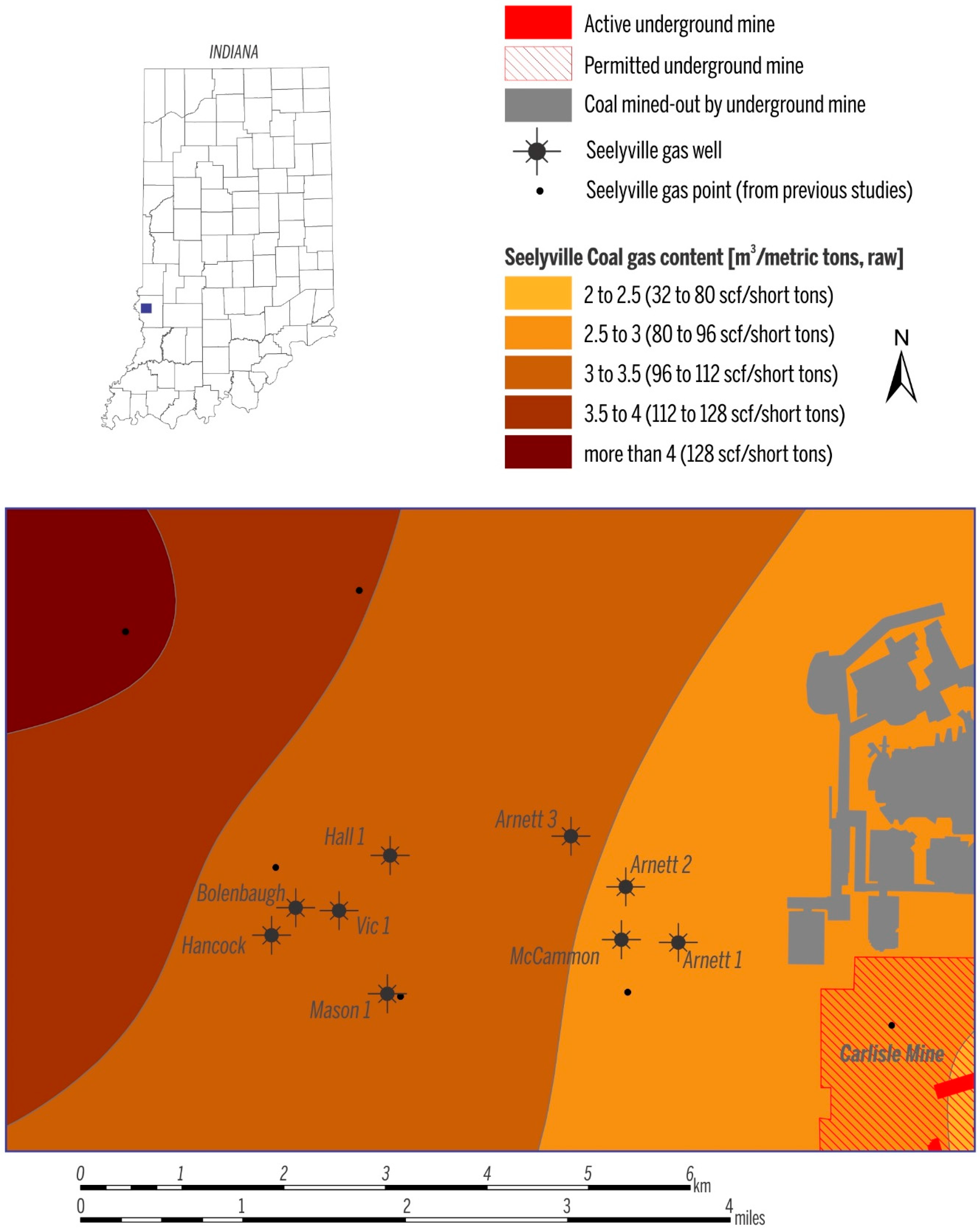
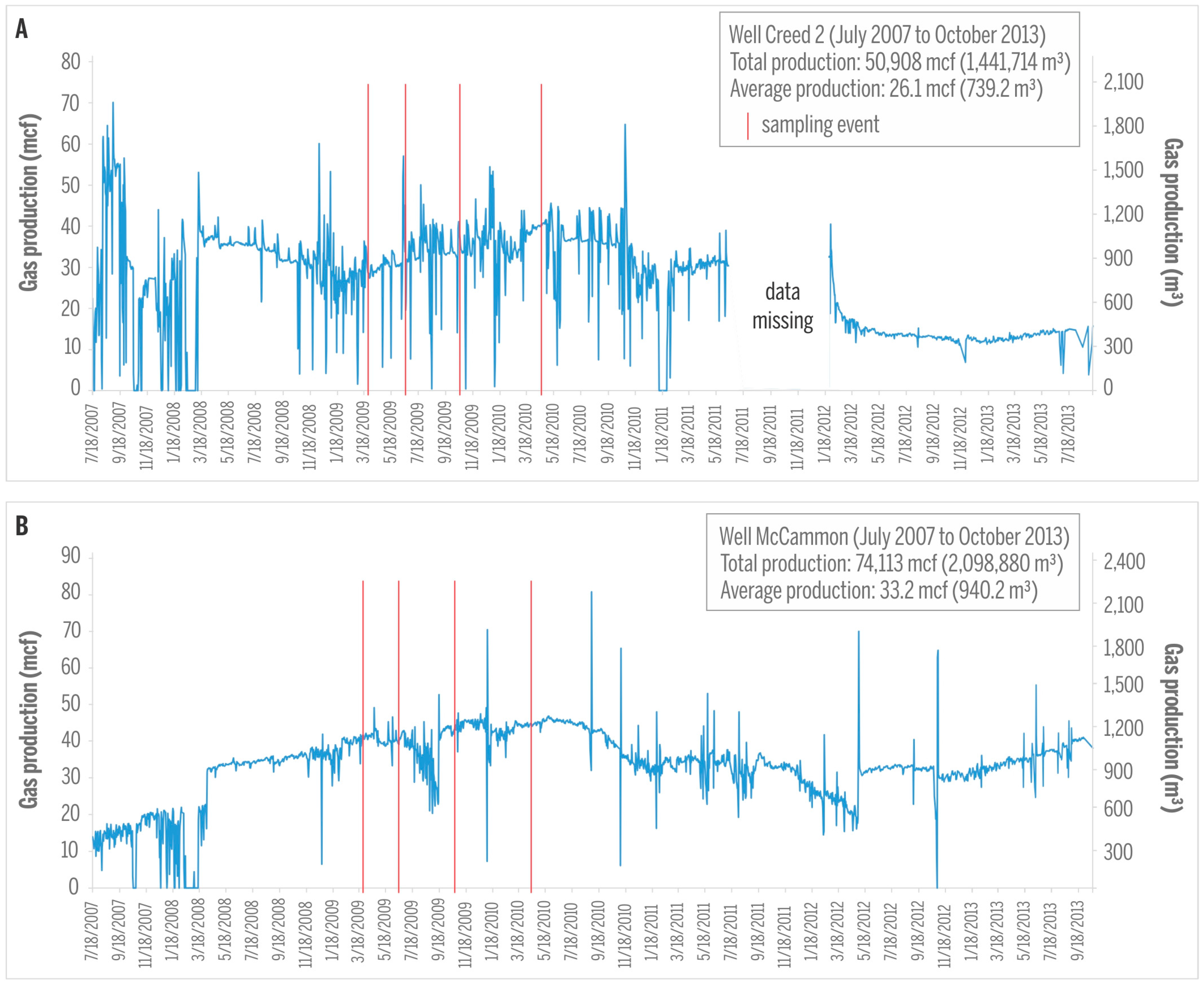

| Gas samples collected in March 2009 | Well | Coal | Concentration (vol %) | δ2H (‰) vs. VSMOW | δ13C (‰) vs. VPDB | C1/(C2 + C3) | ||||
| Methane | Ethane | Propane | CO2 | N2 | ||||||
| Creed 2 | Springfield | 96.3 | 0.09 | b.d.l. | 1.47 | 2.11 | −206 | −61.1 | 1088 | |
| Jackson 1 | Springfield | 96.4 | 0.02 | b.d.l. | 1.48 | 2.11 | −209 | −61.1 | 4530 | |
| Arnett 1 | Seelyville | 95.1 | 0.03 | b.d.l. | 0.38 | 4.50 | −201 | −59.1 | 2964 | |
| Arnett 2 | Seelyville | 95.6 | 0.04 | b.d.l. | 0.45 | 3.85 | −203 | −59.4 | 2444 | |
| Arnett 3 | Seelyville | 95.6 | 0.02 | b.d.l. | 0.50 | 3.81 | −203 | −59.2 | 4437 | |
| Mason 1 | Seelyville | 96.6 | 0.04 | b.d.l. | 0.71 | 2.68 | −205 | −56.0 | 2534 | |
| Vic 1 | Seelyville | 95.9 | 0.03 | b.d.l. | 1.44 | 2.67 | −208 | −55.1 | 3010 | |
| Hall 1 | Seelyville | 97.9 | 0.06 | b.d.l. | 0.91 | 1.75 | −211 | −55.4 | 1686 | |
| Bolenbaugh | Seelyville | 97.2 | 0.05 | b.d.l. | 0.83 | 1.94 | −210 | −55.6 | 1835 | |
| McCammon | Seelyville | 94.9 | 0.04 | b.d.l. | 0.39 | 4.69 | −204 | −59.5 | 2259 | |
| Hancock | Seelyville | 96.4 | 0.03 | b.d.l. | 0.67 | 2.86 | −210 | −57.7 | 3486 | |
| Gas samples collected in June 2009 | Well | Coal | Concentration (vol %) | δ2H (‰) vs. VSMOW | δ13C (‰) vs. VPDB | C1/(C2 + C3) | ||||
| Methane | Ethane | Propane | CO2 | N2 | ||||||
| Creed 2 | Springfield | 96.7 | 0.03 | 0.009 | 1.63 | 1.60 | −217 | −59.2 | 2512 | |
| Jackson 1 | Springfield | 96.5 | 0.00 | b.d.l. | 1.42 | 2.09 | −207 | −61.1 | n.d. | |
| Arnett 1 | Seelyville | 95.2 | 0.04 | b.d.l. | 0.45 | 4.31 | −209 | −59.2 | 2597 | |
| Arnett 2 | Seelyville | 95.5 | 0.03 | 0.008 | 0.28 | 4.20 | −214 | −59.1 | 2787 | |
| Arnett 3 | Seelyville | 95.6 | 0.03 | b.d.l. | 0.51 | 3.84 | n.d. | −59.2 | 3273 | |
| Mason 1 | Seelyville | 96.7 | 0.04 | b.d.l. | 0.65 | 2.62 | n.d. | −55.9 | 2173 | |
| Vic 1 | Seelyville | 95.9 | 0.10 | b.d.l. | 1.40 | 2.51 | −218 | −55.4 | 975 | |
| Hall 1 | Seelyville | 97.3 | 0.02 | b.d.l. | 0.95 | 1.72 | −210 | −55.3 | 4595 | |
| Bolenbaugh | Seelyville | 97.2 | 0.02 | b.d.l. | 0.83 | 1.91 | −218 | −55.5 | 4377 | |
| McCammon | Seelyville | 94.9 | 0.05 | 0.008 | 0.40 | 4.67 | −201 | −59.3 | 1561 | |
| Hancock | Seelyville | 95.7 | 0.03 | 0.015 | 0.31 | 3.95 | - | −57.1 | 2077 | |
| Gas samples collected in October 2009 | Well | Coal | Concentration (vol %) | δ2H (‰) vs. VSMOW | δ13C (‰) vs. VPDB | C1/(C2 + C3) | ||||
| Methane | Ethane | Propane | CO2 | N2 | ||||||
| Creed 2 | Springfield | 96.8 | 0.03 | 0.009 | 1.63 | 1.60 | n.d. | n.d. | 2514 | |
| Jackson 1 | Springfield | 96.7 | 0.01 | 0.006 | 1.67 | 1.48 | n.d. | n.d. | 4647 | |
| Arnett 1 | Seelyville | 95.1 | 0.02 | 0.019 | 0.36 | 4.49 | n.d. | n.d. | 2173 | |
| Arnett 2 | Seelyville | 95. 9 | 0.03 | b.d.l. | 0.45 | 3.62 | n.d. | n.d. | 3159 | |
| Arnett 3 | Seelyville | 95.9 | 0.04 | 0.021 | 0.45 | 3.58 | n.d. | n.d. | 1604 | |
| Mason 1 | Seelyville | 96.7 | 0.05 | 0.023 | 0.69 | 2.48 | n.d. | n.d. | 1417 | |
| Vic 1 | Seelyville | 96.1 | 0.06 | 0.022 | 1.30 | 2.50 | n.d. | n.d. | 1210 | |
| Hall 1 | Seelyville | 97.5 | 0.03 | 0.010 | 0.66 | 1.79 | n.d. | n.d. | 2257 | |
| Bolenbaugh | Seelyville | 97.3 | 0.05 | 0.007 | 0.84 | 1.87 | n.d. | n.d. | 13,788 | |
| McCammon | Seelyville | 95.4 | 0.04 | b.d.l. | 0.40 | 4.20 | n.d. | n.d. | 2623 | |
| Hancock | Seelyville | 95.1 | 0.05 | 0.007 | 0.24 | 4.58 | n.d. | n.d. | 1723 | |
| Gas samples collected in April 2010 | Well | Coal | Concentration (vol %) | δ2H (‰) vs. VSMOW | δ13C (‰) vs. VPDB | C1/(C2 + C3) | ||||
| Methane | Ethane | Propane | CO2 | N2 | ||||||
| Creed 2 | Springfield | samples were not collected | ||||||||
| Jackson 1 | Springfield | samples were not collected | ||||||||
| Arnett 1 | Seelyville | 95.8 | 0.04 | b.d.l. | 0.44 | 3.74 | n.d. | n.d. | 2280 | |
| Arnett 2 | Seelyville | 96.2 | 0.02 | 0.010 | 0.43 | 3.27 | n.d. | n.d. | 2810 | |
| Arnett 3 | Seelyville | 95.8 | 0.05 | 0.027 | 0.45 | 3.67 | n.d. | n.d. | 1277 | |
| Mason 1 | Seelyville | 96.8 | 0.04 | b.d.l. | 0.69 | 2.44 | n.d. | n.d. | 2251 | |
| Vic 1 | Seelyville | 96.9 | 0.02 | 0.019 | 0.66 | 2.38 | n.d. | n.d. | 2400 | |
| Hall 1 | Seelyville | 97.4 | 0.03 | 0.015 | 0.98 | 1.56 | n.d. | n.d. | 2409 | |
| Bolenbaugh | Seelyville | 97.2 | 0.06 | 0.012 | 0.86 | 1.87 | n.d. | n.d. | 1443 | |
| McCammon | Seelyville | 95.2 | 0.04 | 0.008 | 0.42 | 4.35 | n.d. | n.d. | 2073 | |
| Hancock | Seelyville | 94.9 | 0.06 | b.d.l. | 0.25 | 4.81 | n.d. | n.d. | 1520 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastalerz, M.; Drobniak, A.; Schimmelmann, A. Characteristics of Microbial Coalbed Gas during Production; Example from Pennsylvanian Coals in Indiana, USA. Geosciences 2017, 7, 26. https://doi.org/10.3390/geosciences7020026
Mastalerz M, Drobniak A, Schimmelmann A. Characteristics of Microbial Coalbed Gas during Production; Example from Pennsylvanian Coals in Indiana, USA. Geosciences. 2017; 7(2):26. https://doi.org/10.3390/geosciences7020026
Chicago/Turabian StyleMastalerz, Maria, Agnieszka Drobniak, and Arndt Schimmelmann. 2017. "Characteristics of Microbial Coalbed Gas during Production; Example from Pennsylvanian Coals in Indiana, USA" Geosciences 7, no. 2: 26. https://doi.org/10.3390/geosciences7020026





