Applicability of Natural Zeolite for NH-Forms Removal in Enzyme-Mediated Calcite Precipitation Technique
Abstract
:1. Introduction
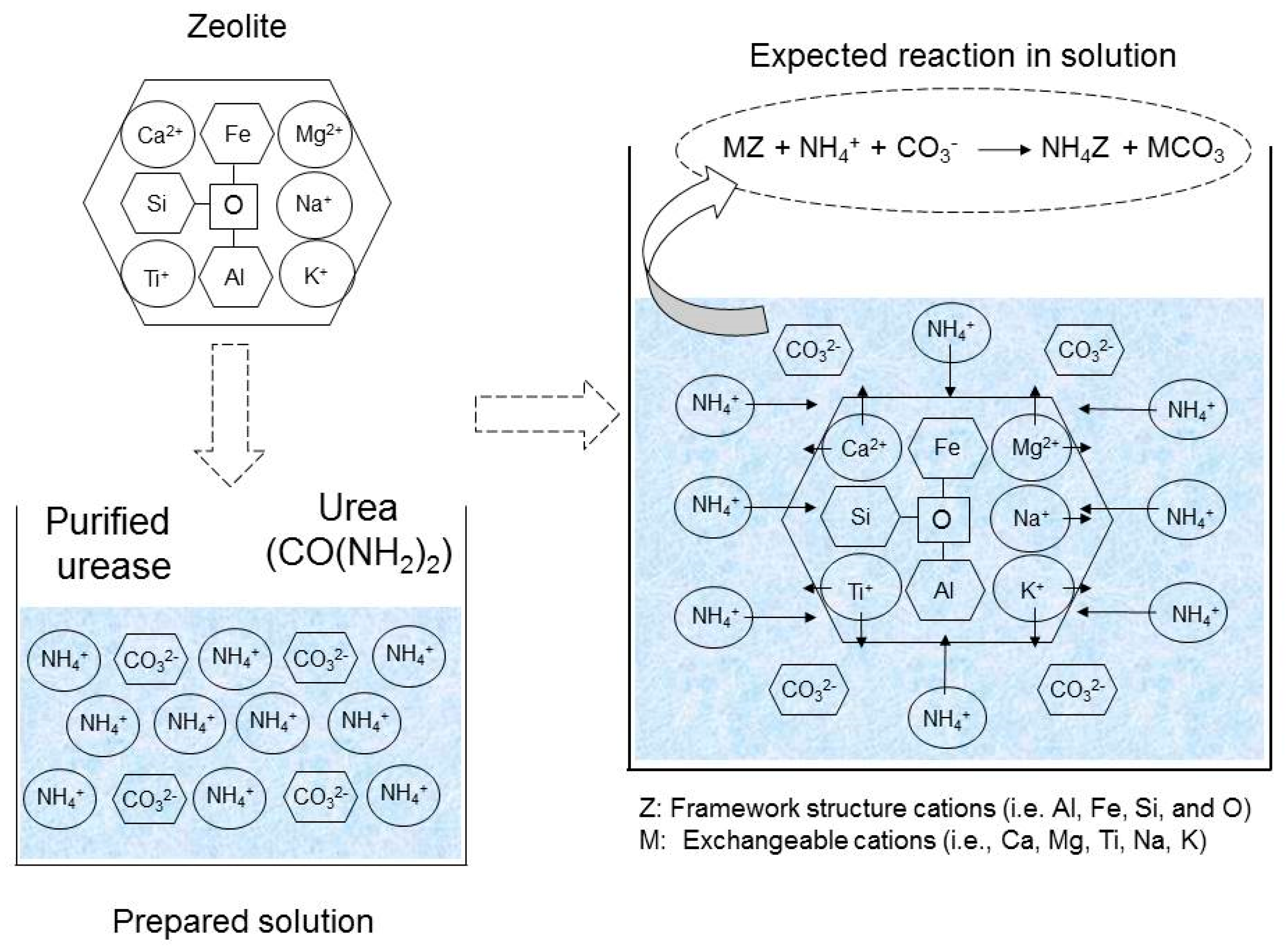
2. Materials and Methods
2.1. Zeolite
2.2. Grouting Materials
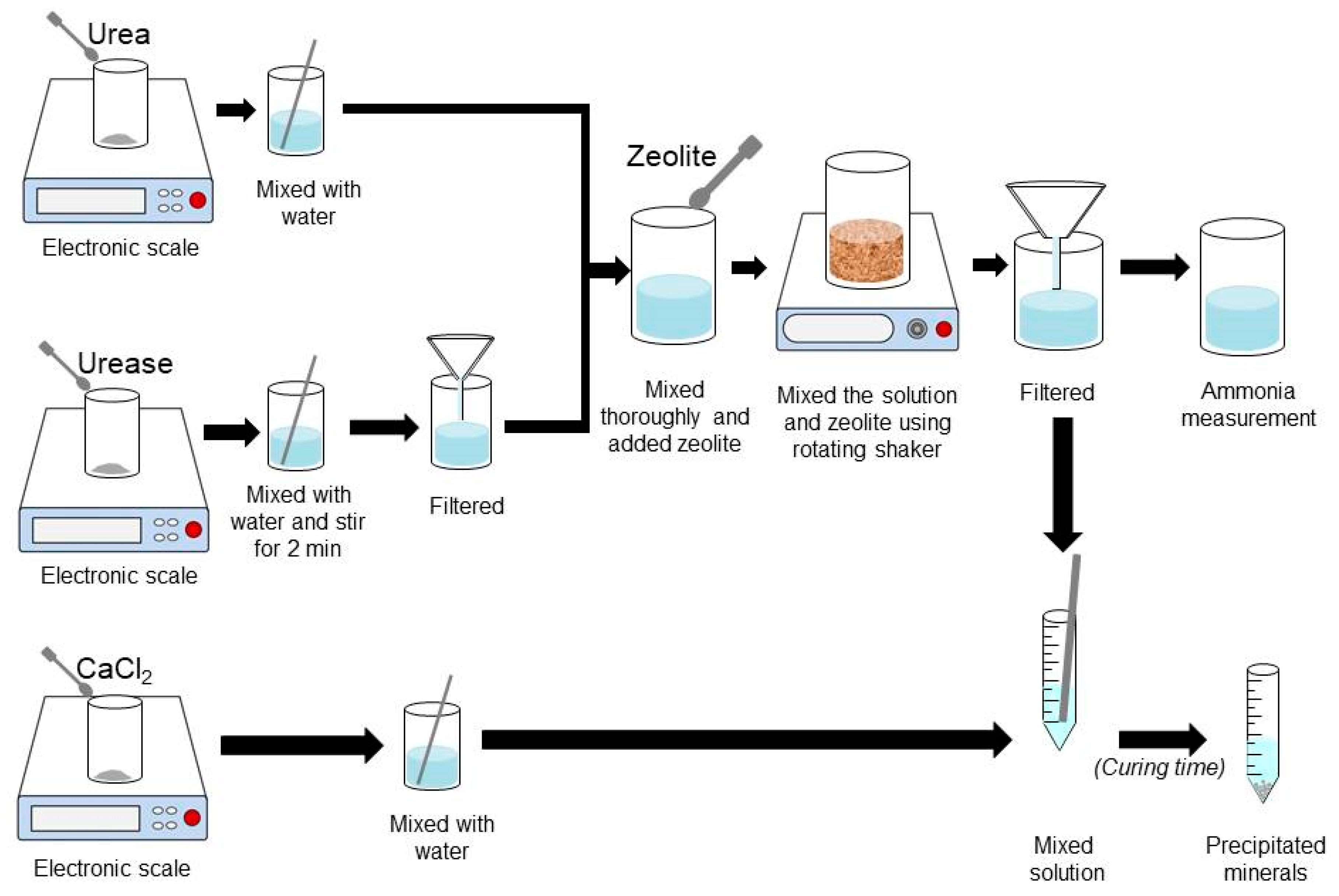
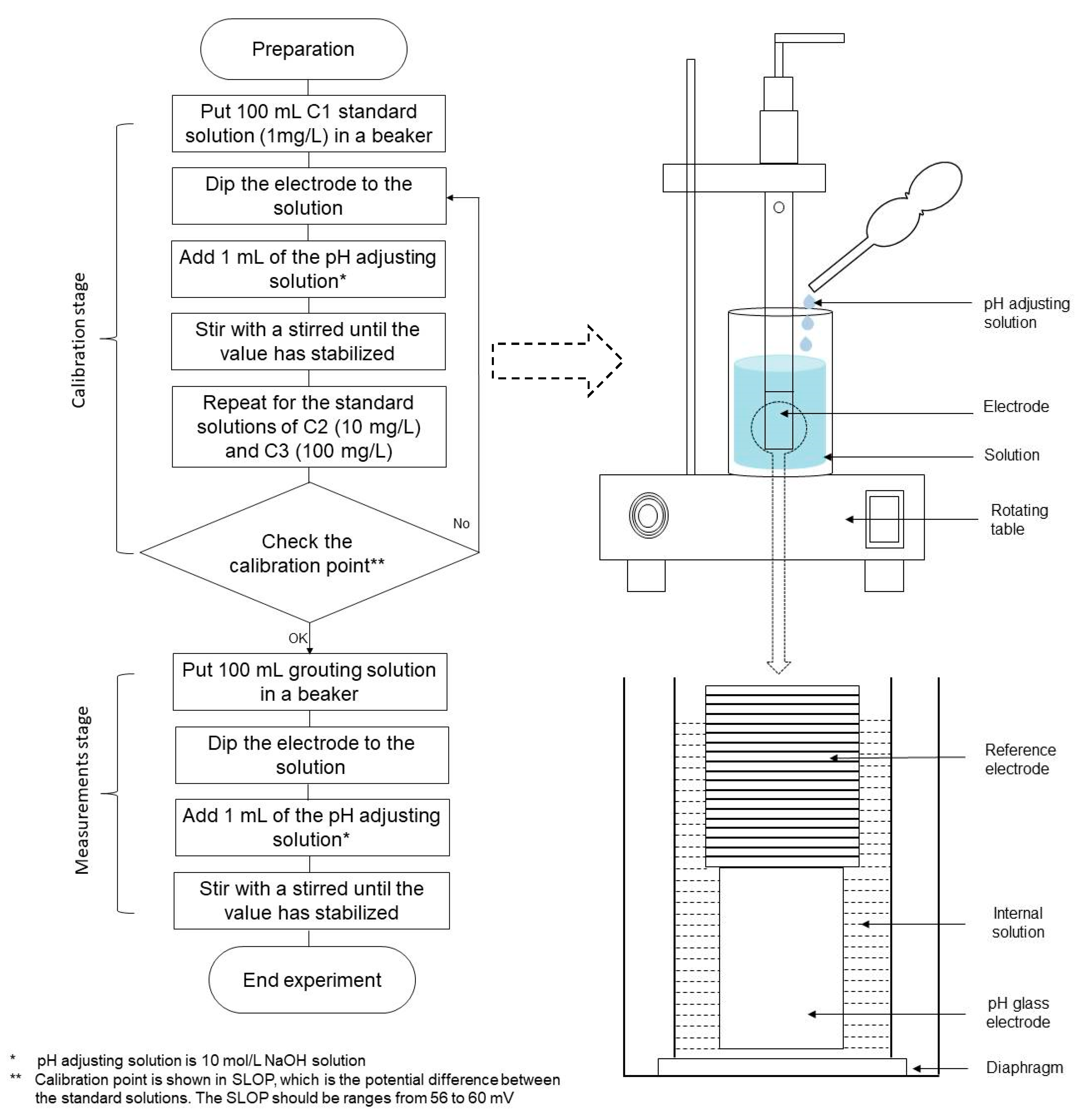
2.3. Experimental Procedures
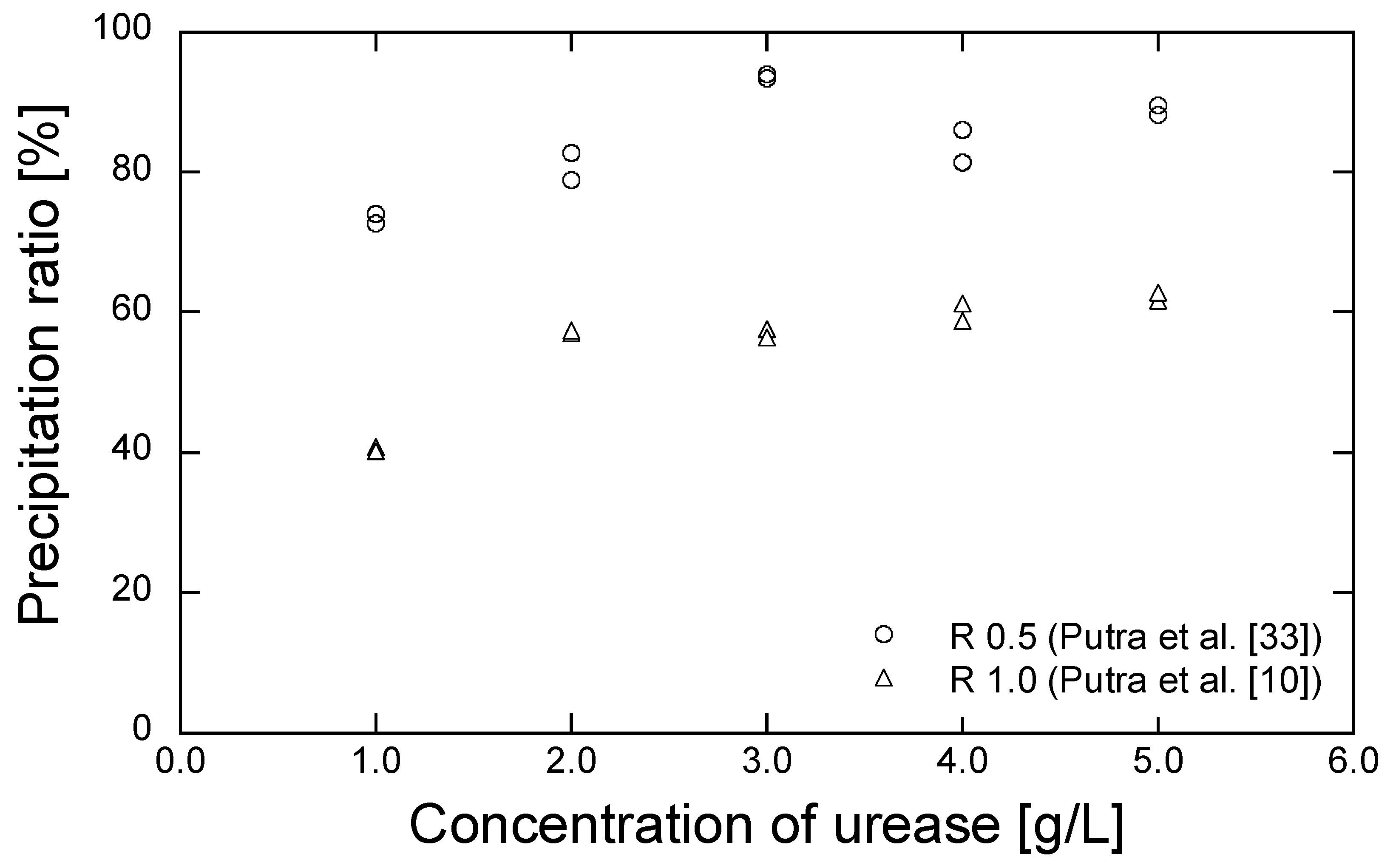
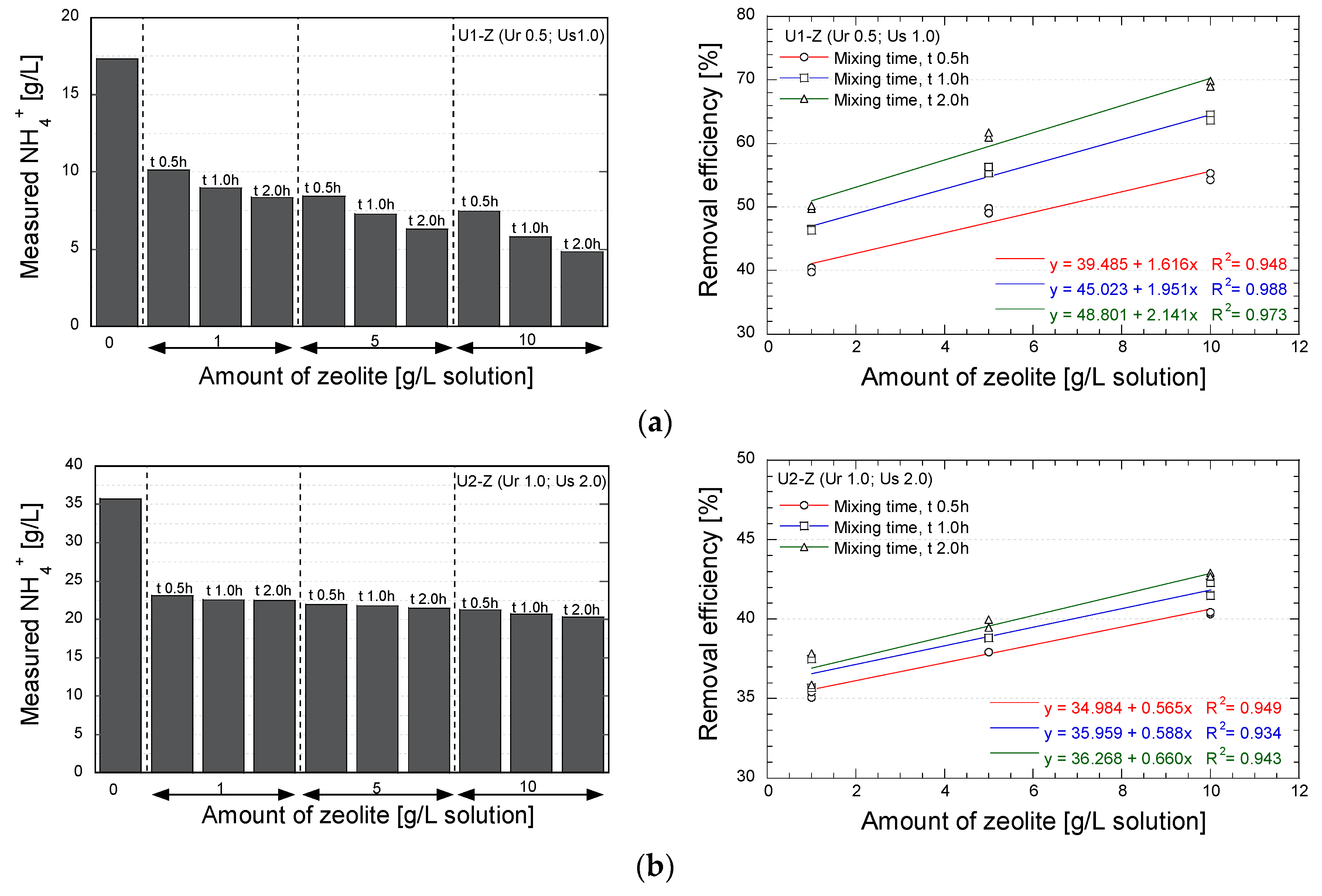
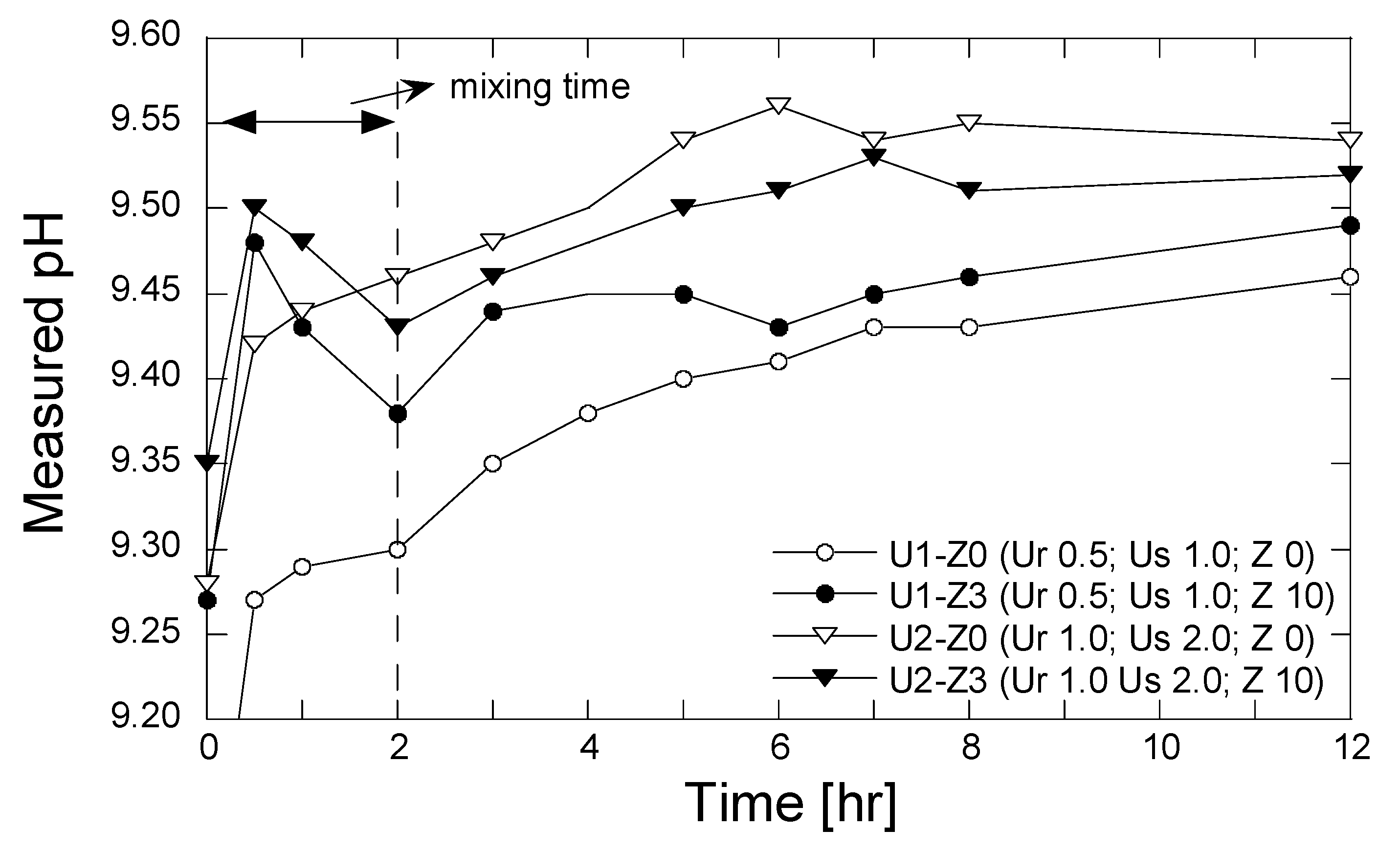
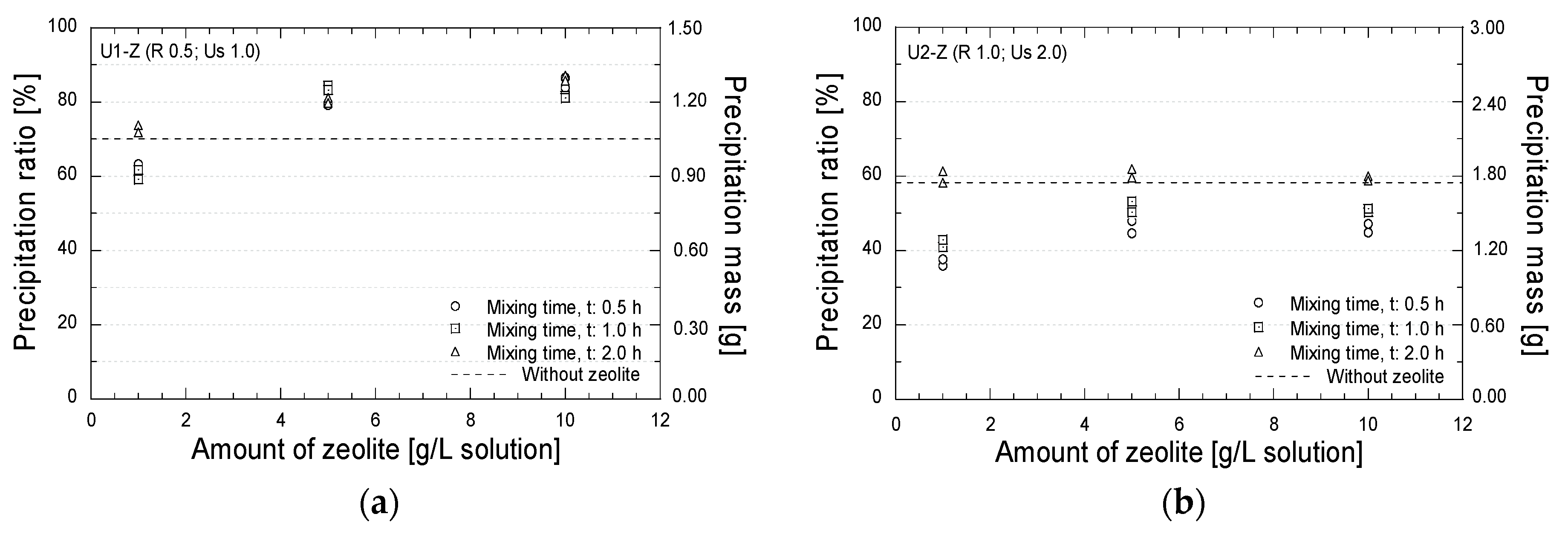
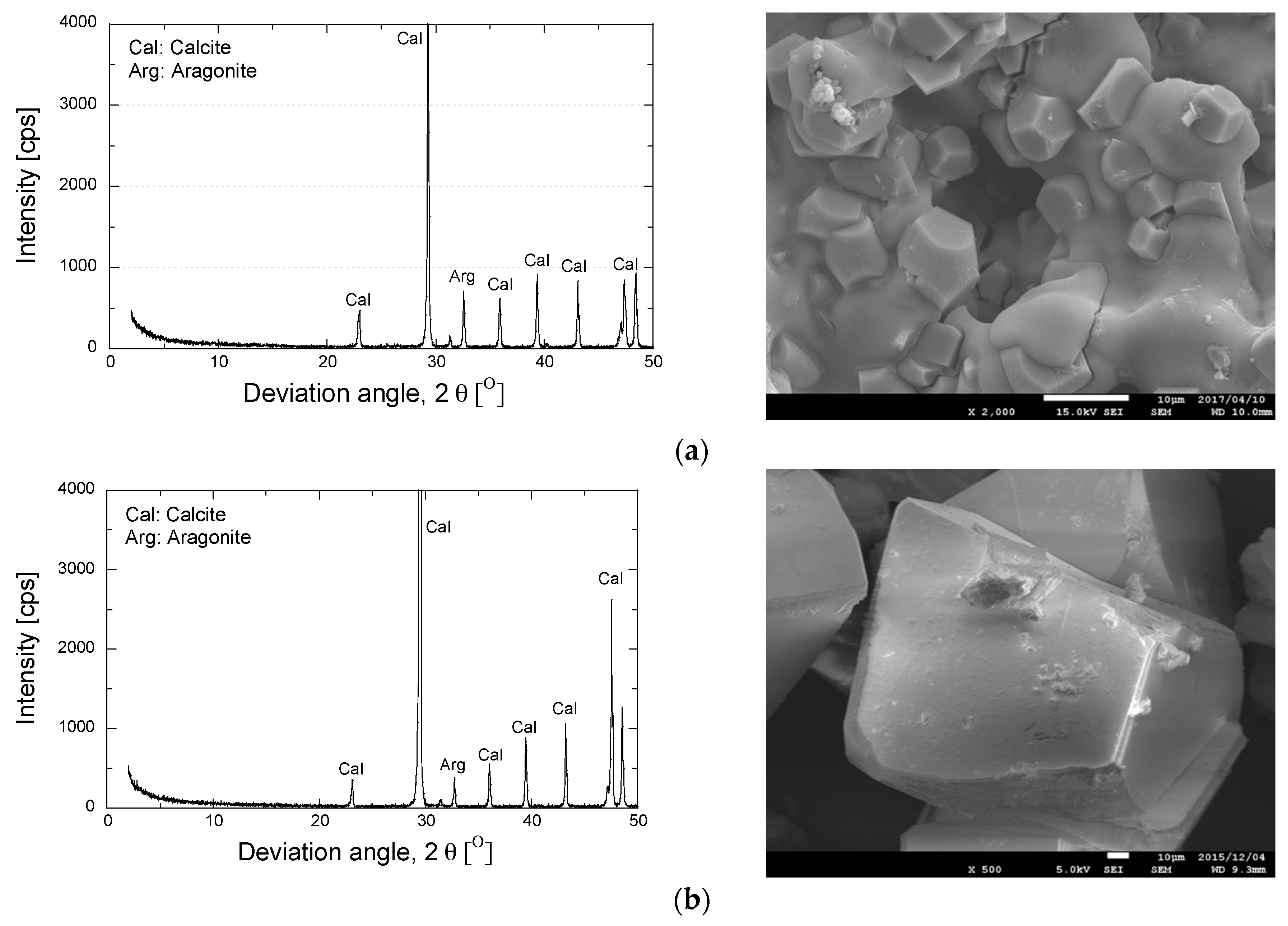
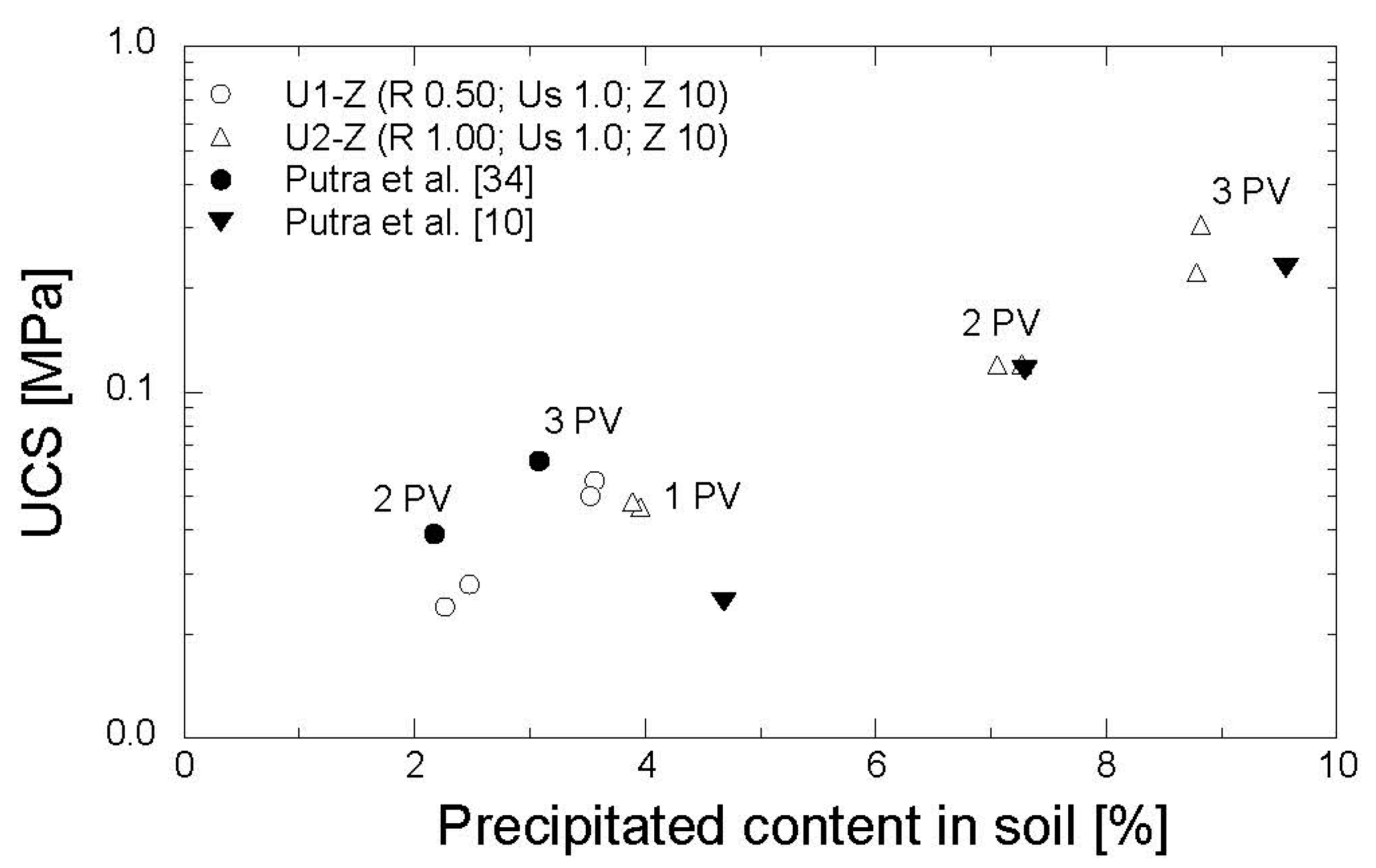
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martinez, B.C.; DeJong, J.T.; Ginn, T.R.; Montoya, B.M.; Barkouki, T.H.; Hunt, C.; Tanyu, B.; Major, D. Experimental Optimization of Microbial-Induced Carbonate Precipitation for Soil Improvement. J. Geotech. Geoenviron. Eng. 2013, 139, 587–598. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Van Paassen, L.A.; Daza, C.M.; Staal, M.; Sorokin, D.Y.; van der Zon, W.; van Loosdrecht, C.M. Potential soil reinforcement by biological denitrification. Ecol. Eng. 2010, 36, 168–175. [Google Scholar] [CrossRef]
- Yasuhara, H.; Neupane, D.; Hayashi, K.; Okamura, M. Experiments and predictions of physical properties of sand cemented by enzymatically-induced carbonate precipitation. Soils Found. 2012, 52, 539–549. [Google Scholar] [CrossRef]
- Neupane, D.; Yasuhara, H.; Kinoshita, N.; Unno, T. Applicability of Enzymatic Calcium Carbonate Precipitation as a Soil-Strengthening Technique. J. Geotech. Geoenviron. Eng. 2013, 139, 2201–2211. [Google Scholar] [CrossRef]
- Okamura, M.; Ishihara, M.; Tamura, K. Degree of Saturation and Liquefaction Resistances of Sand Improved with Sand Compaction Pile. J. Geotech. Geoenviron. Eng. 2006, 132. [Google Scholar] [CrossRef]
- Ivanov, V.; Stabnikov, V. Construction Biotechnology; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Kavazanjian, E.; Hamdan, N. Enzyme Induced Carbonate Precipitation (EICP) Columns for Ground Improvement. In IFCEE 2015; American Society of Civil Engineers: Reston, VA, USA, 2015; pp. 2252–2261. [Google Scholar] [CrossRef]
- Putra, H.; Yasuhara, H.; Kinoshita, N.; Hirata, A. Application of magnesium to improve uniform distribution of precipitated minerals in 1-m column specimens. Geomech. Eng. 2017, 12, 803–813. [Google Scholar] [CrossRef]
- Putra, H.; Yasuhara, H.; Kinoshita, N.; Hirata, A. Optimization of Enzyme-Mediated Calcite Precipitation as a Soil-Improvement Technique: The Effect of Aragonite and Gypsum on the Mechanical Properties of Treated Sand. Crystals 2017, 7, 59. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Ferris, F.G. The coprecipitation of Sr into calcite precipitates induced by bacterial ureolysis in artificial groundwater: Temperature and kinetic dependence. Geochim. Cosmochim. Acta 2005, 69, 4199–4210. [Google Scholar] [CrossRef]
- Ferris, F.; Stehmeier, L.; Kantzas, A.; Mourits, F. Bacteriogenic mineral plugging. J. Can. Pet. Technol. 1996, 35, 56–61. [Google Scholar] [CrossRef]
- Li, M.; Fu, Q.-L.; Zhang, Q.; Achal, V.; Kawasaki, S. Bio-grout based on microbially induced sand solidification by means of asparaginase activity. Sci. Rep. 2015, 5, 16128. [Google Scholar] [CrossRef] [PubMed]
- Soon, N.W.; Lee, L.M.; Khun, T.C.; Ling, H.S. Factors Affecting Improvement in Engineering Properties of Residual Soil through Microbial-Induced Calcite Precipitation. J. Geotech. Geoenviron. Eng. 2014, 140, 4014006. [Google Scholar] [CrossRef]
- Ministry of the Environment of Japan. Water Pollution Control Law; Ministry of the Environment of Japan: Tokyo, Japan, 1970.
- Lin, L.; Yuan, S.; Chen, J.; Xu, Z.; Lu, X. Removal of ammonia nitrogen in wastewater by microwave radiation. J. Hazard. Mater. 2009, 161, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.A.; Abdel-Rahim, M.; Lotfy, A.M.; Abdelaty, B.S.; Sallam, G.M. The Applicability of Activated Carbon, Natural Zeolites, and Probiotics (EM®) and Its Effects on Ammonia Removal Efficiency and Fry Performance of European Seabass Dicentrarchus labrax. J. Aquac. Res. Dev. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.; Song, C.; Li, L.; Zhou, Y. The Mechanism and Performance of Zeolites for Ammonia Removal in the Zeolite Packed Electrolysis Reactor. Electrochemistry 2014, 82, 557–560. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, X.; Yan, B.; Yang, L. Ammonium removal from aqueous solutions by using natural Chinese (Chende) zeolite as adsorbent. J. Hazard. Mater. 2010, 175, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, T.C.; Weatherley, L.R. Ammonia removal from wastewater by ion exchange in the presence of organic contaminants. Water Res. 2003, 37, 1723–1728. [Google Scholar] [CrossRef]
- Karadag, D.; Koc, Y.; Turan, M.; Armagan, B. Removal of ammonium ion from aqueous solution using natural Turkish clinoptilolite. J. Hazard. Mater. 2006, 136, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Saltali, K.; Sari, A.; Aydin, M. Removal of ammonium ion from aqueous solution by natural Turkish (Yildizeli) zeolite for environmental quality. J. Hazard. Mater. 2007, 141, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Chung, Y.C.; Shin, H.S.; Son, D.H. Enhanced ammonia nitrogen removal using consistent biological regeneration and ammonium exchange of zeolite in modified SBR process. Water Res. 2004, 38, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Wang, Y.; Kmiya, Y.; Okuhara, T. Removal of low-concentration ammonia in water by ion-exchange using Na-mordenite. Water Res. 2007, 41, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; An, Y.; Wang, Z.; Yang, S.; Chen, H.; Zhou, Z.; Mai, S. Study on zeolite enhanced contact-adsorption regeneration-stabilization process for nitrogen removal. J. Hazard. Mater. 2008, 156, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari-Hosseini, Z.; Kazemiyan, E.; Tayebee, R.; Shenavaei-Zare, T. Optimization of ammonia removal by natural zeolite from aqueous solution using response surface methodology. Hem. Ind. 2016, 70, 21–29. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Tanner, C.C. Ammonium removal from wastewaters using natural New Zealand zeolites. N. Z. J. Agric. Res. 1998, 41, 427–446. [Google Scholar] [CrossRef]
- Rozic, M.; Cerjan-Stefanovic, S.; Kurajica, S.; Vancina, V.; Hodzic, E. Ammoniacal nitrogen removal from water by treatmenre with clays and zeolites. Water Res. 2000, 34, 3675–3681. [Google Scholar] [CrossRef]
- Margeta, K.; Zabukovec Logar, N.; Šiljeg, M.; Farkas, A. Natural Zeolites in Water Treatment—How Effective is Their Use. In Water Treat; Elshorbagy, W., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Hitachi. Analysis Report of Zeolite; Hitachi: Yasugi, Japan, 2016. [Google Scholar]
- Abdel-Rahim, M. Sustainable Use of Natural Zeolites in Aquaculture: A Short Review. Oceanogr. Fish. 2017, 2. [Google Scholar] [CrossRef]
- Putra, H.; Yasuhara, H.; Kinoshita, N.; Neupane, D. Optimization of Calcite Precipitation as a Soil Improvement Technique. In Proceedings of the 2nd Makassar International Conference on Civil Engineering, Makassar, Indonesia, 11–12 August 2015; pp. 9–14. [Google Scholar] [CrossRef]
- Putra, H.; Yasuhara, H.; Kinoshita, N.; Neupane, D.; Lu, C.-W. Effect of Magnesium as Substitute Material in Enzyme-Mediated Calcite Precipitation for Soil-Improvement Technique. Front. Bioeng. Biotechnol. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Toko Chemical Laboratories, Handy Ion Meter TiN-9001 Instruction Manual, 2010; Toko Chemical Laboratories Co., Ltd.: Tokyo, Japan, 2010.
- Yasuhara, H.; Hayashi, K.; Okamura, M. Evolution in Mechanical and Hydraulic Properties of Calcite-Cemented Sand Mediated by Biocatalyst. In Geo-Frontiers 2011 ©; ASCE: Reston, VA, USA, 2011; pp. 3984–3992. [Google Scholar]
- Lee, W.K.W.; van Deventer, J.S.J. The effect of ionic contaminants on the early-age properties of alkali-activated fly ash-based cements. Cem. Concr. Res. 2002, 32, 577–584. [Google Scholar] [CrossRef]


| Chemical | Content (%) |
|---|---|
| SiO2 | 68.20 |
| Al2O3 | 11.63 |
| Fe2O3 | 1.25 |
| MnO | 0.01 |
| CaO | 1.15 |
| MgO | 0.49 |
| Na2O | 2.77 |
| K2O | 1.24 |
| TiO4 | 0.22 |
| Ignition loss * | 13.20 |
| Parameter | Grouting Material | |
|---|---|---|
| R 0.5 | R 1.0 | |
| Conc. of reagent (mol/L) | ||
| Urea | 0.50 | 1.00 |
| CaCl2 | 0.50 | 1.00 |
| Conc. of urease (g/L) | 1.00 | 2.00 |
| Precipitation amount | ||
| Mass (g) | 1.10 | 1.72 |
| Ratio (%) | 73.45 | 57.15 |
| Sample (-) | Reagent, R (mol/L) | Urease, Us (g/L) | Zeolite (g/L Solution) | Mixing time for Zeolite, t (h) | |
|---|---|---|---|---|---|
| Urea, Ur | CaCl2, Ca * | ||||
| U1-Z0 | 0.5 | 0.5 | 1.0 | - | - |
| U2-Z0 | 1.0 | 1.0 | 2.0 | - | - |
| U1-Z1.1 | 1.0 | 0.5 | |||
| U1-Z1.2 | 0.5 | 0.5 | 1.0 | 1.0 | 1.0 |
| U1-Z1.3 | 1.0 | 2.0 | |||
| U1-Z2.1 | 5.0 | 0.5 | |||
| U1-Z2.2 | 0.5 | 0.5 | 1.0 | 5.0 | 1.0 |
| U1-Z2.3 | 5.0 | 2.0 | |||
| U1-Z3.1 | 10.0 | 0.5 | |||
| U1-Z3.2 | 0.5 | 0.5 | 1.0 | 10.0 | 1.0 |
| U1-Z3.3 | 10.0 | 2.0 | |||
| U2-Z1.1 | 1.0 | 0.5 | |||
| U2-Z1.2 | 1.0 | 1.0 | 2.0 | 1.0 | 1.0 |
| U2-Z1.3 | 1.0 | 2.0 | |||
| U2-Z2.1 | 5.0 | 0.5 | |||
| U2-Z2.2 | 1.0 | 1.0 | 2.0 | 5.0 | 1.0 |
| U2-Z2.3 | 5.0 | 2.0 | |||
| U2-Z3.1 | 10.0 | 0.5 | |||
| U2-Z3.2 | 1.0 | 1.0 | 2.0 | 10.0 | 1.0 |
| U2-Z3.3 | 10.0 | 2.0 | |||
| Sample (-) | Reagent, R (mol/L) | Urease, Us (g/L) | Zeolite, Z (g/L solution) | Mixing Time, t (h) | Number of Injections (PV) |
|---|---|---|---|---|---|
| U1-Z | 0.5 | 1.0 | 10 | 2.0 | 2 and 3 |
| U2-Z | 1.0 | 2.0 | 10 | 2.0 | 1, 2, and 3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putra, H.; Yasuhara, H.; Kinoshita, N. Applicability of Natural Zeolite for NH-Forms Removal in Enzyme-Mediated Calcite Precipitation Technique. Geosciences 2017, 7, 61. https://doi.org/10.3390/geosciences7030061
Putra H, Yasuhara H, Kinoshita N. Applicability of Natural Zeolite for NH-Forms Removal in Enzyme-Mediated Calcite Precipitation Technique. Geosciences. 2017; 7(3):61. https://doi.org/10.3390/geosciences7030061
Chicago/Turabian StylePutra, Heriansyah, Hideaki Yasuhara, and Naoki Kinoshita. 2017. "Applicability of Natural Zeolite for NH-Forms Removal in Enzyme-Mediated Calcite Precipitation Technique" Geosciences 7, no. 3: 61. https://doi.org/10.3390/geosciences7030061