Combination of mTOR and MAPK Inhibitors—A Potential Way to Treat Renal Cell Carcinoma
Abstract
:1. Introduction
2. Renal Cell Carcinoma
3. Etiology: Potential Risk Factors for Renal Cell Carcinoma
4. Classification, Staging and Grading
4.1. Clear Cell Carcinoma
4.2. Papillary Renal Cell Carcinoma
4.3. Chromophobe Rencal Cell Carcinoma
4.4. Collecting Duct Carcinoma
4.5. Unclassified Renal Cell Carcinoma
4.6. Tumor-Node-Metastasis Staging
4.7. Nuclear Grade
5. Diagnosis and Treatment
6. Molecular Markers
6.1. Diagnostic Biomarkers
6.1.1. MN/CA9 and Circulating Cell Detection Biomarkers
6.1.2. Urinary Biomarkers
6.1.3. Serum Nucleic Acids
6.1.4. Composite Biomarkers
6.2. Prognostic Biomarkers
6.2.1. Proliferative Index
6.2.2. Proliferative Biomarker
6.2.3. Adhesion Molecules and Proteases
6.2.4. Apoptotic Regulatory Proteins
6.3. Predictive Biomarkers
7. Treatments
7.1. Surgical Treatment
7.2. Systemic Treatment
8. Molecular Pathways in RCC
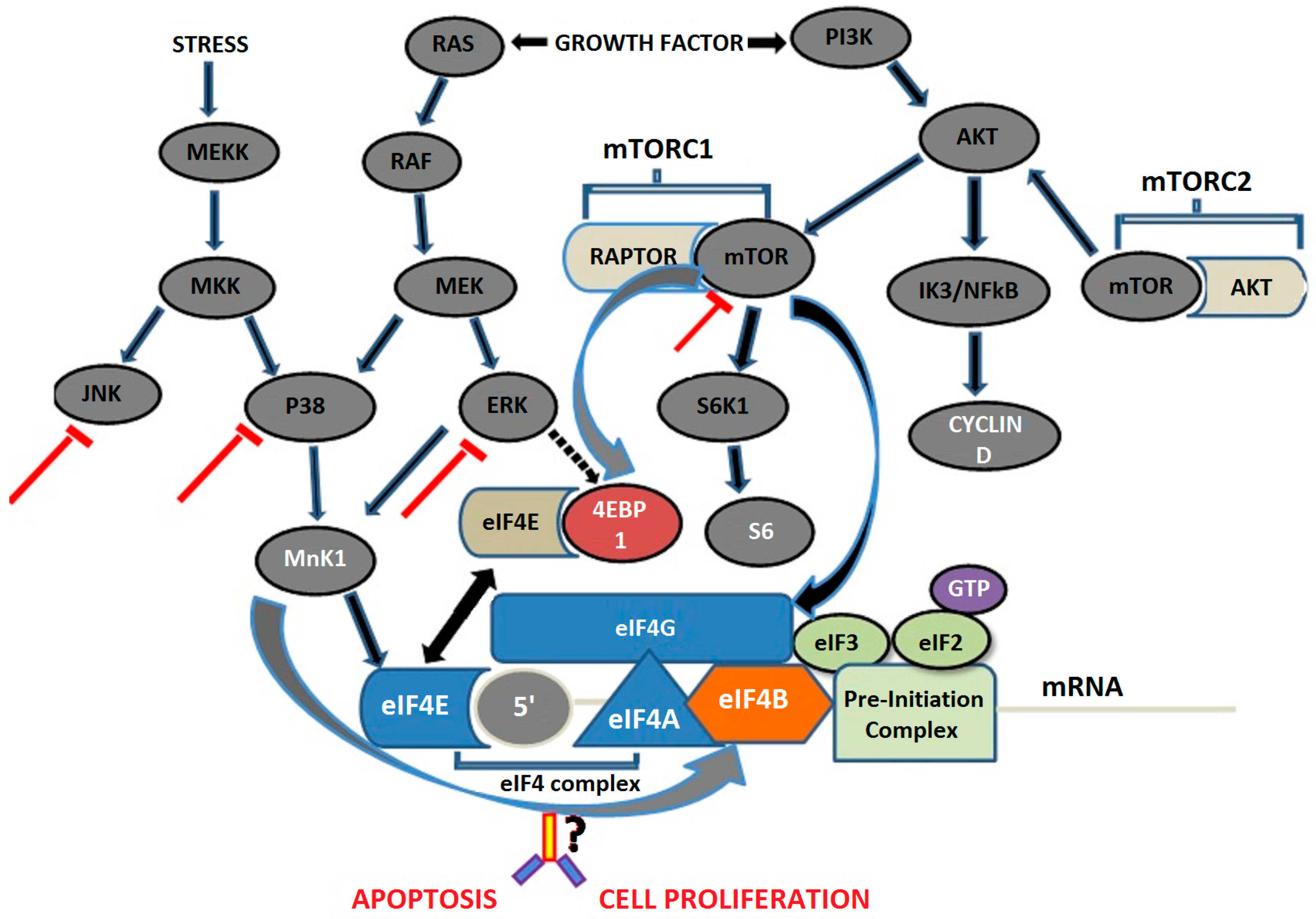
8.1. Mitogen-Activated Protein Kinase Pathway
8.2. Mammalian Target of Rapamycin Pathway
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Sartini, D.; Muzzonigro, G.; Milanese, G.; Pierella, F.; Rossi, V.; Emanuelli, M. Identification of nicotinamide N-methyltransferase as a novel tumor marker for renal clear cell carcinoma. J. Urol. 2006, 176, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Van Spronsen, D.J.; de Weijer, K.J.; Mulders, P.F.; de Mulder, P.H. Novel treatment strategies in clear-cell metastatic renal cell carcinoma. Anticancer Drugs 2005, 16, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Maldazys, J.D.; de Kernion, J.B. Prognostic factors in metastatic renal carcinoma. J. Urol. 1986, 136, 376–379. [Google Scholar] [PubMed]
- De la Taille, A.; Katz, A.; Cao, Y.; McKiernan, J.; Buttyan, R.; Burchardt, M.; Burchardt, T.; Hayek, O.; Olsson, C.A.; Chopin, D.K.; et al. Blood-based RT-PCR assays of MN/CA9 or PSMA: Clinical application in renal cancer patients. Urology 2000, 56, 393–398. [Google Scholar] [CrossRef]
- Dunn, K.L.; Espino, P.S.; Drobic, B.; He, S.; Davie, J.R. The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem. Cell Biol. 2005, 83, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Oka, H.; Chatani, Y.; Hoshino, R.; Ogawa, O.; Kakehi, Y.; Terachi, T.; Okada, Y.; Kawaichi, M.; Kohno, M.; Yoshida, O. Constitutive activation of mitogen-activated protein (MAP) kinases in human renal cell carcinoma. Cancer Res. 1995, 55, 4182–4187. [Google Scholar] [PubMed]
- Atten, M.J.; Attar, B.M.; Holian, O. Decreased MAP kinase activity in human gastric adenocarcinoma. Biochem. Biophys. Res. Commun. 1995, 212, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Kruck, S.; Bedke, J.; Hennenlotter, J.; Ohneseit, P.A.; Kuehs, U.; Senger, E.; Sievert, K.D.; Stenzl, A. Activation of mTOR in renal cell carcinoma is due to increased phosphorylation rather than protein overexpression. Oncol. Rep. 2010, 23, 159–163. [Google Scholar] [PubMed]
- Ljungberg, B.; Campbell, S.C.; Choi, H.Y.; Jacqmin, D.; Lee, J.E.; Weikert, S.; Kiemeney, L.A. The epidemiology of renal cell carcinoma. Eur. Urol. 2011, 60, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Pantuck, A.J.; Zisman, A.; Belldegrun, A.S. The changing natural history of renal cell carcinoma. J. Urol. 2001, 166, 1611–1623. [Google Scholar] [CrossRef]
- Zisman, A.; Pantuck, A.J.; Dorey, F.; Said, J.W.; Shvarts, O.; Quintana, D.; Gitlitz, B.J.; de Kernion, J.B.; Figlin, R.A.; Belldegrun, A.S. Improved prognostication of renal cell carcinoma using an integrated staging system. J. Clin. Oncol. 2001, 19, 1649–1657. [Google Scholar] [PubMed]
- Sanyal, B.; Pant, G.C.; Singhal, G.D.; Tripathi, V.N.; Ambasta, S.S.; Gupta, S.; Mehrotra, M.L. Renal tumours—A review of 54 cases. Indian J. Cancer 1976, 13, 177–182. [Google Scholar] [PubMed]
- Sharma, S.; Nath, P.; Srivastava, A.N.; Singh, K.M. Tumours of the male urogenital tract: A clinicopathologic study. J. Indian Med. Assoc. 1994, 92, 357–360. [Google Scholar] [PubMed]
- Khaitan, A.; Gupta, N.P.; Hemal, A.K.; Dogra, P.N.; Seth, A.; Aron, M. Is there a need for pelvic CT scan in cases of renal cell carcinoma? Int. Urol. Nephrol. 2002, 33, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Flores-Sandoval, N.; Abrams, J. Renal-type clear cell carcinoma occurring in the prostate. Am. J. Surg. Pathol. 2003, 27, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Lambe, S.; Kaler, P.; Pathania, S.; Kumar, S.; Attri, S.; Singh, S.K. Ectopic expression of alkaline phosphatase in proximal tubular brush border membrane of human renal cell carcinoma. Biochim. Biophys. Acta 2005, 1741, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Kakkar, N.; Bal, A.; Singh, S.K.; Joshi, K. Sub-typing of renal cell tumours; contribution of ancillary techniques. Diagn. Pathol. 2009, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Singh, S.K.; Raina, P.; Khullar, M.; Goswami, A.K. MN/CA9 gene expression in peripheral blood of cases with renal cell carcinoma. J. Urol. 2003, 169, 198. [Google Scholar]
- Yap, T.A.; Eisen, T.G. Adjuvant therapy of renal cell carcinoma. Clin. Genitourin. Cancer 2006, 5, 120–130. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.K.; Lipworth, L. Epidemiologic aspects of renal cell cancer. Semin. Oncol. 2000, 27, 115–123. [Google Scholar] [PubMed]
- Lipworth, L.; Tarone, R.E.; McLaughlin, J.K. The epidemiology of renal cell carcinoma. J. Urol. 2006, 176, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.D.; van der Hel, O.L.; McMillan, G.P.; Boffetta, P.; Brennan, P. Renal cell carcinoma in relation to cigarette smoking: Meta-analysis of 24 studies. Int. J. Cancer 2005, 114, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.H.; Dong, L.M.; Devesa, S.S. Epidemiology and risk factors for kidney cancer. Nat. Rev. Urol. 2010, 7, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Tsivian, M.; Moreira, D.M.; Caso, J.R.; Mouraviev, V.; Polascik, T.J. Cigarette smoking is associated with advanced renal cell carcinoma. J. Clin. Oncol. 2011, 29, 2027–2031. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.S.; Cerhan, J.R.; Janney, C.A.; Lynch, C.F.; Cantor, K.P. Smoking cessation and renal cell carcinoma. Ann. Epidemiol. 2003, 13, 245–251. [Google Scholar] [CrossRef]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta- analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Bergstrom, A.; Hsieh, C.C.; Lindblad, P.; Lu, C.M.; Cook, N.R.; Wolk, A. Obesity and renal cell cancer a quantitative review. Br. J. Cancer 2001, 85, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 2004, 4, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Gago-Dominguez, M.; Castelao, J.E.; Yuan, J.M.; Ross, R.K.; Yu, M.C. Lipid peroxidation: A novel and unifying concept of the etiology of renal cell carcinoma (United States). Cancer Causes Control 2002, 13, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.H.; Gridley, G.; Fraumeni, J.F., Jr.; Jarvholm, B. Obesity, hypertension, and the risk of kidney cancer in men. N. Engl. J. Med. 2000, 343, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Weikert, S.; Boeing, H.; Pischon, T.; Weikert, C.; Olsen, A.; Tjonneland, A.; Overvad, K.; Becker, N.; Linseisen, J.; Trichopoulou, A.; et al. Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Am. J. Epidemiol. 2008, 167, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Weikert, S.; Boeing, H.; Pischon, T.; Olsen, A.; Tjonneland, A.; Overvad, K.; Becker, N.; Linseisen, J.; Lahmann, P.H.; Arvaniti, A.; et al. Fruits and vegetables and renal cell carcinoma: Findings from the European prospective investigation into cancer and nutrition (EPIC). Int. J. Cancer 2006, 118, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Spiegelman, D.; Hunter, D.J.; Albanes, D.; Bernstein, L.; van den Brandt, P.A.; Buring, J.E.; Cho, E.; English, D.R.; Freudenheim, J.L.; et al. Fat, protein, and meat consumption and renal cell cancer risk: A pooled analysis of 13 prospective studies. J. Natl. Cancer Inst. 2008, 100, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.R.; Soffer, O.; Nassar, V.H.; Kutner, M.H. Acquired renal cystic disease in end-stage renal disease: An autopsy study of 155 cases. Am. J. Nephrol. 1989, 9, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Port, F.K.; Ragheb, N.E.; Schwartz, A.G.; Hawthorne, V.M. Neoplasms in dialysis patients: A population-based study. Am. J. Kidney Dis. 1989, 14, 119–123. [Google Scholar] [CrossRef]
- Nouh, M.A.; Kuroda, N.; Yamashita, M.; Hayashida, Y.; Yano, T.; Minakuchi, J.; Taniguchi, S.; Nomura, I.; Inui, M.; Sugimoto, M.; Kakehi, Y. Renal cell carcinoma in patients with end-stage renal disease: Relationship between histological type and duration of dialysis. BJU Int. 2010, 105, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Mena, A.C.; Pulido, E.G.; Guillén-Ponce, C. Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: Sunitinib. Anticancer Drugs 2010, 21 (Suppl. 1), S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Pfaffenroth, E.C.; Linehan, W.M. Genetic basis for kidney cancer: Opportunity for disease-specific approaches to therapy. Expert Opin. Biol. Ther. 2008, 8, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Pause, A.; Lee, S.; Worrell, R.A.; Chen, D.Y.; Burgess, W.H.; Linehan, W.M.; Klausner, R.D. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 2156–2161. [Google Scholar] [CrossRef] [PubMed]
- Klatte, T.; Pantuck, A.J. Molecular biology of renal cortical tumors. Urol. Clin. N. Am. 2008, 35, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, B.A.; Schouten, L.J.; Kiemeney, L.A.; Goldbohm, R.A.; van den Brandt, P.A. Relation of height, body mass, energy intake and physical activity to risk of renal cell carcinoma: Results from the Netherlands Cohort Study. Am. J. Epidemiol. 2004, 160, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.H.; Lindblad, P.; Gridley, G.; Nyrén, O.; McLaughlin, J.K.; Linet, M.S.; Pennello, G.A.; Adami, H.O.; Fraumeni, J.F., Jr. Risk of urinary tract cancers following kidney or ureter stones. J. Natl. Cancer Inst. 1997, 89, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Akhtar, M.; Beckwith, B.J.; Bugert, P.; Cooper, C.S.; Delahunt, B.; Eble, J.N.; Fleming, S.; Ljungberg, B.; Medeiros, L.J.; et al. The Heidelberg classification of renal cell tumours. J. Pathol. 1997, 183, 131–133. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer. In AJCC Cancer Staging Manual, 7th ed.; Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. (Eds.) Springer: New York, NY, USA, 2010; pp. 479–489.
- Kaelin, W.G., Jr. Treatment of kidney cancer: Insights provided by the VHL tumor-suppressor protein. Cancer 2009, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Dalgliesh, G.L.; Furge, K.; Greenman, C.; Chen, L.; Bignell, G.; Butler, A.; Davies, H.; Edkins, S.; Hardy, C.; Latimer, C.; et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 2010, 463, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Zhang, N.; Guo, H.F.; Zhang, Z.W.; Xin, D.Q.; Na, Y.Q. Frequent somatic mutations of the von Hippel-Lindau (VHL) tumor suppressor gene and its meaning in sporadic human renal clear cell carcinoma. Beijing Da Xue Xue Bao 2004, 36, 169–172. [Google Scholar] [PubMed]
- Skubitz, K.M.; Zimmermann, W.; Kammerer, R.; Pambuccian, S.; Skubitz, A.P. Differential gene expression identifies subgroups of renal cell carcinoma. J. Lab. Clin. Med. 2006, 147, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Gerashchenko, G.V.; Bogatyrova, O.O.; Rudenko, E.E.; Kondratov, A.G.; Gordiyuk, V.V.; Zgonnyk, Y.M.; Vozianov, O.F.; Pavlova, T.V.; Zabarovsky, E.R.; Rynditch, A.V.; et al. Genetic and epigenetic changes of NKIRAS1 gene in human renal cell carcinomas. Exp. Oncol. 2010, 32, 71–75. [Google Scholar] [PubMed]
- Schmidt, L.; Duh, F.M.; Chen, F.; Kishida, T.; Glenn, G.; Choyke, P.; Scherer, S.W.; Zhuang, Z.; Lubensky, I.; Dean, M.; et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 1997, 16, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Cantley, R.; Gattuso, P.; Cimbaluk, D. Solid variant of papillary renal cell carcinoma with spindle cell and tubular components. Arch. Pathol. Lab. Med. 2010, 134, 1210–1214. [Google Scholar] [PubMed]
- Cohen, H.T.; McGovern, F.J. Renal-cell carcinoma. N. Engl. J. Med. 2005, 353, 2477–2490. [Google Scholar] [CrossRef] [PubMed]
- Skinner, D.G.; Colvin, R.B.; Vermillion, C.D.; Pfister, R.C.; Leadbetter, W.F. Diagnosis and management of renal cell carcinoma. A clinical and pathologic study of 309 cases. Cancer 1971, 28, 1165–1177. [Google Scholar] [CrossRef]
- Fuhrman, S.A.; Lasky, L.C.; Limas, C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am. J. Surg. Pathol. 1982, 6, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Rekha, P.R.; Rajendiran, S.; Rao, S.; Shroff, S.; Joseph, L.D.; Prathiba, D. Histological reclassification, histochemical characterization and c-kit immunoexpression in renal cell carcinoma. Indian J. Urol. 2008, 24, 343–347. [Google Scholar] [PubMed]
- Ficarra, V.; Guillè, F.; Schips, L.; de la Taille, A.; Prayer Galetti, T.; Tostain, J.; Cindolo, L.; Novara, G.; Zigeuner, R.; Bratti, E.; et al. Proposal for revision of the TNM classification system for renal cell carcinoma. Cancer 2005, 104, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, J.M.; Buttyan, R.; Bander, N.H.; de la Taille, A.; Stifelman, M.D.; Emanuel, E.R.; Bagiella, E.; Rubin, M.A.; Katz, A.E.; Olsson, C.A.; et al. The detection of renal carcinoma cells in the peripheral blood with an enhanced reverse transcriptase-polymerase chain reaction assay for MN/CA9. Cancer 1999, 86, 492–497. [Google Scholar] [CrossRef]
- Morrissey, J.J.; Mobley, J.; Song, J. Urinary concentrations of aquaporin-1 and perilipin-2 in patients with renal cell carcinoma correlate with tumor size and stage but not grade. Urology 2014, 83, e9–e14. [Google Scholar] [CrossRef] [PubMed]
- Baer, C.; Claus, R.; Plass, C. Genome-wide epigenetic regulation of miRNAs in cancer. Cancer Res. 2013, 73, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Li, G.; Péoc’h, M.; Genin, C.; Gigante, M. Serum miR-210 as a novel biomarker for molecular diagnosis of clear cell renal cell carcinoma. Exp. Mol. Pathol. 2013, 94, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Wulfken, L.M.; Moritz, R.; Ohlmann, C.; Holdenrieder, S.; Jung, V.; Becker, F.; Herrmann, E.; Walgenbach-Brünagel, G.; von Ruecker, A.; Müller, S.C.; et al. MicroRNAs in renal cell carcinoma: Diagnostic implications of serum miR-1233 levels. PLoS ONE 2011, 6, e25787. [Google Scholar] [CrossRef] [PubMed]
- Redova, M.; Poprach, A.; Besse, A.; Iliev, R.; Nekvindova, J.; Lakomy, R.; Radova, L.; Svoboda, M.; Dolezel, J.; Vyzula, R.; et al. MiR-210 expression in tumor tissue and in vitro effects of its silencing in renal cell carcinoma. Tumour Biol. 2013, 34, 481–491. [Google Scholar] [CrossRef] [PubMed]
- De Martino, M.; Klatte, T.; Haitel, A.; Marberger, M. Serum cell-free DNA in renal cell carcinoma: A diagnostic and prognostic marker. Cancer 2012, 118, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Su-Kim, D.; Choi, Y.D.; Moon, M. Composite three-marker assay for early detection of kidney cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Elson, P.J.; Witte, R.S.; Trump, D.L. Prognostic factors for survival in patients with recurrent or metastatic renal cell carcinoma. Cancer Res. 1988, 48, 7310–7313. [Google Scholar] [PubMed]
- Raj, G.V.; Thompson, R.H.; Leibovich, B.C.; Blute, M.L.; Russo, P.; Kattan, M.W. Preoperative nomogram predicting 12-year probability of metastatic renal cancer. J. Urol. 2008, 179, 2146–2151. [Google Scholar] [CrossRef] [PubMed]
- Hutterer, G.C.; Patard, J.J.; Jeldres, C.; Perrotte, P.; de La Taille, A.; Salomon, L.; Verhoest, G.; Tostain, J.; Cindolo, L.; Ficarra, V.; et al. Patients with distant metastases from renal cell carcinoma can be accurately identified: External validation of a new nomogram. BJU Int. 2008, 101, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Delahunt, B.; Nacey, J.N. Renal cell carcinoma. II. Histological indicators of prognosis. Pathology 1987, 19, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.J.; Albers, M.W.; Shin, T.B.; Ichikawa, K.; Keith, C.T.; Lane, W.S.; Schreiber, S.L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 1994, 369, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Gayed, B.A.; Youssef, R.F.; Bagrodia, A.; Kapur, P.; Darwish, O.M.; Krabbe, L.M.; Sagalowsky, A.; Lotan, Y.; Margulis, V. Prognostic role of cell cycle and proliferative biomarkers in patients with clear cell renal cell carcinoma. J. Urol. 2013, 190, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, A.; Watanabe, R.; Tomita, Y. E-cadherin expression in renal cell cancer and its significance in metastasis and survival. Br. J. Cancer 1995, 71, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Shimazui, T.; Giroldi, L.A.; Bringuier, P.P.; Oosterwijk, E.; Schalken, J.A. Complex cadherin expression in renal cell carcinoma. Cancer Res. 1996, 56, 3234–3237. [Google Scholar] [PubMed]
- Chauhan, S.S.; Goldstein, L.J.; Gottesman, M.M. Expression of cathepsin L in human tumors. Cancer Res. 1991, 51, 1478–1481. [Google Scholar] [PubMed]
- Yoshino, S.; Kato, M.; Okada, K. Prognostic significance of microvessel count in low stage renal cell carcinoma. Int. J. Urol. 1995, 2, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.; Yang, G.; Brown, R.W.; Cao, J.; D’Agati, V.; Thompson, T.S.; Truong, L.D. Apoptosis in renal cell carcinoma: Detection by in situ end-labeling of fragmented DNA and correlation with other prognostic factors. Hum. Pathol. 1996, 27, 1012–1017. [Google Scholar] [CrossRef]
- Wenzel, M.; Mahotka, C.; Krieg, A.; Bachmann, A.; Schmitt, M.; Gabbert, H.E.; Gerharz, C.D. Novel survivin-related members of the inhibitor of apoptosis (IAP) family. Cell Death Differ. 2000, 7, 682–683. [Google Scholar] [CrossRef] [PubMed]
- Mahotka, C.; Krieg, T.; Krieg, A.; Wenzel, M.; Suschek, C.V.; Heydthausen, M.; Gabbert, H.E.; Gerharz, C.D. Distinct in vivo expression patterns of survivin splice variants in renal cell carcinomas. Int. J. Cancer 2002, 100, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Byun, B.; Tak, H.; Joe, C.O. BTB/POZ domain of speckle-type POZ protein (SPOP) confers proapoptotic function in HeLa cells. Biofactors 2007, 31, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Baytekin, F.; Tuna, B.; Mungan, U.; Aslan, G.; Yorukoglu, K. Significance of P-glycoprotein, p53, and survivin expression in renal cell carcinoma. Urol. Oncol. 2011, 29, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Kamel, D.; Turpeenniemi-Hujanen, T.; Vahakangas, K.; Paakka, P.; Soini, Y. Proliferating cell nuclear antigen but not p53 or human papillomavirus DNA correlates with advanced clinical stage in renal cell carcinoma. Histopathology 1994, 25, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Shalitin, C.; Epelbaum, R.; Moskovitz, B.; Segal, R.; Valansi, C.; Werner, M.; Livne, P.M. Increased levels of a 21-kDa protein in the circulation of tumor-bearing patients. Cancer Detect. Prev. 1994, 18, 357–365. [Google Scholar] [PubMed]
- Nishikawa, M.; Miyake, H.; Harada, K.; Fujisawa, M. Expression level of phosphorylated-4E-binding protein 1 in radical nephrectomy specimens as a prognostic predictor in patients with metastatic renal cell carcinoma treated with mammalian target of rapamycin inhibitors. Med. Oncol. 2014, 31, 792. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.C.; Wood, C.G.; Karam, J.A. Biomarkers of renal cell carcinoma. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Pantuck, A.J.; Seligson, D.B.; Klatte, T.; Yu, H.; Leppert, J.T.; Moore, L.; O’Toole, T.; Gibbons, J.; Belldegrun, A.S.; Figlin, R.A. Prognostic relevance of the mTOR pathway in renal cell carcinoma: Implications for molecular patient selection for targeted therapy. Cancer 2007, 109, 2257–2267. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.; Regan, M.; McDermott, D.; Mier, J.; Stanbridge, E.; Youmans, A.; Febbo, P.; Upton, M.; Lechpammer, M.; Signoretti, S. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin. Cancer Res. 2005, 11, 3714–3721. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; Liu, Y.; Zurita, A.J.; Lin, Y.; Baker-Neblett, K.L.; Martin, A.M.; Figlin, R.A.; Hutson, T.E.; Sternberg, C.N.; Amado, R.G.; et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: A retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012, 13, 827–837. [Google Scholar] [CrossRef]
- Zurita, A.J.; Jonasch, E.; Wang, X.; Khajavi, M.; Yan, S.; Du, D.Z.; Xu, L.; Herynk, M.H.; McKee, K.S.; Tran, H.T.; et al. A cytokine and angiogenic factor (CAF) analysis in plasma for selection of sorafenib therapy in patients with metastatic renal cell carcinoma. Ann. Oncol. 2012, 23, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Yuen, J.S. Molecular targeted therapy in advanced renal cell carcinoma: A review of its recent past and a glimpse into the near future. Indian J. Urol. 2009, 25, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Drucker, B.J. Renal cell carcinoma: Current status and future prospects. Cancer Treat. Rev. 2005, 31, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.A.; Rini, B.I. Recent progress in the management of advanced renal cell carcinoma. CA Cancer J. Clin. 2007, 57, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Yagoda, A.; Petrylak, D.; Thompson, S. Cytotoxic chemotherapy for advanced renal cell carcinoma. Urol. Clin. N. Am. 1993, 20, 303–321. [Google Scholar]
- Hartmann, J.T.; Bokemeyer, C. Chemotherapy for renal cell carcinoma. Anticancer Res. 1999, 19, 1541–1543. [Google Scholar] [PubMed]
- Mickisch, G.H. Chemoresistance of renal cell carcinoma: 1986–1994. World J. Urol. 1994, 12, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Greef, B.; Eisen, T. Medical treatment of renal cancer: New horizons. Br. J. Cancer 2016, 115, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hudes, G.R.; Curti, B.D.; McDermott, D.F.; Escudier, B.J.; Negrier, S.; Duclos, B.; Moore, L.; O’Toole, T.; Boni, J.P.; et al. Phase I/II trial of temsirolimus combined with interferon alfa for advanced renal cell carcinoma. J. Clin. Oncol. 2007, 25, 3958–3964. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.Y.; Kollmannsberger, C.; Chi, K.N. Targeted therapy for metastatic renal cell carcinoma: Current treatment and future directions. Ther. Adv. Med. Oncol. 2010, 2, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Samaras, V.; Tsopanomichalou, M.; Stamatelli, A.; Arnaoutoglou, C.; Samaras, E.; Arnaoutoglou, M.; Poulias, H.; Barbatis, C. Is there any potential link among caspase-8, p-p38 MAPK and bcl-2 in clear cell renal cell carcinomas? A comparative immunohistochemical analysis with clinical connotations. Diagn Pathol. 2009, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ding, Y.; Luo, W.M.; Bender, S.; Qian, C.N.; Kort, E.; Zhang, Z.F.; VandenBeldt, K.; Duesbery, N.S.; Resau, J.H.; et al. Inhibition of MAPK kinase signaling pathways suppressed renal cell carcinoma growth and angiogenesis in vivo. Cancer Res. 2008, 68, 81–88. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Liu, H.; Magyar, C.E.; Guo, Y.; Veena, M.S.; Srivatsan, E.S.; Huang, J.; Rettig, M.B. Hyperactivated JNK is a therapeutic target in pVHL-deficient renal cell carcinoma. Cancer Res. 2013, 73, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.M.; Simeone, E.; Festino, L.; Vanella, V.; Palla, M.; Ascierto, P.A. Novel mechanisms and therapeutic approaches in melanoma: Targeting the MAPK pathway. Discov. Med. 2015, 19, 455–461. [Google Scholar] [PubMed]
- LoRusso, P.M.; Krishnamurthi, S.S.; Rinehart, J.J.; Nabell, L.M.; Malburg, L.; Chapman, P.B.; DePrimo, S.E.; Bentivegna, S.; Wilner, K.D.; Tan, W.; et al. Phase I pharmacokinetic and pharmacodynamic study of the oral MAPK/ERK kinase inhibitor PD-0325901 in patients with advanced cancers. Clin. Cancer Res. 2010, 16, 1924–1937. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Ma, L.; Teruya-Feldstein, J. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Investig. 2008, 118, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Corradetti, M.N.; Guan, K.L. Dysregulation of the TSC-mTOR pathway in human disease. Nat. Genet. 2005, 37, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Loewith, R.; Jacinto, E.; Wullschleger, S.; Lorberg, A.; Crespo, J.L.; Bonenfant, D.; Oppliger, W.; Jenoe, P.; Hall, M.N. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 2002, 10, 457–468. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Ali, S.M.; Sengupta, S.; Sheen, J.H.; Hsu, P.P.; Bagley, A.F.; Markhard, A.L.; Sabatini, D.M. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 2006, 22, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Maruki, Y.; Long, X.; Yoshino, K.; Oshiro, N.; Hidayat, S.; Tokunaga, C.; Avruch, J.; Yonezawa, K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 2002, 110, 177–189. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [PubMed]
- Hresko, R.C.; Mueckler, M. mTOR, RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J. Biol. Chem. 2005, 280, 40406–40416. [Google Scholar] [CrossRef] [PubMed]
- Toschi, A.; Edelstein, J.; Rockwell, P.; Ohh, M.; Foster, D.A. HIF alpha expression in VHL-deficient renal cancer cells is dependent on phospholipase D. Oncogene 2008, 27, 2746–2753. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.V.; Tran, C.; Mellinghoff, I.K.; Welsbie, D.S.; Chan, E.; Fueger, B.; Czernin, J.; Sawyers, C.L. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat. Med. 2006, 12, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Toral-Barza, L.; Discafani, C.; Zhang, W.G.; Skotnicki, J.; Frost, P.; Gibbons, J.J. A novel target in breast cancer: The effect of CCI-779, an mTOR inhibitor, in preclinical models of breastcancer. Endocr. Relat. Cancer. 2001, 8, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Figlin, R.A. Targeted inhibition of mammalian target of rapamycin for the treatment of advanced renal cell carcinoma. Cancer 2009, 115, 3618–3630. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.V. mTOR and cancer: Reason for dancing at the crossroads? Curr. Opin. Genet. Dev. 2006, 16, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Easton, J.B.; Houghton, P.J. mTOR and cancer therapy. Oncogene 2006, 25, 6436–6446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Duan, Y.; Zheng, X.F. Targeting the mTOR kinase domain: The second generation of mTOR inhibitors. Drug Discov. Today 2011, 16, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Berel, D.; Wang, Y.; Li, P.; Bhowmick, N.A.; Figlin, R.A.; Kim, H.L. A comparison of Ku0063794, a dual mTORC1 and mTORC2 inhibitor, and temsirolimus in preclinical renal cellcarcinoma models. PLoS ONE 2013, 8, e54918. [Google Scholar]
- Lasithiotakis, K.G.; Sinnberg, T.W.; Schittek, B.; Flaherty, K.T.; Kulms, D.; Maczey, E.; Garbe, C.; Meier, F.E. Combined inhibition of MAPK and mTOR signaling inhibits growth, induces cell death, and abrogates invasive growth of melanoma cells. J. Investig. Dermatol. 2008, 128, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.N.; Srinivas, S.; Hauke, R.J.; Amato, R.J.; Esteves, B.; Cotreau, M.M.; Strahs, A.L.; Slichenmyer, W.J.; Bhargava, P.; Kabbinavar, F.F. Phase Ib study of tivozanib (AV-951) in combination with temsirolimus in patients with renal cell carcinoma. Eur. J. Cancer 2013, 49, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Mohri, D.; Ijichi, H.; Miyabayashi, K.; Takahashi, R.; Kudo, Y.; Sasaki, T.; Asaoka, Y.; Tanaka, Y.; Ikenoue, T.; Tateishi, K.; et al. A potent therapeutics for gallbladder cancer by combinatorial inhibition of the MAPK and mTOR signaling networks. J. Gastroenterol. 2015, 51, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, O.; Hitora, T.; Yamagami, Y.; Mori, M.; Nishimura, H.; Horie, R.; Yamaguchi, K.; Yamamoto, T. The combination of rapamycin and MAPK inhibitors enhances the growth inhibitory effect on Nara-H cells. Int. J. Mol. Med. 2014, 33, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Vijapurkar, U.; Robillard, L.; Zhou, S.; Degtyarev, M.; Lin, K.; Truong, T.; Tremayne, J.; Ross, L.B.; Pei, Z.; Friedman, L.S.; et al. mTOR kinase inhibitor potentiates apoptosis of PI3K and MEK inhibitors in diagnostically defined subpopulations. Cancer Lett. 2012, 326, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, Z.; Tawbi, H.A.; Hu, J.; Guan, M.; Frankel, P.H.; Ruel, N.H.; Wilczynski, S.; Christensen, S.; Gandara, D.R.; Chow, W.A. A randomised phase II trial of selumetinib vs. selumetinib plus temsirolimus for soft-tissue sarcomas. Br. J. Cancer 2015, 112, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Temraz, S.; Mukherji, D.; Shamseddine, A. Dual Inhibition of MEK and PI3K Pathway in KRAS and BRAF Mutated Colorectal Cancers. Int. J. Mol. Sci. 2015, 16, 22976–22988. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Loh, K.; Yap, Y.S. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol. Med. 2015, 12, 342–354. [Google Scholar] [PubMed]
- Chauhan, A. Evolution of Speckle-Type POZ Protein (SPOP), a Biomarker and Its Inhibition in the Combination Therapy in Renal Cell Carcinoma in Vitro. Doctoral Thesis, Postgraduate Institute of Medical Education & Research, Chandigarh, India, 2014. [Google Scholar]
- Hudes, G.; Carducci, M.; Tomczak, P. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.J.; Jac, J.; Giessinger, S.; Saxena, S.; Willis, J.P. A phase 2 study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastaticclear cell renal cell cancer. Cancer 2009, 115, 2438–2446. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Kroemer, G.; Raymond, E. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 2006, 5, 671–688. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Buckner, J.C.; Maurer, M.J.; Kreisberg, J.I.; Ballman, K.; Boni, J.; Peralba, J.M.; Jenkins, R.B.; Dakhil, S.R.; Morton, R.F.; et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: A north central cancer treatment group study. J. Clin. Oncol. 2005, 23, 5294–5304. [Google Scholar] [CrossRef] [PubMed]
| S. No. | Risk Factor | Finding | Reference |
|---|---|---|---|
| 1 | Cigarette Smoking | Cigarette smoking has been considered as the most consistent causal risk factor for RCC which accounts for up to 30% of RCC in men and 20% in women. | [21,22] |
| A meta-analysis from 19 case-control studies on 1,457,754 participants with 1,326 RCC, revealed a relative risk of RCC as 1.54 in male and 1.22 in female smokers | [23] | ||
| There is significant relationship between smoking and incidence rate of RCC | [24] | ||
| Higher risk of RCC is associated with heavier smoking | [25] | ||
| Quitting smoking reduces the risk of RCC | [26] | ||
| 2 | Alcohol Consumption | There is a positive correlation between kidney cancer and consumption of alcohol | [21] |
| 3 | Obesity | Excessive weight is a risk factor for RCC in several patients | [27] |
| The proportion of RCC attributable to overweight is estimated to be >40% in USA whereas >30% in Europe | [28,29] | ||
| Obesity with hypertension may lead to increase in lipid peroxidation, which forms DNA adducts and ultimately leads to RCC | [30] | ||
| 4 | Hypertension | There is a relationship between RCC and blood pressure, and people with high blood pressure have higher tendency for RCC | [31,32] |
| 5 | Diet | Diet plays a key role in etiology of RCC but investigations have shown no protective effect of vegetables and/or fruits consumption on RCC | [32,33] |
| Epidemiologic study suggested high protein consumption as a risk factor for RCC as well as an inducer of renal tubular hypertrophy | [34] | ||
| 6 | Acquired Cystic/Chronic Dialysis | Acquired renal cystic disease develops in patients mostly with end-stage renal disease, and long-term haemodialysis increases the chance of RCC | [35] |
| The incidence of RCC in acquired renal cystic disease is found to be 3–6 times higher than that of other cases | [36] | ||
| Long term use of dialysis is reported to be associated with higher incidence of RCC | [37] | ||
| 7 | Inherited Susceptibility | Genetic diseases such as von Hippel-Lindau syndrome (VHL), hereditary papillary renal carcinoma, tuberous sclerosis, Birt-Hogg-Dubé syndrome (BHD) and hereditary leiomyoma are also associated with RCC | [38,39,40,41] |
| 8 | Additional Risk Factor | Urinary tract infection | [26] |
| Low physical activity | [42] | ||
| Dialysis treatment also increases the risk for developing RCC | [24] | ||
| Radiations appear to increase the chances of occurring RCC | [21] | ||
| Kidney stones increase risk of RCC | [43] |
| TX | T Cannot Assess. |
|---|---|
| T0 | No evidence. |
| T1 | T ≤ 7 cm in greatest dimension, limited to kidney. |
| T1a | T ≤ 4 cm in greatest dimension, limited to kidney. |
| T1b | T > 4 cm but not >7 cm in greatest dimension, limited to kidney. |
| T2 | T > 7 cm in greatest dimension, limited to kidney. |
| T2a | T > 7 cm but ≤10 cm in greatest dimension, limited to kidney. |
| T2b | T > 10 cm, limited to kidney. |
| T3 | T extends into major veins or perinephric tissues but not into the ipsilateral adrenal gland and not beyond Gerota fascia. |
| T3a | T grossly extends into the renal vein or its segmental branches, or T invades perirenal and/or renal sinus fat but not beyond Gerota fascia. |
| T3b | T grossly extends into the vena cava below the diaphragm. |
| T3c | T grossly extends into vena cava above diaphragm or invades wall of vena cava. |
| T4 | T invades beyond Gerota fascia (including contiguous extension into ipsilateral adrenal gland). |
| NX | N Cannot Assess. |
|---|---|
| N0 | No N metastasis. |
| N1 | Metastases in N. |
| M0 | No Metastasis (M). |
| M1 | M appears |
| Stage | T | N | M |
|---|---|---|---|
| I | T1 | N0 | M0 |
| II | T2 | N0 | M0 |
| III | T1 or T2 | N1 | M0 |
| T3 | N0 or N1 | M0 | |
| IV | T4 | Any N | M0 |
| Any T | Any N | M1 |
| Grade | Nucleus | Nuclear Size (μm) | Nucleoli |
|---|---|---|---|
| 1 | Round, uniform | 10 | Absent/inconspicuous |
| 2 | Slightly irregular | 15 | Evident |
| 3 | Very irregular | 20 | Large and prominent |
| 4 | Bizarre & multilobated | >20 | Prominent, chromatin clumped |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, A.; Semwal, D.K.; Mishra, S.P.; Goyal, S.; Marathe, R.; Semwal, R.B. Combination of mTOR and MAPK Inhibitors—A Potential Way to Treat Renal Cell Carcinoma. Med. Sci. 2016, 4, 16. https://doi.org/10.3390/medsci4040016
Chauhan A, Semwal DK, Mishra SP, Goyal S, Marathe R, Semwal RB. Combination of mTOR and MAPK Inhibitors—A Potential Way to Treat Renal Cell Carcinoma. Medical Sciences. 2016; 4(4):16. https://doi.org/10.3390/medsci4040016
Chicago/Turabian StyleChauhan, Ashutosh, Deepak Kumar Semwal, Satyendra Prasad Mishra, Sandeep Goyal, Rajendra Marathe, and Ruchi Badoni Semwal. 2016. "Combination of mTOR and MAPK Inhibitors—A Potential Way to Treat Renal Cell Carcinoma" Medical Sciences 4, no. 4: 16. https://doi.org/10.3390/medsci4040016






