Comparison of the Mineral Element Content of Public Drinking Fountains and Bottled Water: A Case Study of Ferrara City
Abstract
:1. Introduction
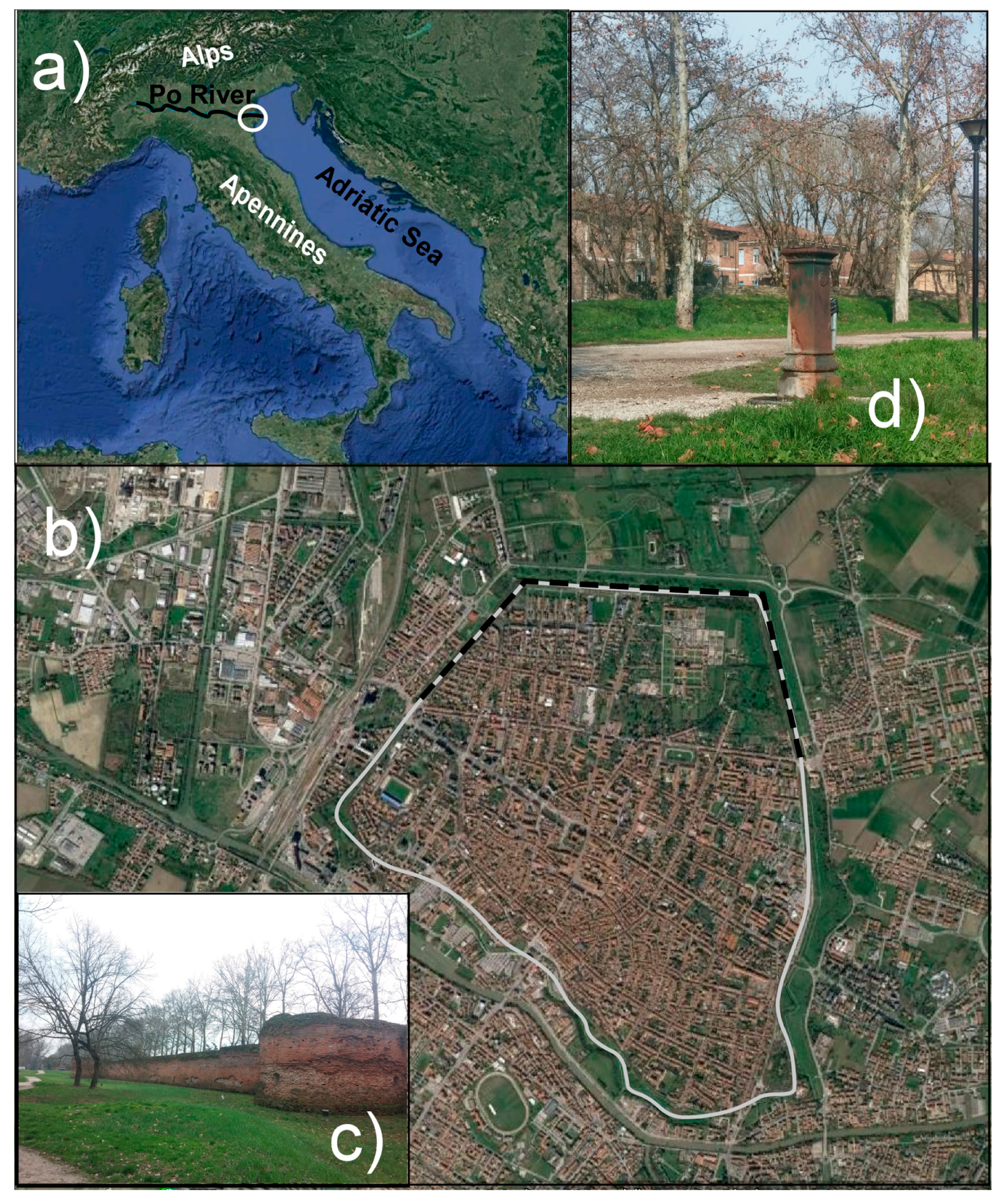
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Maughan, R.J. Role of micronutrients in sport and physical activity. Br. Med. Bull. 1999, 55, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Speich, M.; Pineau, A.; Ballereau, F. Minerals, trace elements and related biological variables in athletes and during physical activity. Clin. Chim. Acta 2001, 312, 1–11. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Lukaski, H.C. Magnesium, zinc, chromium nutrition and athletic performance. Can. J. Appl. Physiol. 2001, 26, S13–S22. [Google Scholar] [CrossRef] [PubMed]
- Kara, E.; Mustafa, A.; Yalçinkaya, O. The effect of aerobic exercise programme on trace element levels of young men. Afr. J. Microbiol. Res. 2012, 6, 165–168. [Google Scholar]
- Azoulay, A.; Garzon, P.; Eisenberg, M.J. Comparison of the mineral content of tap water and bottled waters. J. Gen. Intern. Med. 2001, 16, 168–175. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics. Clinical Report–Sports Drinks and Energy Drinks for Children and Adolescents: Are They Appropriate? Pediatrics 2011, 127, 1182–1189. [Google Scholar]
- Milosevic, A. Sports drinks hazard to teeth. Br. J. Sports Med. 1997, 31, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S.; Vanderkooy, P.S.; McLennan, C.E.; Mercer, N.M. Contribution of tap water to mineral intakes of Canadian preschool children. Arch. Environ. Health 1987, 42, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Doria, M.F.; Pidgeon, N.; Hunter, P.R. Perceptions of drinking water quality and risk and its effect on behaviour: A cross-national study. Sci. Total Environ. 2009, 407, 5455–5464. [Google Scholar] [CrossRef] [PubMed]
- Botto, S.; Niccolucci, V.; Rugani, B.; Nicolardi, V.; Bastianoni, S.; Gaggi, C. Towards lower Carbon Footprint patterns of consumption: The case of drinking water in Italy. Environ. Sci. Policy 2011, 14, 388–395. [Google Scholar] [CrossRef]
- Cidu, R.; Frau, F.; Tore, P. Drinking water quality: Comparing inorganic components in bottled water and Italian tap water. J. Food Compos. Anal. 2011, 24, 184–193. [Google Scholar] [CrossRef]
- Carstea, M.E.; Levei, E.A.; Hoaghia, M.A.; Savastru, R. Quality assessment of Romanian bottled mineral water and tap water. Environ. Monit. Assess. 2016, 188, 521. [Google Scholar] [CrossRef] [PubMed]
- Saylor, A.; Prokopy, L.S.; Amberg, S. What’s wrong with the tap? Examining perceptions of tap water and bottled water at Purdue University. Environ. Manag. 2011, 48, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Saenz, L.; Irigoyen, M.; Benavides, J.; Mendoza, M. Tap or bottled water: Drinking preferences among urban minority children and adolescents. J. Community Health 2012, 37, 54–58. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, O.; Quaranta, A.; Lovero, G.; Caggiano, G.; Montagna, M.T. Mineral water or tap water? An endless debate. Ann. Ig. 2015, 27, 58–65. [Google Scholar] [PubMed]
- Lagioia, G.; Calabrò, G.; Amicarellia, V. Empirical study of the environmental management of Italy’s drinking water supply. Resour. Conserv. Recycl. 2012, 60, 119–130. [Google Scholar] [CrossRef]
- Torretta, V. Environmental and economic aspects of water kiosks: Case study of a medium-sized Italian town. Waste Manag. 2013, 33, 1057–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, P.; Bernardes, A.M. Carbon emissions and embodied energy as tools for evaluating environmental aspects of tap water and bottled water in Brazil. Desalination Water Treat. 2016, 57, 13020–13029. [Google Scholar] [CrossRef]
- Carson, H.S. The incidence of plastic ingestion by fishes: From the prey’s perspective. Mar. Pollut. Bull. 2013, 74, 170–174. [Google Scholar] [CrossRef] [PubMed]
- National Academy of Sciences. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; The National Academies Press: Washington, DC, USA, 2005; p. 638. [Google Scholar]
- Van der Aa, M. Classification of mineral water types and comparison with drinking water standards. Environ. Geol. 2003, 44, 554–563. [Google Scholar] [CrossRef] [Green Version]
- Cuoco, E.; Colombani, N.; Darrah, T.H.; Mastrocicco, M.; Tedesco, D. Geolithological and anthropogenic controls on the hydrochemistry of the Volturno River (Southern Italy). Hydrol. Process. 2017, 31, 627–638. [Google Scholar] [CrossRef]
- Rosborg, I.; Nihlgård, B.; Ferrante, M. Mineral Composition of Drinking Water and Daily Uptake. In Drinking Water Minerals and Minerals Balance Importance, Health Significance, Safety Precautions; Rosborg, I., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 25–30. [Google Scholar]
- Dinelli, E.; Lima, A.; De Vivo, B.; Albanese, S.; Cicchella, D.; Valera, P. Hydrogeochemical analysis on Italian bottled mineral waters: Effects of geology. J. Geochem. Explor. 2010, 107, 317–335. [Google Scholar] [CrossRef]
- Convertino, V.A.; Armstrong, L.E.; Coyle, E.F.; Mackm, G.W.; Sawka, M.N.; Senay, L.C.; Sherman, W.M. American College of Sports Medicine position stand. Exercise and fluid replacement. Med. Sci. Sports Exerc. 1996, 28, i–vii. [Google Scholar] [CrossRef] [PubMed]
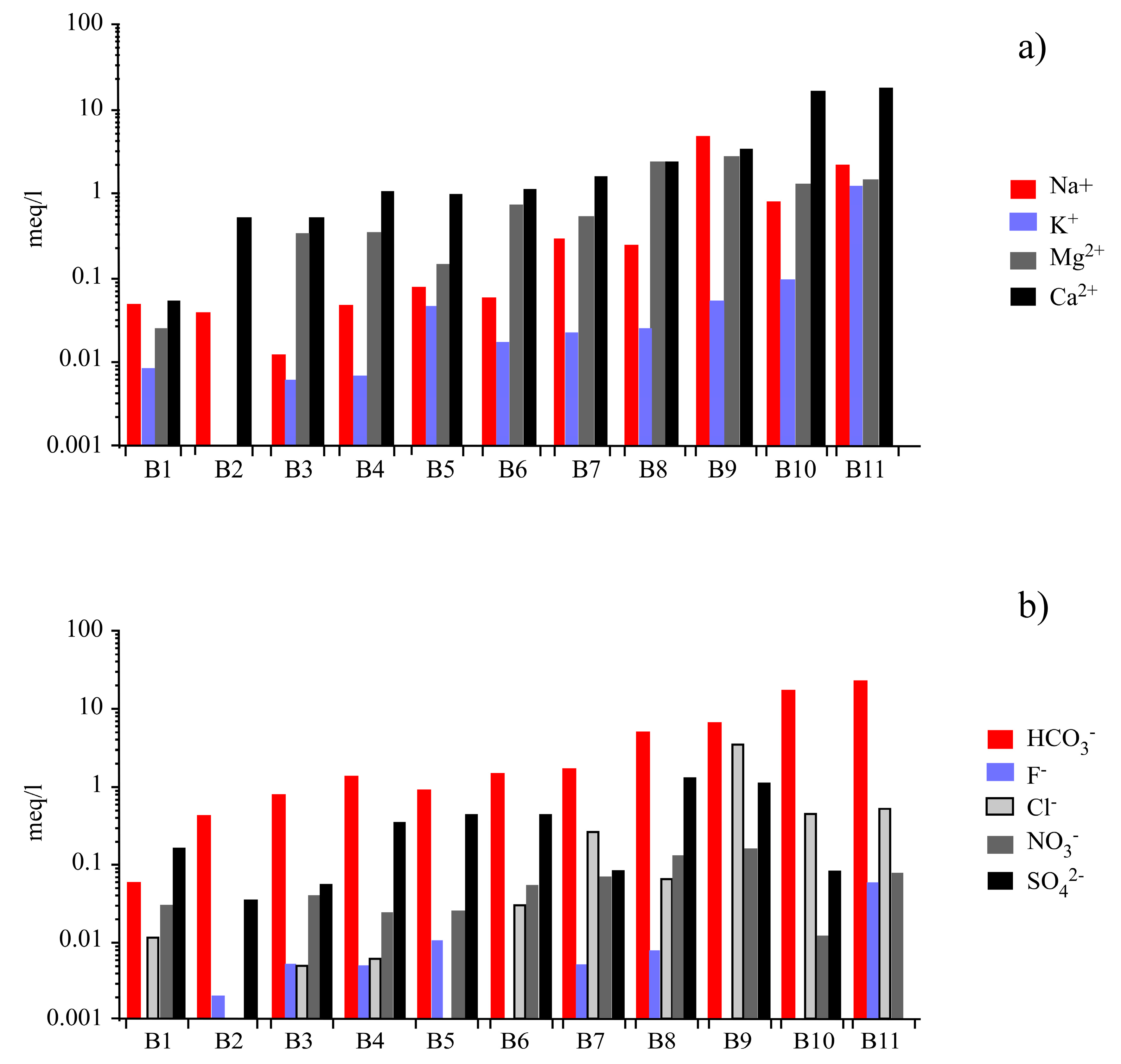
| Samples | TDS | EC | pH | HCO3− | F− | Cl− | NO3− | SO42− | ΣAnion | Na+ | K+ | Mg2+ | Ca2+ | ΣCation | Charge Imbalance |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | µS/cm | - | mg/L | mg/L | mg/L | mg/L | mg/L | meq/L | mg/L | mg/L | mg/L | mg/L | meq/L | % | |
| Group 1 | |||||||||||||||
| B1 | 14.0 | 17.6 | 5.82 | 3.60 | bdl | 0.4 | 1.90 | 1.40 | 0.13 | 1.10 | 0.33 | 0.31 | 1.05 | 0.13 | 1.33 |
| B2 | 39.2 | 65.8 | 7.40 | 26.2 | 0.04 | bdl | bdl | 7.8 | 0.59 | 0.90 | bdl | bdl | 10.5 | 0.56 | −2.66 |
| B3 | 47.0 | 71.0 | 6.90 | 48.8 | 0.10 | 0.2 | 2.50 | 1.7 | 0.89 | 0.28 | 0.25 | 4.00 | 10.2 | 0.86 | −1.68 |
| B4 | 69.0 | 119 | 8.34 | 84.0 | 0.10 | 0.22 | 1.50 | 2.80 | 1.47 | 1.10 | 0.27 | 4.20 | 21.5 | 1.47 | 0.08 |
| B5 | 80.5 | 124 | 7.70 | 57.1 | 0.20 | bdl | 1.60 | 16.9 | 1.32 | 1.90 | 1.85 | 1.73 | 21.0 | 1.32 | −0.15 |
| Group 2 | |||||||||||||||
| B6 | 114 | 178 | 8.18 | 94.6 | bdl | 1.1 | 3.40 | 22.0 | 2.09 | 1.30 | 0.70 | 8.70 | 23.8 | 1.98 | −2.87 |
| B7 | 142 | 223 | 7.90 | 106 | 0.10 | 9.0 | 4.30 | 21.1 | 2.50 | 6.40 | 0.92 | 6.52 | 32.9 | 2.48 | −0.51 |
| B8 | 272 | 415 | 7.42 | 301 | 0.15 | 2.4 | 8.50 | 4.1 | 5.23 | 5.80 | 1.02 | 29.4 | 48.6 | 5.12 | −1.06 |
| Group 3 | |||||||||||||||
| B9 | 695 | 1065 | 7.57 | 395 | bdl | 120 | 10.0 | 64.2 | 11.4 | 112 | 2.10 | 33.1 | 70.1 | 11.1 | −0.96 |
| B10 | 988 | 1513 | 6.41 | 1021 | bdl | 16.3 | 0.76 | 55.2 | 18.4 | 19.6 | 3.79 | 15.2 | 325 | 18.4 | 0.19 |
| B11 | 1283 | 1800 | 6.20 | 1430 | 1.13 | 19.0 | 5.00 | 4.0 | 24.2 | 49.0 | 49.0 | 18.0 | 365 | 23.1 | −2.36 |
| Samples | TDS | EC | pH | HCO3− | F− | Cl− | NO3− | SO42− | ΣAnion | Na+ | K+ | Mg2+ | Ca2+ | ΣCation | Charge Imbalance |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | µS/cm | - | mg/L | mg/L | mg/L | mg/L | mg/L | meq/L | mg/L | mg/L | mg/L | mg/L | meq/L | % | |
| F1 | 337 | 436 | 7.89 | 179 | 0.09 | 29.4 | 8.35 | 33.9 | 4.61 | 16.1 | 2.82 | 11.5 | 58.0 | 4.61 | 0.07 |
| F2 | 312 | 407 | 7.75 | 164 | 0.14 | 28.1 | 7.44 | 31.3 | 4.26 | 14.0 | 2.47 | 10.3 | 53.2 | 4.17 | −1.05 |
| F3 | 315 | 414 | 7.91 | 163 | 0.14 | 28.6 | 7.75 | 31.5 | 4.26 | 14.4 | 2.49 | 10.3 | 52.7 | 4.16 | −1.22 |
| F4 | 340 | 441 | 7.97 | 183 | 0.09 | 29.6 | 7.42 | 34.0 | 4.67 | 14.6 | 2.49 | 10.5 | 58.0 | 4.46 | −2.30 |
| F5 | 303 | 353 | 7.95 | 183 | 0.12 | 22.9 | 7.45 | 33.5 | 4.47 | 14.3 | 2.45 | 12.0 | 59.1 | 4.62 | 1.67 |
| F6 | 321 | 423 | 7.87 | 166 | 0.10 | 29.6 | 7.25 | 32.3 | 4.35 | 14.9 | 2.49 | 10.3 | 52.8 | 4.19 | −1.82 |
| F7 | 317 | 421 | 7.67 | 162 | 0.20 | 29.3 | 7.06 | 31.7 | 4.27 | 14.8 | 2.53 | 10.2 | 51.3 | 4.11 | −1.90 |
| F8 | 337 | 450 | 7.71 | 171 | 0.09 | 30.9 | 7.53 | 34.7 | 4.52 | 15.8 | 2.70 | 11.0 | 54.6 | 4.38 | −1.55 |
| F9 | 326 | 431 | 7.88 | 168 | 0.10 | 29.2 | 7.54 | 34.0 | 4.41 | 15.1 | 2.61 | 10.6 | 53.4 | 4.26 | −1.80 |
| F10 | 325 | 436 | 7.8 | 164 | 0.10 | 29.7 | 7.21 | 32.7 | 4.33 | 15.0 | 2.50 | 10.5 | 51.8 | 4.16 | −1.94 |
| F11 | 339 | 441 | 7.71 | 185 | 0.11 | 29.1 | 6.82 | 31.7 | 4.63 | 15.2 | 2.47 | 12.1 | 56.1 | 4.50 | −1.41 |
| F12 | 353 | 442 | 7.82 | 196 | 0.11 | 32.1 | 7.40 | 36.1 | 4.99 | 17.2 | 2.53 | 13.1 | 60.2 | 4.87 | −1.24 |
| F13 | 356 | 434 | 7.25 | 203 | 0.12 | 30.1 | 6.90 | 44.1 | 5.21 | 14.3 | 2.50 | 13.2 | 63.1 | 4.89 | −3.18 |
| F14 | 388 | 517 | 7.90 | 201 | 0.11 | 33.1 | 7.15 | 42.1 | 5.22 | 17.2 | 2.54 | 13.1 | 61.3 | 4.92 | −2.99 |
| F15 | 340 | 442 | 7.73 | 185 | 0.11 | 28.1 | 7.48 | 34.1 | 4.66 | 16.2 | 2.45 | 13.2 | 52.1 | 4.42 | −2.57 |
| F16 | 320 | 413 | 7.85 | 175 | 0.14 | 27.1 | 7.42 | 30.1 | 4.38 | 13.2 | 2.59 | 10.2 | 57.1 | 4.30 | −0.96 |
| F17 | 344 | 439 | 7.75 | 188 | 0.18 | 30.2 | 5.55 | 39.1 | 4.84 | 13.3 | 2.55 | 12.1 | 58.3 | 4.51 | −3.51 |
| F18 | 315 | 412 | 7.74 | 172 | 0.10 | 27.1 | 6.7 | 30.1 | 4.32 | 12.2 | 2.52 | 11.2 | 51.2 | 4.04 | −3.39 |
| F19 | 334 | 419 | 7.90 | 185 | 0.14 | 29.7 | 6.47 | 39.1 | 4.79 | 15.2 | 3.47 | 11.1 | 55.2 | 4.39 | −4.38 |
| F20 | 315 | 412 | 7.72 | 172 | 0.10 | 27.1 | 6.45 | 30.1 | 4.31 | 12.2 | 2.41 | 11.4 | 51.2 | 4.03 | −3.37 |
| Average | 332 | 429 | 7.79 | 178 | 0.12 | 29.1 | 7.17 | 34.3 | 4.57 | 14.8 | 2.58 | 11.4 | 55.5 | 4.40 | −1.94 |
| Maximun | 388 | 517 | 7.97 | 203 | 0.20 | 33.1 | 8.35 | 44.1 | 5.22 | 17.2 | 3.47 | 13.2 | 63.1 | 4.92 | 1.67 |
| Minimun | 303 | 353 | 7.25 | 162 | 0.09 | 22.9 | 5.55 | 30.1 | 4.26 | 12.2 | 2.41 | 10.2 | 51.2 | 4.03 | −4.38 |
| Standard deviation | 18.9 | 28.8 | 0.15 | 12.3 | 0.03 | 2.06 | 0.57 | 3.90 | 0.29 | 1.36 | 0.22 | 1.07 | 3.57 | 0.27 | 1.34 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Giuseppe, D. Comparison of the Mineral Element Content of Public Drinking Fountains and Bottled Water: A Case Study of Ferrara City. Geosciences 2017, 7, 76. https://doi.org/10.3390/geosciences7030076
Di Giuseppe D. Comparison of the Mineral Element Content of Public Drinking Fountains and Bottled Water: A Case Study of Ferrara City. Geosciences. 2017; 7(3):76. https://doi.org/10.3390/geosciences7030076
Chicago/Turabian StyleDi Giuseppe, Dario. 2017. "Comparison of the Mineral Element Content of Public Drinking Fountains and Bottled Water: A Case Study of Ferrara City" Geosciences 7, no. 3: 76. https://doi.org/10.3390/geosciences7030076





