Evidence of Road Salt in New Hampshire’s Snowpack Hundreds of Meters from Roadways
Abstract
:1. Introduction
2. Materials and Methods
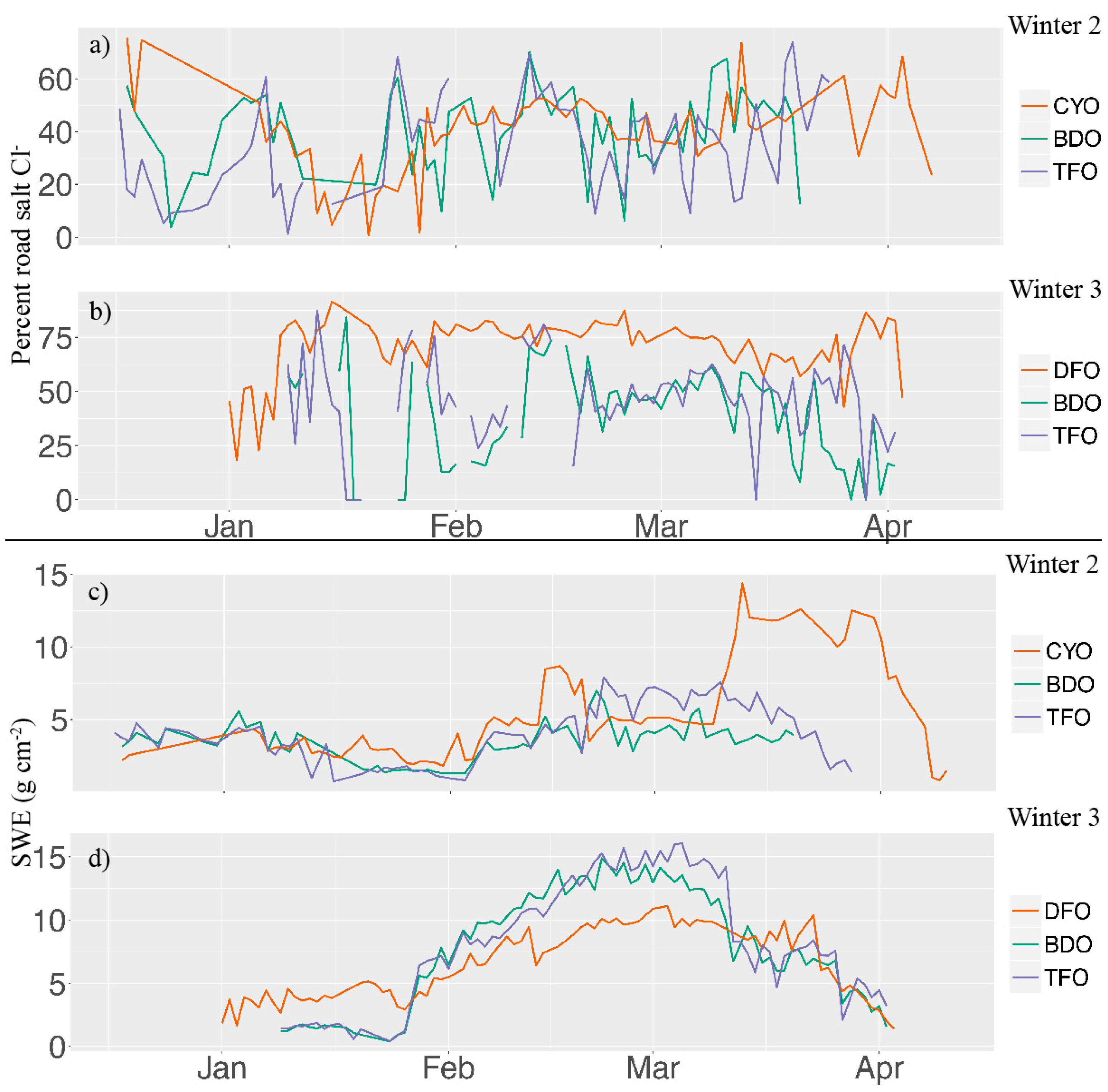
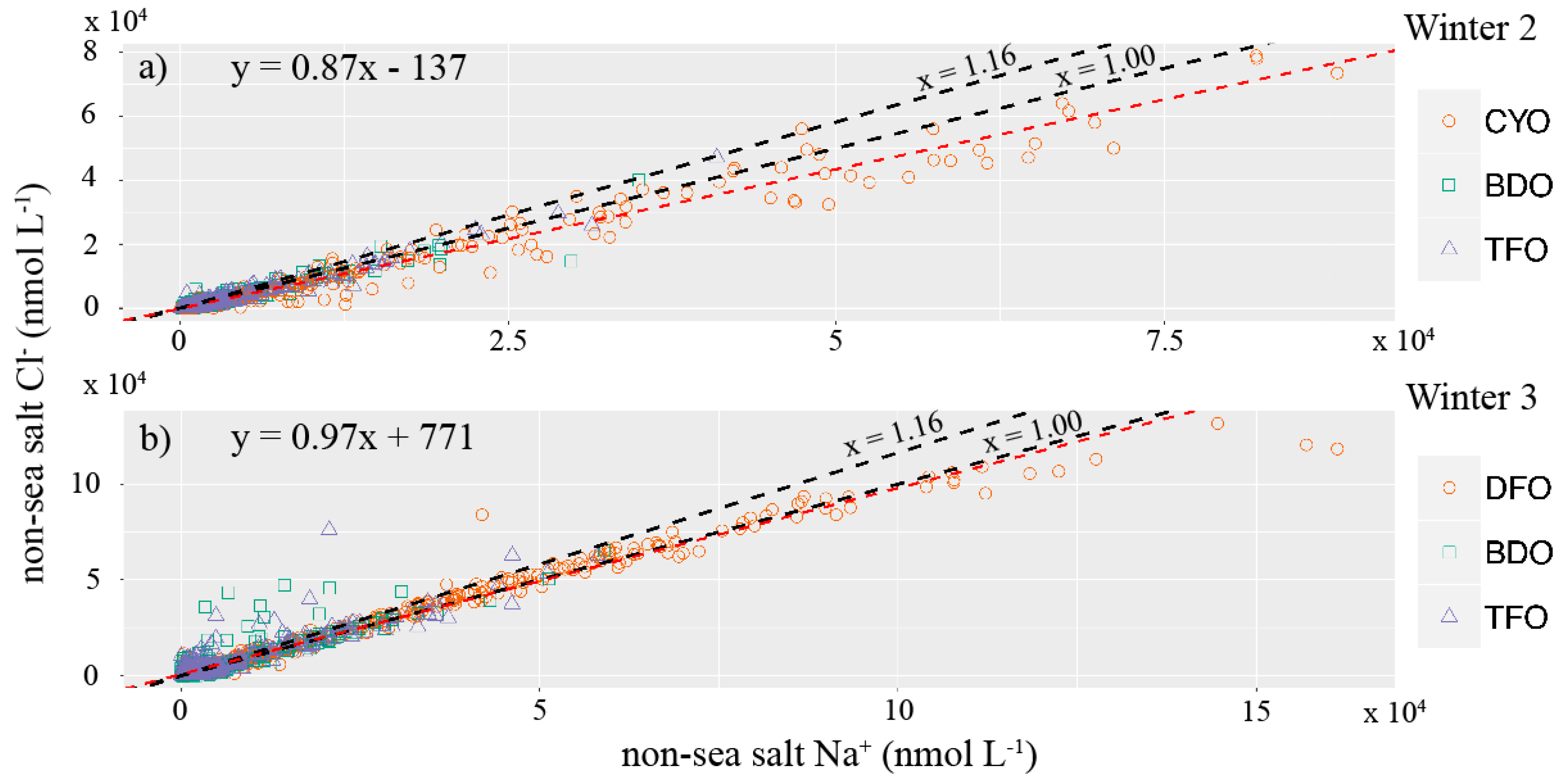
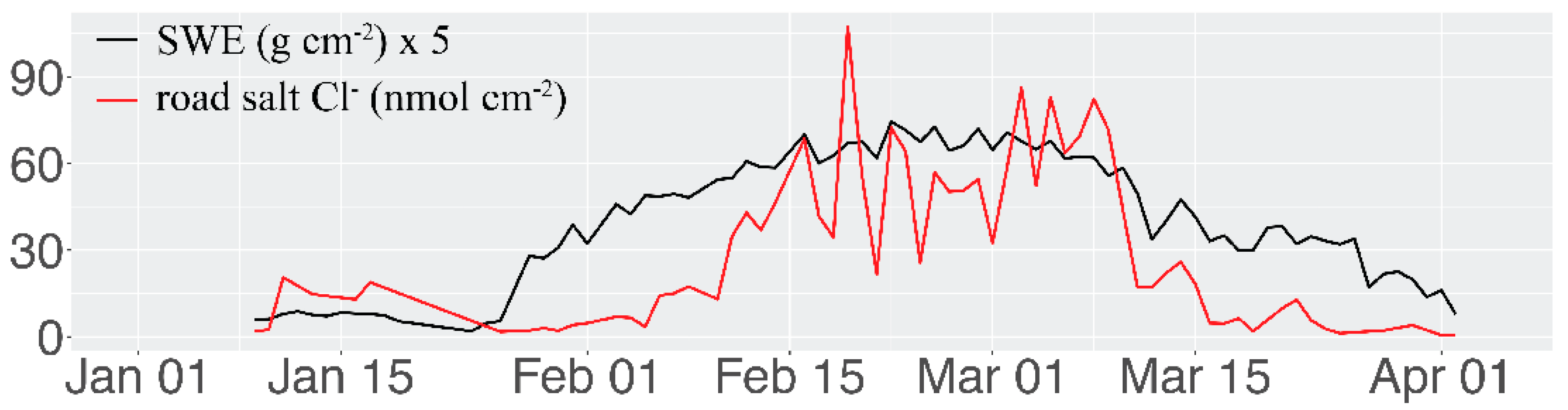
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Daley, M.L.; Potter, J.D.; McDowell, W.H. Salinization of urbanizing New Hampshire streams and groundwater: effects of road salt and hydrologic variability. J. N. Am. Benthol. Soc. 2009, 28, 929–940. [Google Scholar] [CrossRef]
- Trowbridge, P. Data Report for the Total Maximum Daily Loads for Chloride for Waterbodies in the Vicinity of the I-93 Corridor from Massachusetts to Manchester, NH: Policy—Porcupine Brook, Beaver Brook, Dinsmore Brook, North Tributary to Canobie Lake; State of New Hampshire, Department of Environmental Services: Concord, NH, USA, 2007.
- Kaushal, S.S.; Groffman, P.M.; Likens, G.E.; Belt, K.T.; Stack, W.P.; Kelly, V.R.; Band, L.E.; Fisher, G.T. Increased salinization of fresh water in the northeastern United States. Proc. Natl. Acad. Sci. USA 2005, 102, 13517–13520. [Google Scholar] [CrossRef] [PubMed]
- Godwin, K.S.; Hafner, S.D.; Buff, M.F. Long-term trends in sodium and chloride in the Mohawk River, New York: the effect of fifty years of road-salt application. Environ. Pollut. 2003, 124, 273–281. [Google Scholar] [CrossRef]
- Corsi, S.R.; Graczyk, D.J.; Geis, S.W.; Booth, N.L.; Richards, K.D. A Fresh Look at Road Salt: Aquatic Toxicity and Water-Quality Impacts on Local, Regional, and National Scales. Environ. Sci. Technol. 2010, 44, 7376–7382. [Google Scholar] [CrossRef] [PubMed]
- Corsi, S.R.; De Cicco, L.A.; Lutz, M.A.; Hirsch, R.M. River chloride trends in snow-affected urban watersheds: increasing concentrations outpace urban growth rate and are common among all seasons. Sci. Total Environ. 2015, 508, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Cassanelli, J.P.; Robbins, G.A. Effects of Road Salt on Connecticut’s Groundwater: A Statewide Centennial Perspective. J. Environ. Qual. 2013, 42, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Dugan, H.A.; Bartlett, S.L.; Burke, S.M.; Doubek, J.P.; Krivak-Tetley, F.E.; Skaff, N.K.; Summers, J.C.; Farrell, K.J.; McCullough, I.M.; Morales-Williams, A.M.; et al. Salting our freshwater lakes. Proc. Natl. Acad. Sci. USA 2017, 114, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Bierwagen, B.G.; Theobald, D.M.; Pyke, C.R.; Choate, A.; Groth, P.; Thomas, J.V.; Morefield, P. National housing and impervious surface scenarios for integrated climate impact assessments. Proc. Natl. Acad. Sci. USA 2010, 107, 20887–20892. [Google Scholar] [CrossRef] [PubMed]
- New Hampshire Department of Transportation Bureau of Highway Maintenance. Statewide Report: FY 2015 Salt and Sand Report (November 2014–April 2015); Report Week No. 21, Report Date 21 November 2014–2 April 2015, Page 1; New Hampshire Department of Transportation: Concord, NH, USA.
- Blomqvist, G.; Johansson, E.-L. Airborne spreading and deposition of de-icing salt—A case study. Sci. Total Environ. 1999, 235, 161–168. [Google Scholar] [CrossRef]
- Kelsey, P.D.; Hootman, R.G. Deicing Salt Dispersion and Effects on Vegetation along Highways. Case Study: Deicing Salt Deposition on the Morton Arboretum. In Chemical Deicers and the Environment Proceedings of Alternative Deicing Technologies and the Environment, East Lansing, MI, USA, 25–26 March 1991; Lewis Publishers, Inc.: Boca Raton, FL, USA, 1992. [Google Scholar]
- Lazarcik, J.; Dibb, J.E.; Adolph, A.C.; Amante, J.M.; Wake, C.P.; Scheuer, E.; Mineau, M.M.; Albert, M.R. Major fraction of black carbon is flushed from the melting New Hampshire snowpack nearly as quickly as soluble impurities. J. Geophys. Res. Atmos. 2017, 122, 537–553. [Google Scholar] [CrossRef]
- Contosta, A.R.; Adolph, A.; Burchsted, D.; Burakowski, E.; Green, M.; Guerra, D.; Albert, M.; Dibb, J.; Martin, M.; McDowell, W.H.; et al. A longer vernal window: the role of winter coldness and snowpack in driving spring transitions and lags. Glob. Chang. Biol. 2017, 23, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, A.H.; Audorff, V.; Beierkuhnlein, C. Salt in the wound: The interfering effect of road salt on acidified forest catchments. Sci. Total Environ. 2015, 532, 595–604. [Google Scholar] [CrossRef] [PubMed]
- McBean, E.A.; Al-Nassri, S. Migration pattern of de-icing salts from roads. J. Environ. Manag. 1987, 25, 231–238. [Google Scholar]
- Hautala, E.L.; Rekilä, R.; Tarhanen, J.; Ruuskanen, J. Deposition of motor vehicle emissions and winter maintenance along roadside assessed by snow analyses. Environ. Pollut. 1995, 87, 45–49. [Google Scholar] [CrossRef]
- Lundmark, A.; Olofsson, B. Chloride Deposition and Distribution in Soils Along a Deiced Highway—Assessment Using Different Methods of Measurement. Water Air Soil Pollut. 2007, 182, 173–185. [Google Scholar] [CrossRef]
- Liang, T.; Chamecki, M.; Yu, X. Sea salt aerosol deposition in the coastal zone: A large eddy simulation study. Atmos. Res. 2016, 180, 119–127. [Google Scholar] [CrossRef]
- Adolph, A.C.; Albert, M.R.; Lazarcik, J.; Dibb, J.E.; Amante, J.M.; Price, A. Dominance of grain size impacts on seasonal snow albedo at open sites in New Hampshire. J. Geophys. Res. Atmos. 2017, 122, 121–139. [Google Scholar] [CrossRef]
- Keene, W.C.; Pszenny, A.A.P.; Galloway, J.N.; Hawley, M.E. Sea-salt correction and interpretation of constituent ratios in marine precipitation. J. Geophys. Res. Atmos. 1986, 91, 6647–6658. [Google Scholar] [CrossRef]
- Pilson, M.E.Q. Major Constituents of Seawater. In An Introduction to the Chemistry of the Sea, 1st ed.; Prentice Hall (Pearson Education Inc.): London, UK, 1998; p. 58. [Google Scholar]
- Perry, K.D.; Cahill, T.A.; Eldred, R.A.; Dutcher, D.D.; Gill, T.E. Long-range transport of North African dust to the eastern United States. J. Geophys. Res. Atmos. 1997, 102, 11225–11238. [Google Scholar] [CrossRef]
- VanCuren, R.A.; Cahill, T.A. Asian aerosols in North America: Frequency and concentration of fine dust. J. Geophys. Res. Atmos. 2002, 107, AAC 19-1–AAC 19-16. [Google Scholar] [CrossRef]
- Mahalinganathan, K.; Thamban, M.; Laluraj, C.M.; Redkar, B.L. Relation between surface topography and sea-salt snow chemistry from Princess Elizabeth Land, East Antarctica. Cryosphere 2012, 6, 505–515. [Google Scholar] [CrossRef]
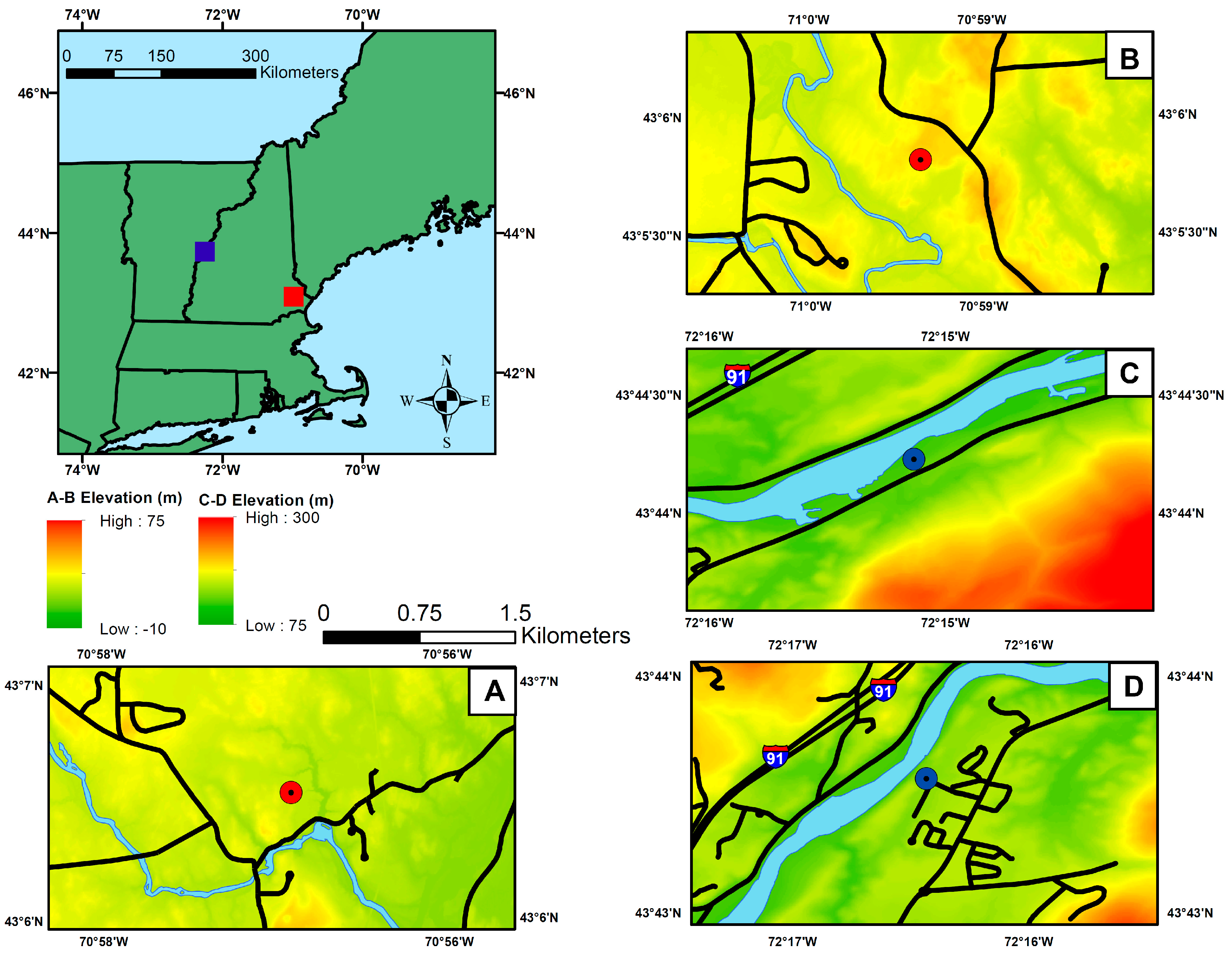
| Site (Abbr.) | Distance to Nearest Maintained Road (m) | Distance to Atlantic Ocean (km) | Elevation (m) | Latitude and Longitude |
|---|---|---|---|---|
| CRREL Yard Open (CYO) a | 290 | 150 | 143 | N 43°43′ |
| W 72°16′ | ||||
| Dartmouth Farm Open (DFO) a | 114 | 150 | 119 | N 43°44′ |
| W 72°15′ | ||||
| Burley-Demeritt Open (BDO) | 330 | 23 | 35 | N 43°05′ |
| W 70°59′ | ||||
| Thompson Farm Open (TFO) | 348 | 22 | 19 | N 43°06′ |
| W 70°56′ |
| Site | Average Cl−/Na+ | % Non-Sea Salt Cl− | Cumulative Non-Sea Salt Cl− (nmol cm−2) |
|---|---|---|---|
| Winter 2 1 | |||
| CYO | 0.94 | 61 ± 21 | 510 ± 173 |
| BDO | 1.02 | 63 ± 21 | 140 ± 48 |
| TFO | 0.99 | 60 ± 20 | 188 ± 64 |
| Winter 3 1 | |||
| DFO | 1.02 | 81 ± 28 | 1780 ± 605 |
| BDO | 0.97 | 63 ± 21 | 434 ± 148 |
| TFO | 1.02 | 66 ± 22 | 667 ± 227 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarcik, J.; Dibb, J.E. Evidence of Road Salt in New Hampshire’s Snowpack Hundreds of Meters from Roadways. Geosciences 2017, 7, 54. https://doi.org/10.3390/geosciences7030054
Lazarcik J, Dibb JE. Evidence of Road Salt in New Hampshire’s Snowpack Hundreds of Meters from Roadways. Geosciences. 2017; 7(3):54. https://doi.org/10.3390/geosciences7030054
Chicago/Turabian StyleLazarcik, James, and Jack E. Dibb. 2017. "Evidence of Road Salt in New Hampshire’s Snowpack Hundreds of Meters from Roadways" Geosciences 7, no. 3: 54. https://doi.org/10.3390/geosciences7030054





