Evaluation of Near-Surface Gases in Marine Sediments to Assess Subsurface Petroleum Gas Generation and Entrapment
Abstract
:1. Introduction
2. Origin of Near-Surface Marine Sediment Gases
2.1. Shallow Microbial Activity
2.2. Thermal Cracking of Organic Matter
2.3. Thermal Cracking of Inorganic Material
2.4. Magmatic and Mantle Degassing
3. Near-Surface Seep Distribution and Activity
3.1. Seepage Activity: Active versus Passive
3.2. Seepage Type: Macro versus Micro
3.3. Near-Surface Alteration Processes
4. Sediment Sample Collection
4.1. Targeted Sampling (Site-Specific)
4.2. Sub-Sample Collection
5. Sediment Gas Analysis
5.1. Interstitial
5.2. Bound
5.3. Impact of Sediment Gas Extraction
6. Interpretation of Seabed Gases
6.1. Defining Background and Anomalous Sediment Gases
6.2. Determination of Gas Origin
- Gas composition: C1 to C5 and non-hydrocarbon components such as CO2, O2, and N2;
- Compound-specific carbon isotopic ratio (δ13Cn) and hydrogen isotopic ratio (δDCH4).
6.2.1. Gas Composition
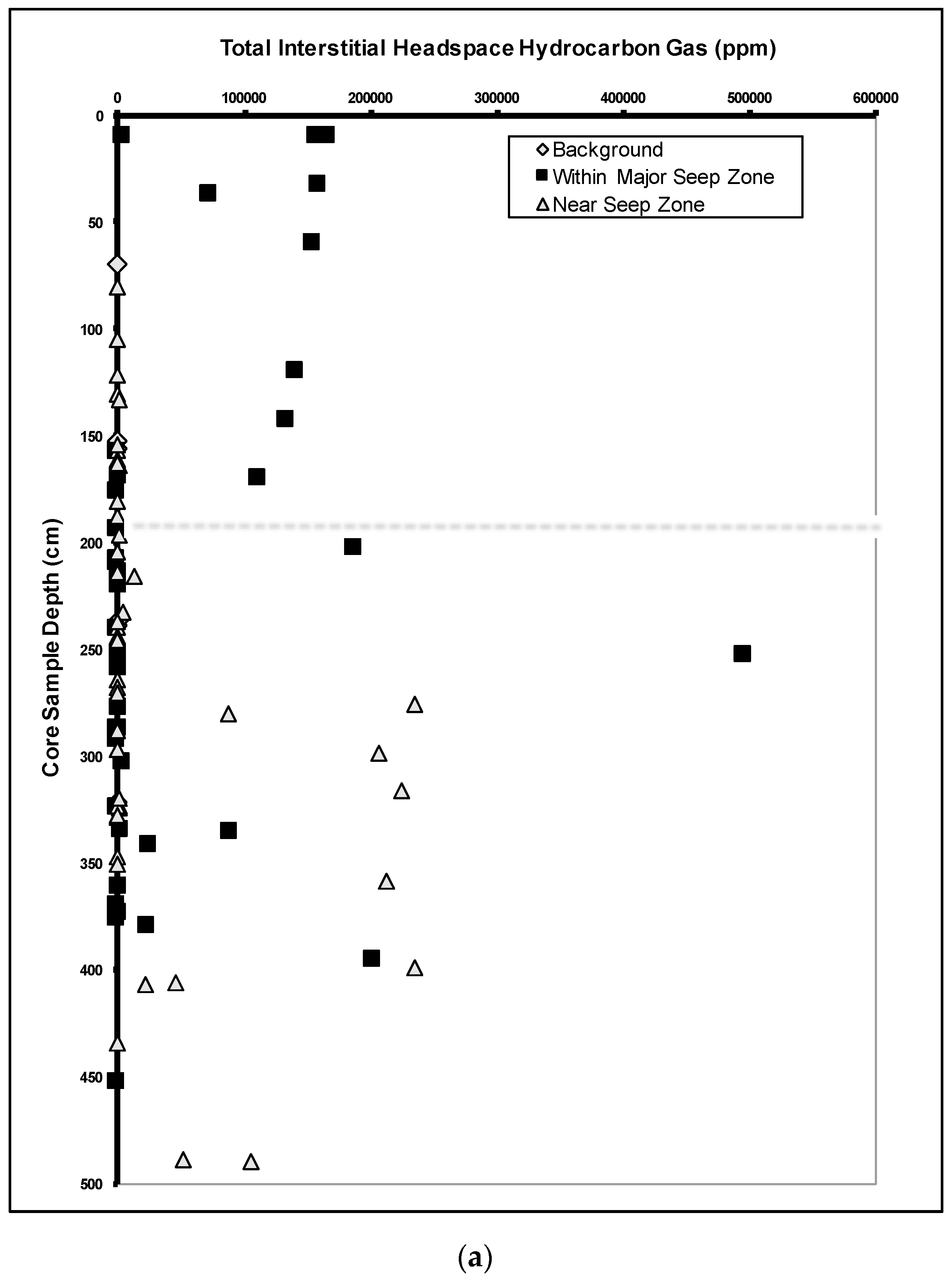
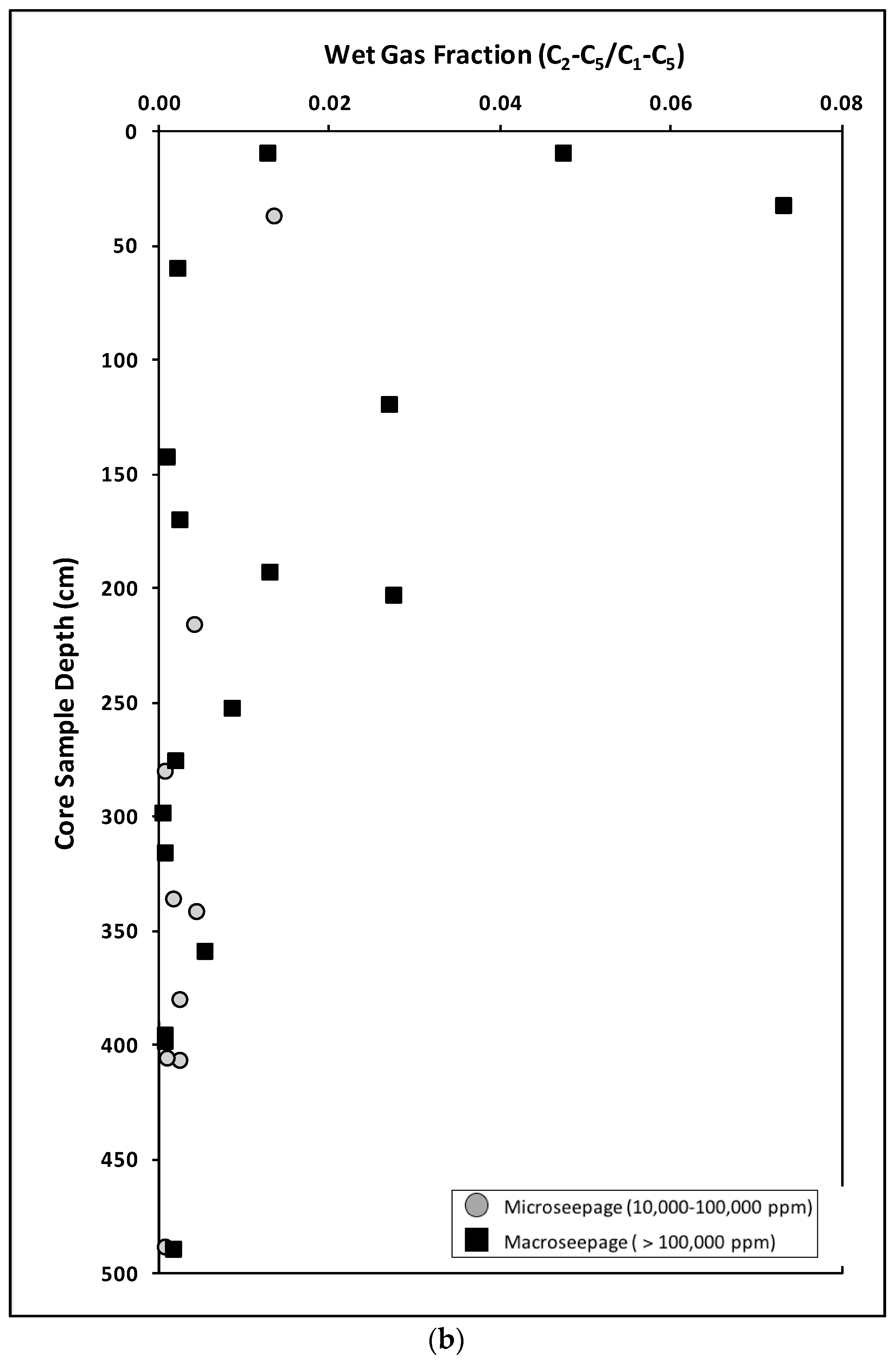
Interstitial Gases
- Group 1:
- Low total interstitial headspace hydrocarbon gas concentrations (∑C1–C5 less than 1000 ppm) with low Wet Gas Fraction (less than 0.05).
- Group 2:
- Very low total interstitial headspace hydrocarbon gas concentrations (∑C1–C5 less than 100 ppm) with elevated Wet Gas Fraction (greater than 0.05).
- Group 3:
- Very high total interstitial headspace gas concentrations (∑C1–C5 greater than 10,000 ppm) with variable Wet Gas Fraction (less than 0.01 to 0.14).
- Group 1:
- Low total interstitial headspace gas hydrocarbon concentrations (∑C1–C5 less than 1000 ppm) with low Wet Gas Fraction (less than 0.05).
- Group 2:
- Very low total interstitial headspace hydrocarbon gas concentrations (∑C1–C5 less than 100 ppm) with elevated Wet Gas Fraction (greater than 0.05).
- Group 3:
- Elevated total interstitial headspace hydrocarbon gas concentrations (∑C1–C5 greater than 10,000 ppm) with very low Wet Gas Fraction (less than 0.01).
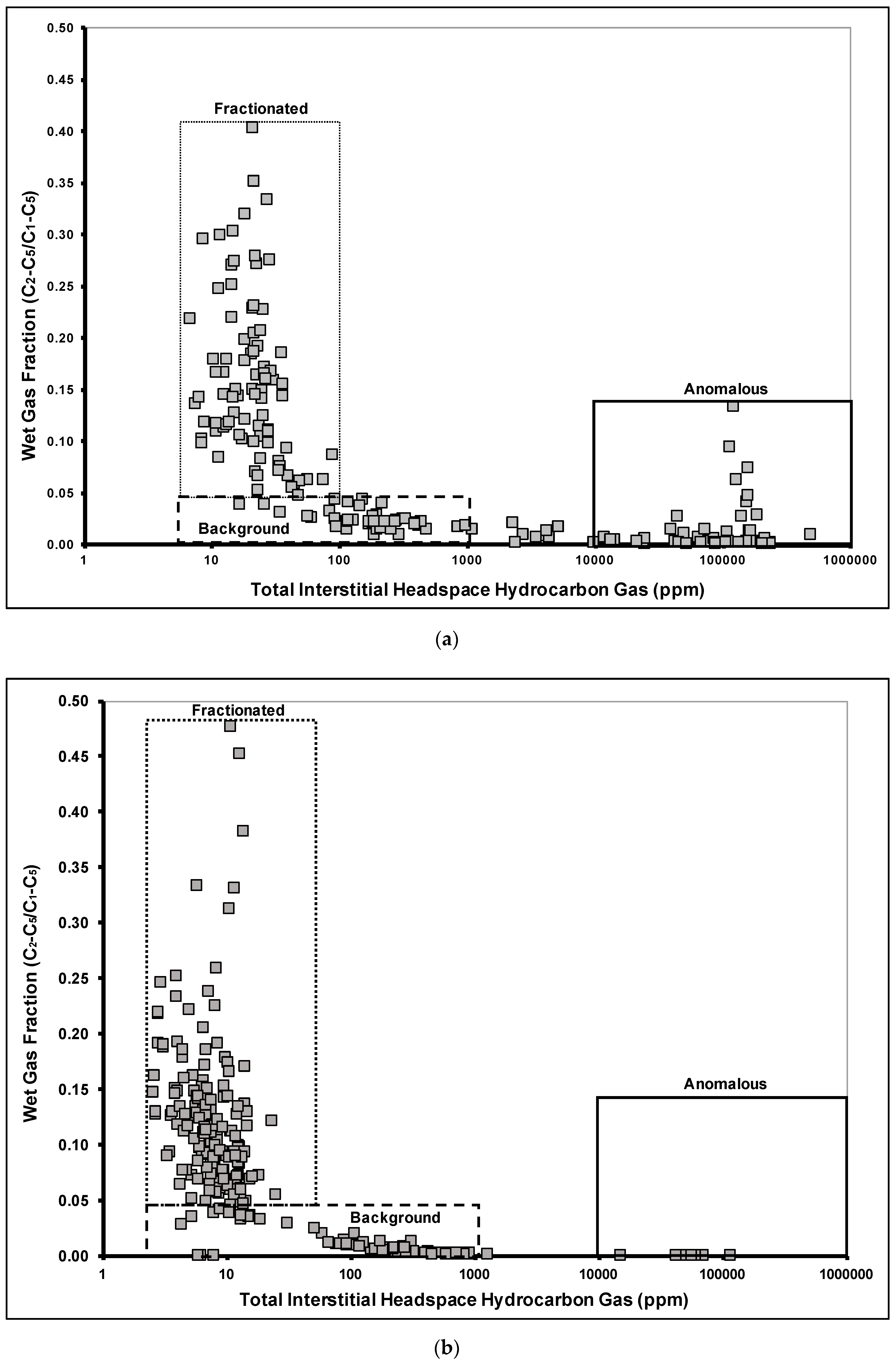
Bound Gases
Significance of Wet Gases
Ethene and Propene
Non-Hydrocarbon Gas—Carbon Dioxide
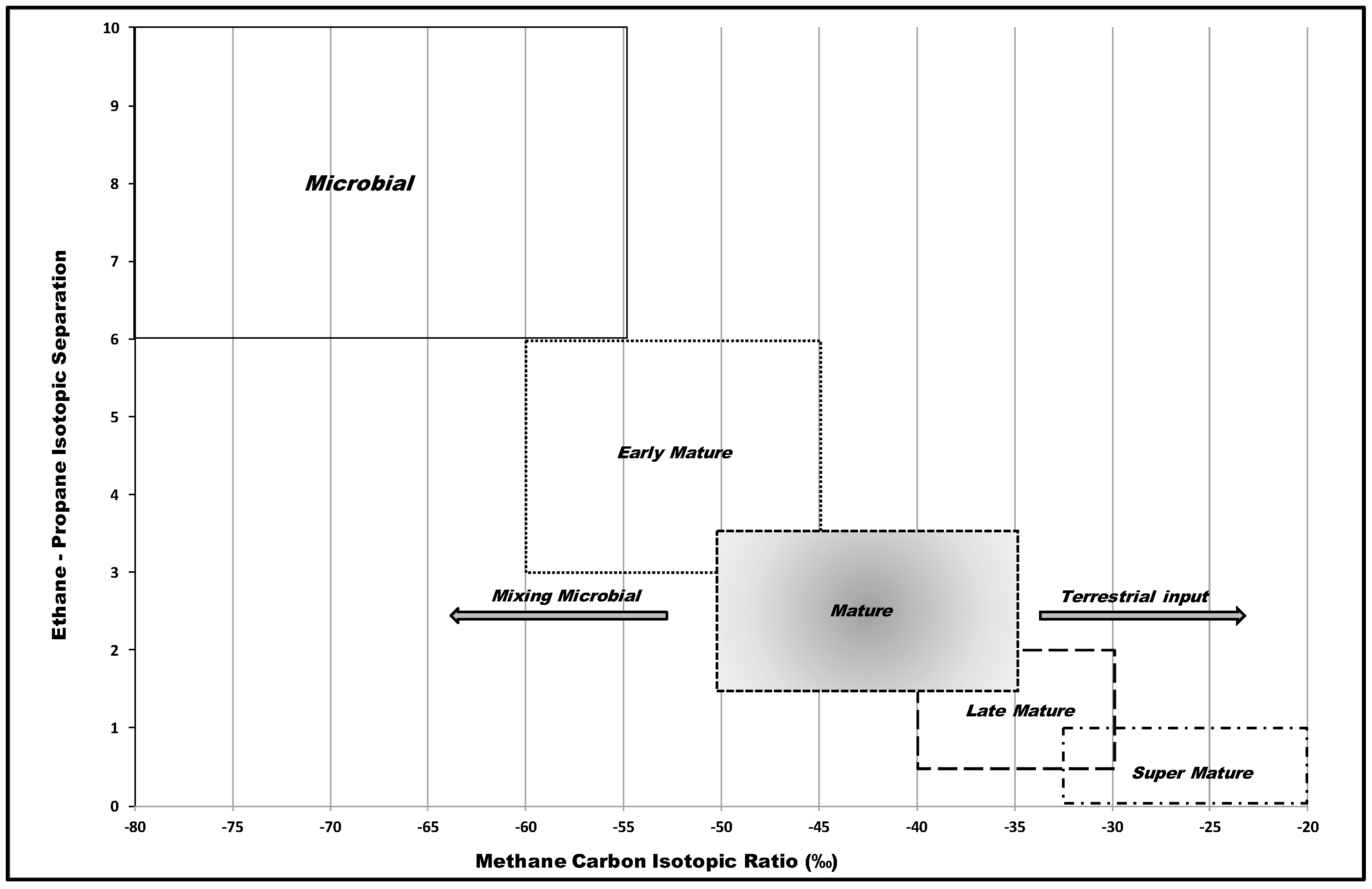
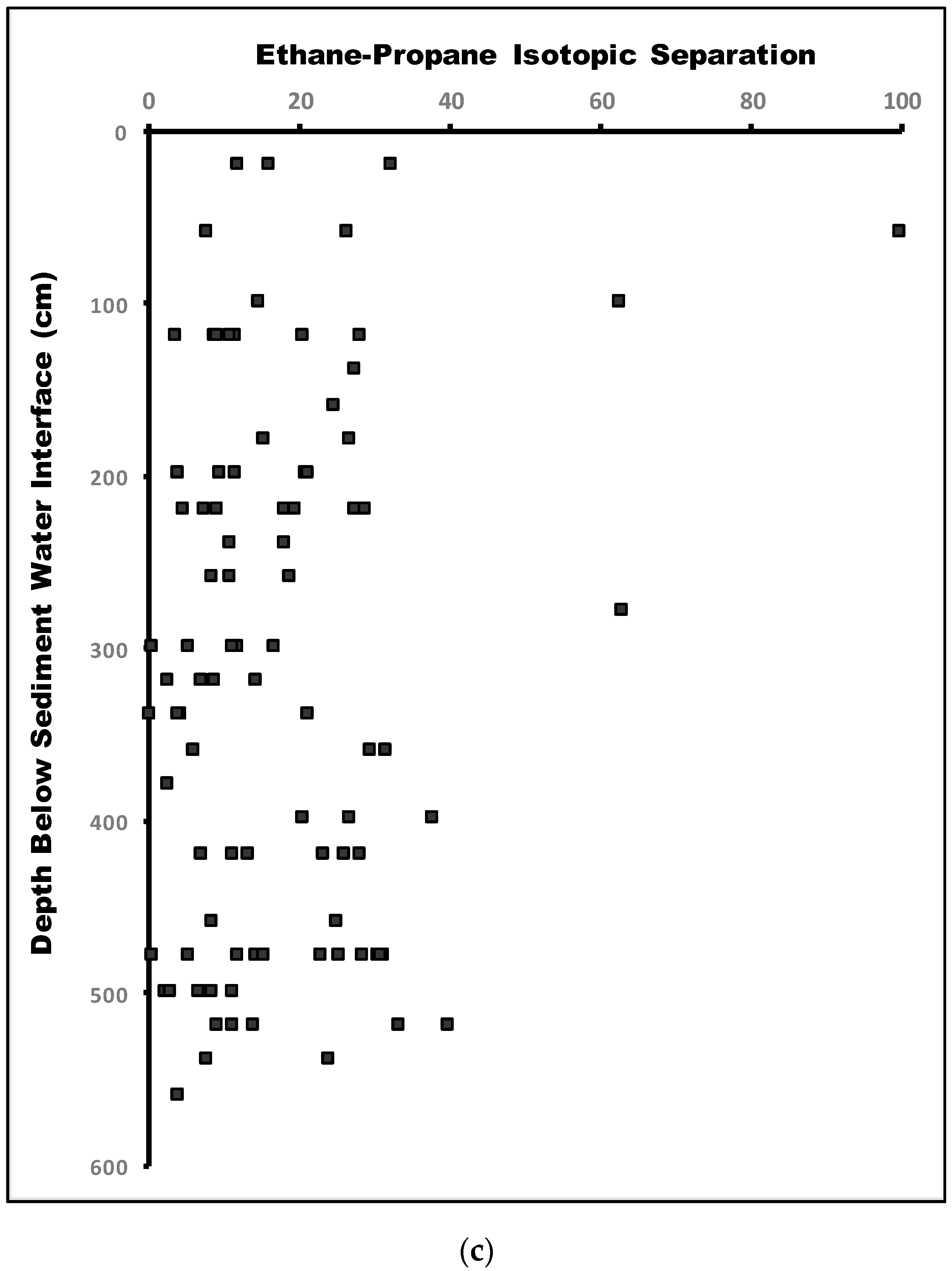
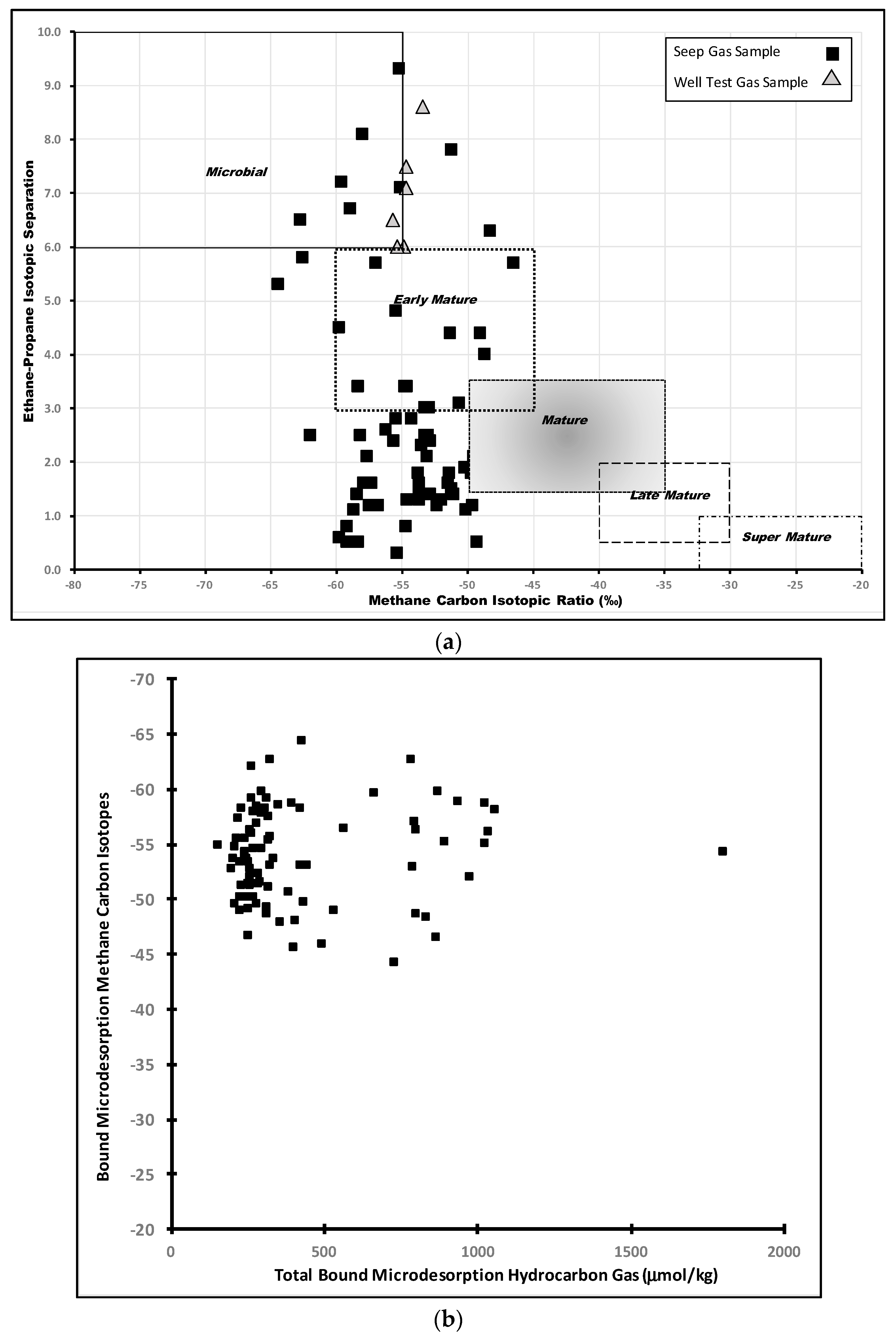
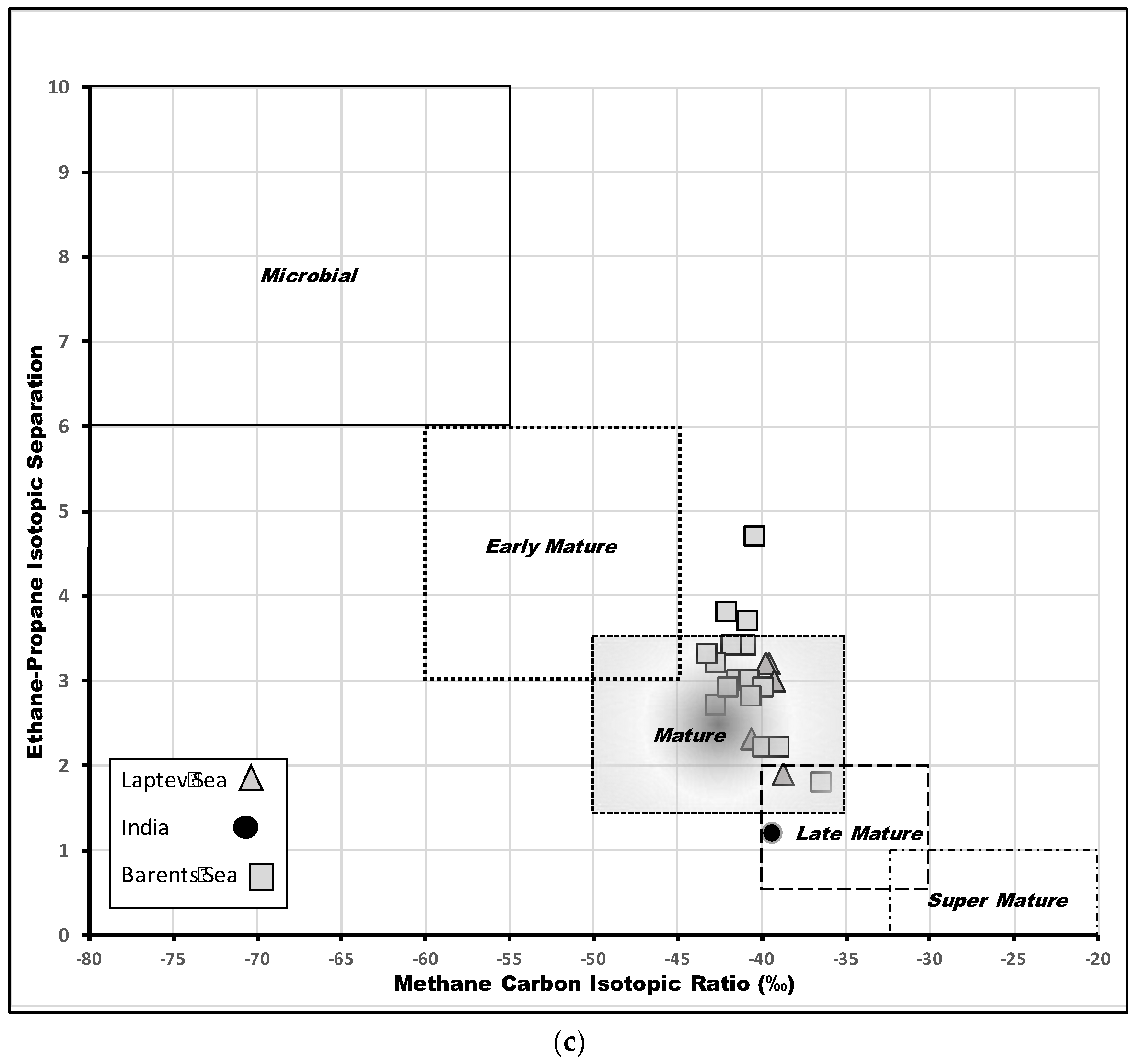
6.2.2. Compound-Specific Isotopes
7. Discussion
8. Conclusions
- Gases contained within near-surface marine sediments can be derived from multiple sources: shallow microbial activity, thermal cracking of organic matter and inorganic materials or magmatic-mantle degassing.
- Not all gas seepage is alike; seep type and activity will have an impact on the near-surface signal and the best way to collect, extract, analyse and interpret results.
- Sediment gas concentration and character will change with depth below the water sediment interface and proximity to the near-surface migration pathways.
- Near-surface alteration from microbial activity and pore water flushing will impact sediment gas concentration and composition as well as compound-specific isotopes.
- Sediment gases can reside in the interstitial spaces, bound to mineral or organic surfaces and/or entrapped in carbonate inclusions.
- The interstitial sediment gases are those gases contained within the sediment pore space, either dissolved in the pore waters (solute) or as free gas (vapour).
- The bound gases are believed to be attached to organic and/or mineral surfaces, entrapped in structured water or entrapped in authigenic carbonate inclusions.
- There are different sediment gas extraction methods to remove the interstitial and bound sediment gases, each providing different results.
- The differences could be related to gas origin (thermogenic versus microbial), storage (bound vs. interstitial) and/or the sediment gas extraction process.
- Interstitial sediment gases will be impacted by degassing during core retrieval from deep water, resulting in compound fractionation (preferential loss of methane).
- Interstitial and bound sediment gases can be impacted by compound fractionation during field sub-sampling and preparation.
- Interstitial or bound sediment gases are different from the gases collected at the well head and thus require a different interpretation approach.
- Examination of the interstitial gas concentration versus wet gas fraction is required to help define background, fractionated and anomalous (migrated) populations.
- Compound-specific isotopes on anomalous interstitial sediment gas samples will provide the most reliable isotopic data to evaluate gas origin.
- The sorption process and how best to remove the bound gases are not well understood.
- Laboratory and field studies suggest that the bound gases do not always reflect the migrated thermogenic hydrocarbons, in part due to fractionation related to sample preparation and the desorption process, in situ re-equilibration with locally derived microbial gases, and possible reworking of gas inclusions.
- Given the complexities of bound gases, the author recommends a simple headspace interstitial gas analysis to evaluate anomalous near-surface sediment gases.
- Gas interpretation should not be based on gas composition alone; compound-specific carbon isotopes and other supporting data are required to provide a fully integrated sediment gas interpretation.
- Non-hydrocarbon sediment gases such as carbon dioxide can provide critical information on the potential for reservoirs with elevated CO2 if there is good communication between the subsurface and near-surface sediments.
Acknowledgments
Conflicts of Interest
References
- Abrams, M.A. Distribution of subsurface hydrocarbon seepage in near-surface marine sediments. In Hydrocarbon Migration and Its Near Surface Effects; Schumacher, D., Abrams, M.A., Eds.; American Association Petroleum Geology Memoir No. 66; American Association of Petroleum Geologists: Tulsa, OK, USA, 1996; pp. 1–14. [Google Scholar]
- Abrams, M.A. Significance of hydrocarbon seepage relative to sub-surface petroleum generation and entrapment. Mar. Pet. Geol. Bull. 2005, 22, 457–478. [Google Scholar] [CrossRef]
- Abrams, M.; Dahdah, N. Surface sediment gases as indicators of subsurface hydrocarbons—Examining the record in laboratory and field studies. Mar. Pet. Geol. 2010, 27, 273–284. [Google Scholar] [CrossRef]
- Bernard, B.D. Light Hydrocarbons in Marine Sediments. Ph.D. Thesis, Texas A & M University, College Station, TX, USA, 1978; p. 144. [Google Scholar]
- Bjoroy, M.; Ferriday, I. Surface geochemistry as an exploration tool: A comparison of results using different analytical techniques. In Proceedings of the American Association Petroleum Geology Hedberg Conference “Near-Surface Hydrocarbon Migration: Mechanisms and Seepage Rates”, Vancouver, BC, Canada, 16–19 September 2001. [Google Scholar]
- Horvitz, L. Vegetation and geochemical prospecting for petroleum. Am. Assoc. Pet. Geol. Bull. 1972, 56, 925–940. [Google Scholar]
- Whiticar, M.J. Characterization and Application of Sorbed Gas by Microdesorption CF-IRMS. In Proceedings of the Near-Surface Hydrocarbon Migration: Mechanisms and Seepage Rates, American Association Petroleum Geology Hedberg Conference, Vancouver, BC, Canada, 7–10 April 2002. [Google Scholar]
- Horvitz, L. Geochemical exploration for petroleum. Science 1985, 229, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Horvitz, L. Hydrocarbon prospecting after forty years. In Unconventional Exploration for Petroleum and Natural Gas II; Gottlieb, B.M., Ed.; Southern Methodist University Press: Dallas, TX, USA, 1981; pp. 83–95. [Google Scholar]
- Abrams, M.A.; Segall, M.P.; Burtell, S.G. Best practices for detecting, identifying, and characterizing near-surface migration of hydrocarbons within marine sediments. In Proceedings of the 2001 Offshore Technology Conference, Houston, TX, USA, 30 April–3 May 2001. OTC Paper No. 13039. [Google Scholar]
- Abrams, M.A. Interpretation of surface methane carbon isotopes extracted from surficial marine sediments for detection of subsurface hydrocarbons. Assoc. Pet. Geochem. Explor. Bull. 1989, 5, 139–166. [Google Scholar]
- Abrams, M.A. Surface geochemical calibration research study: An example of research partnership between academia and industry. In Proceedings of the New Insights into Petroleum Geoscience Research through Collaboration between Industry and Academia, Geological Society, London, UK, 8–9 May 2002. [Google Scholar]
- Abrams, M.A.; Dahdah, N.F. Surface sediment hydrocarbons as indicators of subsurface hydrocarbons—Field calibration of existing and new surface geochemistry methods in the Marco Polo Area Gulf of Mexico. Am. Assoc. Pet. Geol. Bull. 2011, 95, 1907–1935. [Google Scholar] [CrossRef]
- Whiticar, M.J.; Faber, E.; Schoell, M. Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation—Isotope evidence. Geochim. Cosmochim. Acta 1986, 50, 693–709. [Google Scholar] [CrossRef]
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Oremland, R.S.; Whiticar, M.J.; Strohmaier, F.F.; Kliene, R.P. Bacterial ethane formation from reduced, ethylated sulfur compounds in anoxic sediments. Geochim. Cosmochim. Acta 1988, 51, 1895–1904. [Google Scholar] [CrossRef]
- Larter, S.; Koopmans, M.P.; Head, I. Biodegradation rates assessed geologically in a heavy oilfield—Implications for a deep, slow (largo) biosphere. In Proceedings of the GeoCanada 2000—The Millennium Geosciences Summit, Calgary, AB, Canada, 29 May–2 June 2000. [Google Scholar]
- James, A.T. Correlation of natural gas by use of carbon isotopic distribution between hydrocarbon components. Am. Assoc. Pet. Geol. Bull. 1983, 67, 1176–1191. [Google Scholar]
- James, A.T. Correlation of reservoired gases using the carbon isotopic composition of wet gas components. Am. Assoc. Pet. Geol. Bull. 1990, 74, 1441–1448. [Google Scholar]
- Schoell, M. Genetic classification of natural gases. Am. Assoc. Pet. Geol. Bull. 1983, 67, 2225–2238. [Google Scholar]
- Stahl, W. Carbon isotope ratios of German natural gases in comparison with isotope data of gaseous hydrocarbons from other parts of the worlds. Adv. Org. Geochim. 1973, 12, 453–462. [Google Scholar]
- Imbus, S.; Katz, B.; Urwongse, T. Predicting CO2 occurrence on a regional scale: Southeast Asia example. Org. Geochem. 1998, 29, 325–346. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Relative importance of thermodynamic and kinetic processes in governing the chemical and isotopic composition of carbon gases in high heat flow sedimentary basins. Geochim. Cosmochim. Acta 1997, 61, 3763–3785. [Google Scholar] [CrossRef]
- Wycherley, H.; Fleet, A.; Shaw, H. Some observations on the origins of large volumes of carbon dioxide accumulations in sedimentary basins. Mar. Pet. Geol. 1999, 16, 489–494. [Google Scholar] [CrossRef]
- Sherwood Lollar, B.; Ballentine, C.J. Insights into deep carbon derived from noble gases. Nat. Geosci. 2009, 2, 543–547. [Google Scholar] [CrossRef]
- MacDonald, I.R.; Reilly, J.F.; Best, S.E.; Venkataramaiah, R.; Sassen, R.; Guinasso, N.L.; Amos, J. Remote sensing inventory of active oil seeps and chemosynthetic communities in the Northern Gulf of Mexico. In Hydrocarbon Migration and Its Near Surface Effects; Schumacher, D., Abrams, M.A., Eds.; American Association of Petroleum Geologist Memoir No. 66; American Association of Petroleum Geologists: Tulsa, OK, USA, 1996; pp. 27–39. [Google Scholar]
- Cameron, N.R.; Brooks, J.M.; Zumberge, J.E. Deepwater Petroleum Systems in Nigeria: Their identification and characterisation ahead of the drill bit using SGE technology. In Proceedings of the IBC Nigeria Energy Summit, London, UK, 15–16 June 1999; p. 20. [Google Scholar]
- Van Graas, G.; Abrams, M.A.; Bilbo, M.; Narimanov, A.A. The use of integrated seepage detection tools in the South Caspian. In Proceedings of the American Association Petroleum Geology International Regional Conference Abstracts, Oil and Gas Business of the Greater Caspian Area—Present and Future Exploration and Production Operations, Istanbul, Turkey, 9–12 July 2000. [Google Scholar]
- Serie, C.; Huuse, M.; Schødt, N.H.; Brooks, J.M.; Williams, A. Subsurface fluid flow in the deep-water Kwanza Basin, offshore Angola. Basin Res. 2016, 29, 1–31. [Google Scholar] [CrossRef]
- Kessler, J.D.; Reeburgh, W.S.; Southon, J.; Seifert, R.; Michaelis, W.; Tyler, S.C. Basin-wide estimates of the input of methane from seeps and clathrates to the Black Sea. Earth Planet. Sci. Lett. 2006, 243, 366–375. [Google Scholar] [CrossRef]
- Reeburgh, W.S.; Ward, B.B.; Whalen, S.C.; Sandbeck, K.A.; Kilpatrick, K.A.; Kerkhof, L.J. Black Sea methane geochemistry. Deep-Sea Res. 1991, 38, S1189–S1210. [Google Scholar] [CrossRef]
- James, A.T.; Burns, B.J. Microbial alteration of subsurface natural gases accumulations. Am. Assoc. Pet. Geol. Bull. 1984, 68, 957–960. [Google Scholar]
- Hovland, M.; Judd, A.G. Seabed Pockmarks and Seepages: Impact on Geology, Biology and the Marine Environments; Graham & Trottman: London, UK, 1988. [Google Scholar]
- MacDonald, I.; Buthman, D.B.; Sager, W.W.; Peccini, M.B.; Guinasso, N.L., Jr. Pulsed oil discharge from a mud volcano. Geology 2000, 28, 907–910. [Google Scholar] [CrossRef]
- Quigley, D.; Hornafius, J.S.; Luyendyk, B.P.; Francis, R.D.; Clark, J.; Washburn, L. Decrease in natural marine hydrocarbon seepage near Coal Oil Point, California, associated with offshore oil production. Geology 1999, 27, 1047–1050. [Google Scholar] [CrossRef]
- Roberts, H.; Carney, R.S. Evidence of episodic fluid, gas, and sediment venting on the northern Gulf of Mexico continental slope. Econ. Geol. 1997, 92, 863–879. [Google Scholar] [CrossRef]
- Piggott, N.; Abrams, M.A. Near surface coring in the Beaufort and Chukchi Seas. In Hydrocarbon Migration and Its Near Surface Effects; Schumacher, D., Abrams, M.A., Eds.; American Association of Petroleum Geologist Memoir No. 66; American Association of Petroleum Geologists: Tulsa, OK, USA, 1996; pp. 385–400. [Google Scholar]
- Abrams, M.A. Geophysical and geochemical evidence for subsurface hydrocarbon leakage in the Bering Sea, Alaska. Mar. Pet. Geol. Bull. 1992, 9, 208–221. [Google Scholar] [CrossRef]
- Macgregor, D.S. Relationships between seepage, tectonics, and subsurface petroleum reservoirs. Mar. Pet. Geol. 1993, 10, 606–619. [Google Scholar] [CrossRef]
- Bernard, B.B.; Brooks, J.M.; Orange, D.L.; Decker, J. Interstitial Light Hydrocarbon Gases in Jumbo Piston Cores Offshore Indonesia: Thermogenic or Biogenic? In Proceedings of the 2013 Offshore Technology Conference, Houston, TX, USA, 6–9 May 2013. Document ID—24228-MS. [Google Scholar]
- Abrams, M.A. Best Practices for the Collection, Analysis, and Interpretation of Seabed Geochemical Samples to Evaluate Subsurface Hydrocarbon Generation and Entrapment. OTC-24219 2013. Available online: http://www.apachecorp.com/resources/upload/file/innovation/abrams-best-practices_for_the_collection_analysis_and_interpretation_of_seabed_geochemical_samples.pdf (accessed on 19 April 2017).
- Pape, T.; Bahr, A.; Klapp, S.A.; Abegg, F.; Bohrmann, G. High-intensity gas seepage causes rafting of shallow gas hydrates in the southeastern Black Sea. Earth Planet. Sci. Lett. 2011, 307, 35–46. [Google Scholar] [CrossRef]
- Abrams, M.A.; Boettcher, S.B. Mapping migration pathway using geophysical data, seabed core geochemistry, and submersible observations in the central Gulf of Mexico. In Proceedings of the Annual AAPG Convention, New Orleans, LA, USA, 16–19 April 2000; American Association Petroleum Geology Convention Abstracts. Volume 41, p. 7. [Google Scholar]
- Bolchert, G.; Weimer, P.; McBride, B.C. Structural and stratigraphic controls on petroleum seeps, Green Canyon and Ewing Bank, Northern Gulf of Mexico: Implications for petroleum migration. Gulf Coast Assoc. Geol. Soc. Trans. I 2000, 50, 65–74. [Google Scholar]
- Dembicki, H., Jr.; Samuels, B.M. Identification, characterization, and groundtruthing of deep-water thermogenic hydrocarbon macroseepage utilizing high- resolution AUV geophysical data. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 30 April–3 May 2007; OTC Paper 18556. p. 11. [Google Scholar]
- Dembicki, H., Jr.; Samuels, B.M. Improving the detection and analysis of seafloor macro-seeps: An example from the Marco Polo Field. In Proceedings of the Gulf of Mexico: Inter- national Petroleum Research Conference, Kuala Lumpur, Malaysia, 3–5 December 2008. IPTC Paper 12124. [Google Scholar]
- Toki, T.; Maegawa, K.; Tsunogai, U.; Kawagucci, S.; Takahata, N.; Sano, Y.; Ashi, J.; Kinoshita, M.; Gamo, T. Gas chemistry of pore fluids from Oomine Ridge on the Nankai accretionary prism. In Accretionary Prisms and Convergent Margin Tectonics in the Northwest Pacific Basin; Ogawa, Y., Anma, R., Dilek, Y., Eds.; Springer: Heidelberg, Germany, 2011; Volume 8, pp. 247–262. [Google Scholar]
- Ertefai, T.F.; Heuer, V.B.; Prieto-Mollar, X.; Vogt, C.; Sylva, S.P.; Seewald, J.; Hinrichs, K.-U. The biogeochemistry of sorbed methane in marine sediments. Geochim. Cosmochim. Acta 2010, 74, 6033–6048. [Google Scholar] [CrossRef]
- Zhang, L. Vacuum desoprtion of light hydrocarbons adsorbed on soil particles: A new method in geochemical exploration for petroleum. Am. Assoc. Pet. Geol. Bull. 2003, 87, 89–97. [Google Scholar]
- Hinrichs, K.U.; Hayes, J.M.; Bach, W.; Spivack, A.J.; Hmelo, L.R.; Holm, N.G.; Johnson, C.G.; Sylva, S.P. Biological formation of ethane and propane in the deep marine subsurface. Proc. Natl. Acad. Sci. USA 2006, 103, 14684–14689. [Google Scholar] [CrossRef] [PubMed]
- Faber, E.; Berner, U.; Hollerbach, A.; Gerling, P. Isotope geochemistry in surface exploration for hydrocarbons. Geol. Rund. 1997, D103, 103–127. [Google Scholar]
- Woltemate, I. Isotopische Untersuchungen zur bak- Teriellen Gasbildung in Cinem SuBwasseree. Diploma–beit, Universitat Clausthal, Clausthal Zellerfeld, Germany, 1982; p. 90. [Google Scholar]
- Badeira de Mello, C.S.; Goncalves, R.C.S.; Miller, D.J.; Francolin, J.B.L. Blender: A surface geochemistry tool to sample interstitial hydrocarbons in soils and piston core sediments. In Proceedings of the IMOG 2005 Meeting, Seville, Spain, 12–16 September 2005. [Google Scholar]
- Pflaum, R.E. Gaseous Hydrocarbons Bound in Marine Sediments. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 1989; p. 156. [Google Scholar]
- Horvitz, E.P.; Ma, S. Hydrocarbons in near-surface sand, a geochemical survey of the Dolphin field in North Dakota. Assoc. Pet. Geochem. Explor. Bull. 1988, 4, 30–46. [Google Scholar]
- Cole, G.; Yu, A.; Peel, F.; Requejo, R.; DeVay, J.; Brooks, J.; Bernard, B.; Zumberge, J.; Brown, S. Constraining source and charge risk in deepwater areas. World Oil 2001, 222, 69–77. [Google Scholar]
- Faber, E.; Stahl, W. Geochemical surface exploration for hydrocarbons in the North Sea. Assoc. Pet. Geol. Bull. 1984, 68, 363–386. [Google Scholar]
- Faber, E.; Stahl, W.J.; Whiticar, M.J.; Lietz, J.; Brooks, J.M. Thermal Hydrocarbons in Gulf Coast Sediments. In Gulf Coast Oils and Gases: Their Characteristics, Origin, Distribution, and Exploration and Production Significance, Proceedings of the 9th Annual Research Conference, Gulf Coast Section, Society of Economic Paleontologists and Mineralogists Foundation; Earth Enterprises: New York, NY, USA, 1990; pp. 297–308. [Google Scholar]
- Knies, J.; Damm, E.; Gutt, J.; Mann, U.; Pinturier, L. Near-surface hydrocarbon anomalies in shelf sediments off Spitsbergen: Evidences for past seepages. Geochem. Geophys. Geosyst. 2004, 5, Q06003. [Google Scholar] [CrossRef]
- Cramer, B.; Franke, D. Indications for an active petroleum system in the Laptev Sea, NE Siberia. J. Pet. Geol. 2005, 28, 369–384. [Google Scholar] [CrossRef]
- Patil, D.J.; Mani, D.; Madhavi, T.; Sudarshan, V.; Srikarni, C.; Kalpana, M.S.; Sreenivas, B.; Dayal, A.M. Near surface hydrocarbon prospecting in Mesozoic Kutch sedimentary basin, Gujarat, Western India—A reconnaissance study using geochemical and isotopic approach. J. Pet. Sci. Eng 2013, 108, 393–403. [Google Scholar] [CrossRef]
- Blumenberg, M.; Lutz, R.; Schlömer, S.; Krüger, M.; Scheeder, G.; Berglar, K.; Heyde, I.; Weniger, P. Hydrocarbons from near-surface sediments of the Barents Sea north of Svalbard—Indication of subsurface hydrocarbon generation? Mar. Pet. Geol. Bull. 2016, 76, 432–443. [Google Scholar] [CrossRef]
- Bernard, B.B.; Brooks, J.M.; Zumberge, J. Determining the origin of gases in near- surface sediments. In Proceedings of the Near-Surface Hydrocarbon Migration: Mechanisms and Seepage Rates AAPG Hedberg Conference, Vancouver, BC, Canada, 16–19 September 2001; pp. 16–19. [Google Scholar]
- Ullom, W.L. Ethylene and propylene in soil gas: Occurrences: Sources, and impact on interpretation of exploration geochemical data. Assoc. Pet. Geochem. Explor. Bull. 1988, 4, 62–81. [Google Scholar]
- Vogel, T.M.; Oremland, R.S.; Kvenvolden, K.A. Low-temperature formation of hydrocarbon gases in San Francisco bay sediment (California USA). Chem. Geol. 1982, 37, 289–298. [Google Scholar] [CrossRef]
- Davis, J.B.; Squires, R.M. Detection of Microbially Produced Gaseous Hydrocarbons Other than Methane. Science 1954, 119, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Oremland, R.S. Microbial formation of ethane in anoxic estuarine sediments. Appl. Environ. Microbiol. 1981, 42, 122–129. [Google Scholar] [PubMed]
- Clayton, C.J. Controls on the carbon isotope ratio of carbon dioxide in oil and gas fields. In Organic Geochemistry: Developments and Applications to Energy, Climate, Environment and Human History, Proceedings 17th International Meeting on Organic Geochemistry, San Sebastian, Spain, 4–8 September 1995; Grimalt, J.O., Dorronsoro, C., Eds.; AIGOA: San Sebastián, Spain, 1995; pp. 1073–1074. [Google Scholar]
- Cooper, B.A.; Raven, M.J.; Samuel, L.; Hadjono; Satoto, W. Origin and geological controls on subsurface CO2 distribution with examples from Western Indonesia. In Proceedings of the International Conference on Petroleum Systems of SE Asia and Australasia, Jakarta, Indonesia, 21–23 May 1997; pp. 877–892. [Google Scholar]
- Kvenvolden, K.A.; Vogel, T.M.; Gardner, J.V. Geochemical prospecting for hydrocarbons in the outer continental shelf, Southern Bering Sea, Alaska. J. Geochem. Explor. 1981, 14, 209–219. [Google Scholar] [CrossRef]
- Kvenvolden, K.A.; Weliky, K.; Nelson, H.; DesMarais, D.J. Submarine seep of carbon dioxide in Norton Sound. Alsk. Sci. 1979, 205, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Faber, E.; Stahl, W. Analytical procedure and results of an isotope geochemical surface survey in an area of the British North Sea. Geol. Soc. Lond. Spec. Publ. 1983, 11, 51–63. [Google Scholar] [CrossRef]
- Milkov, A.V. Worldwide distribution and significance of secondary microbial methane formed during petroleum biodegradation in conventional reservoirs. Org. Geochem. 2011, 42, 184–207. [Google Scholar] [CrossRef]
- Chung, H.M.; Gormly, J.R.; Squires, R.M. Origin of gaseous hydrocarbons in subsurface environments: Theoretical considerations of carbon isotope distribution. Chem. Geol. 1988, 71, 97–103. [Google Scholar] [CrossRef]
- Quistada, S.D.; Valentine, D.L. Anaerobic propane oxidation in marine hydrocarbon seep sediments. Geochim. Cosmochim. Acta 2011, 75, 2159–2169. [Google Scholar] [CrossRef]
- Kniemeyer, O.; Musat, F.; Sievert, S.M.; Knittel, K.; Wilkes, H.; Blumenberg, M.; Michaelis, W.; Classen, A.; Bolm, C.; Joye, S.B.; et al. Anaerobic oxidation of short-chain hydrocarbons by marine sulphate-reducing bacteria. Nature 2007, 449, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Abrams, M.A. Modifications for Increasing Recovery and Penetration in an Open Barrel Gravity Corer; OCEANS 82 Conference Record; 82CH18 27.5; IEE/MTS: Washington, DC, USA, 1982; pp. 661–666. [Google Scholar]




| Analysis | Max | Min | Area | Reference |
|---|---|---|---|---|
| Acid extraction | 561 ppb | 208 ppb | Offshore California | [9] |
| Acid extraction | 1563 ppb | 166 ppb | U.S. Gulf of Mexico | [9] |
| Acid extraction | 562 ppb | 4 ppb | North Sea | [57] |
| Acid extraction | 1120 ppb | 160 ppb | U.S. Gulf of Mexico | [58] |
| Acid extraction | 6605 ppb | 120 ppb | Barents Sea shelf | [59] |
| Acid extraction | 461 ppb | 38 ppb | Laptev Sea | [60] |
| Acid extraction | 551 ppb | 46 ppb | Kutch Basin India | [61] |
| Acid extraction | 603 ppb | 75 ppb | Barents Sea | [62] |
| Microdesorption | 1736 nmol/g | 67 nmol/g | U.S. Gulf of Mexico | [13] |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrams, M.A. Evaluation of Near-Surface Gases in Marine Sediments to Assess Subsurface Petroleum Gas Generation and Entrapment. Geosciences 2017, 7, 35. https://doi.org/10.3390/geosciences7020035
Abrams MA. Evaluation of Near-Surface Gases in Marine Sediments to Assess Subsurface Petroleum Gas Generation and Entrapment. Geosciences. 2017; 7(2):35. https://doi.org/10.3390/geosciences7020035
Chicago/Turabian StyleAbrams, Michael A. 2017. "Evaluation of Near-Surface Gases in Marine Sediments to Assess Subsurface Petroleum Gas Generation and Entrapment" Geosciences 7, no. 2: 35. https://doi.org/10.3390/geosciences7020035





