Efficacy of Pancreatic Endotherapy In Pancreatic Ascites And Pleural Effusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Analyses
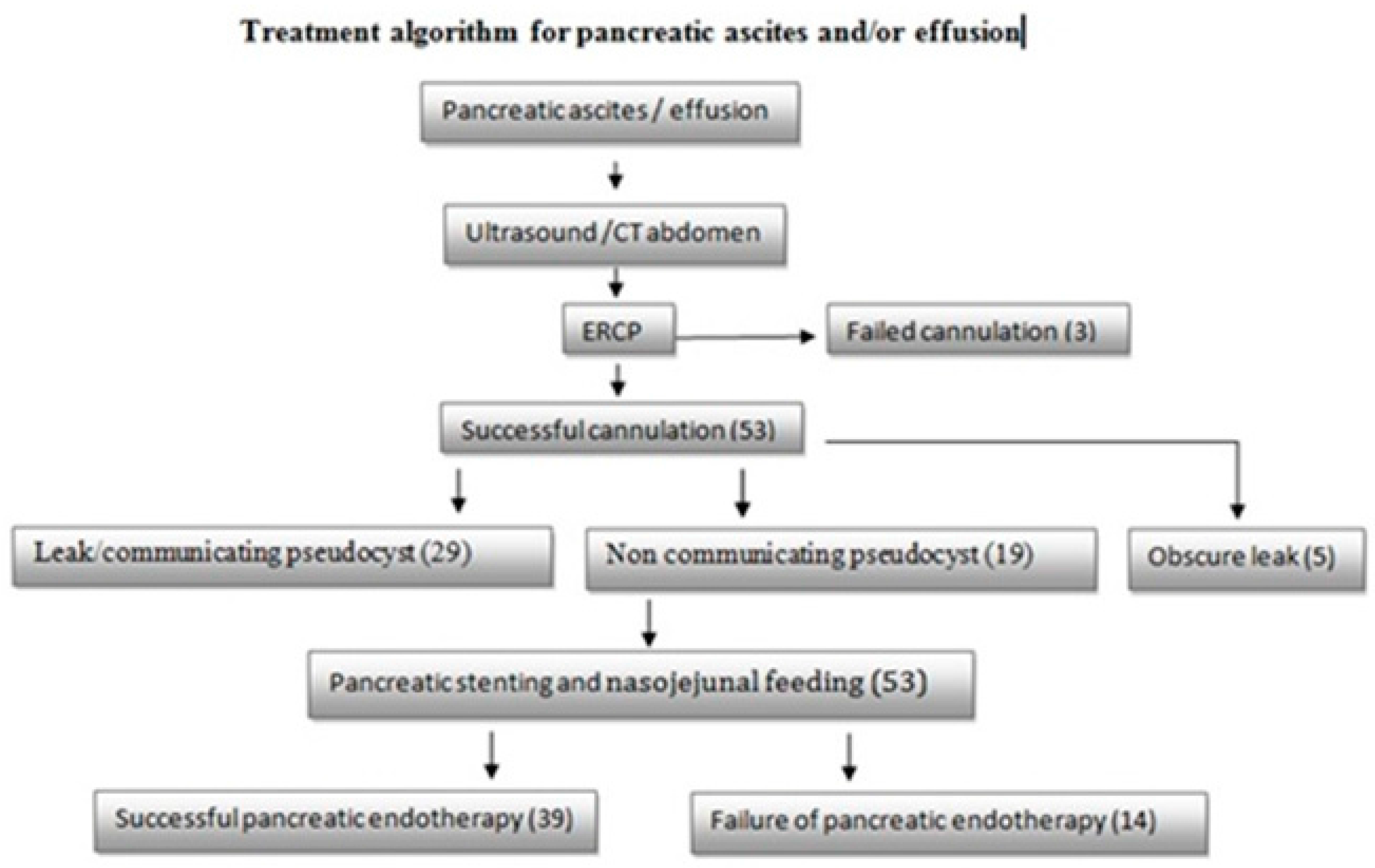
2.3. Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gomez-Cerezo, J.; Cano, A.B.; Suarez, I.; Soto, A.; Rios, J. Pancreatic ascites: Study of therapeutic options by analysis of case reports and case series between the years 1975 and 2000. Am. J. Gastroenterol. 2003, 98, 568–577. [Google Scholar] [CrossRef]
- Bracher, G.A.; Manocha, A.P.; DeBanto, J.R.; Gates, L.K.; Slivka, A.; Whitcomb, D.C.; Martin, S.P. Endoscopic pancreatic duct stenting to treat pancreatic ascites. Gastrointest. Endosc. 1999, 49, 710–715. [Google Scholar] [CrossRef]
- Smith, E.B. Hemorrhagic ascites and hemothorax associated with benign pancreatic disease. AMA Arch. Surg. 1953, 67, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Tomey, S.; Jain, P.; Azfar, M.; Sachdev, A.; Chaudhary, A. Internal pancreatic fistulae with serous effusions in chronic pancreatitis. Aust. N. Z. J. Surg. 1996, 66, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Kozarek, R.A. Management of Pancreatic Ascites. Gastroenterol. Hepatol. 2007, 3, 362–364. [Google Scholar]
- Schoefl, R.; Haefner, M.; Pongratz, S.; Pfeffel, F.; Stain, C.; Poetzi, R.; Gangl, A. Endoscopic treatment of fistulae and abcess in pancreatitis: Three case reports. Endoscopy 1996, 28, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Moosa, A.R. Surgical treatment of chronic pancreatitis: An overview. Br. J. Surg. 1987, 74, 661–667. [Google Scholar] [CrossRef]
- Cameron, J.L.; Keiffer, R.S.; Anderson, W.J.; Zuidema, G.D. Internal pancreatic fistulas: Pancreatic ascites and pleural effusions. Ann. Surg. 1976, 184, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Parekh, D.; Segal, I. Pancreatic ascites and effusion. Risk factors for failure of conservative therapy and the role of octreotide. Arch. Surg. 1992, 127, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Burgess, N.A.; Moore, H.E.; Williams, J.O.; Lewis, M.H. A review of pancreatico-pleural fistula in pancreatitis and its management. HPB Surg. 1992, 5, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, J.E.; Machado, M.; Bacchella, T.; Penteado, S.; Mott, C.B.; Jukemura, J.; Pinotti, H.W. Surgical treatment of pancreatic ascites and pancreatic pleural effusions. Hepato-Gastroenterology 1995, 42, 748–751. [Google Scholar]
- Eckhauser, F.; Raper, S.E.; Knol, J.A.; Mulholland, M.W. Surgical management of pancreatic pseudocysts, pancreatic ascites, and pancreatopleural fistulae. Pancreas 1991, 6, S66–S75. [Google Scholar] [CrossRef] [PubMed]
- Pai, C.G.; Suvarna, D.; Bhat, G. Endoscopic treatment as first-line therapy for pancreatic ascites and pleural effusion. J. Gastroenterol. Hepatol. 2009, 24, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, D.K.; Rana, S.S.; Siyad, I.; Poddar, U.; Thapa, B.R.; Sinha, S.K.; Nagi, B. Endoscopic transpapillary nasopancreatic drainage alone to treat pancreatic ascites and pleural effusion. J. Gastroenterol. Hepatol. 2006, 21, 1059–1064. [Google Scholar] [CrossRef] [PubMed]

| Total Patients | 53 |
|---|---|
| Male:female | 7.8:1 |
| Mean age in years | 43.89 ± 8.1 |
| Acute pancreatitis | 11/53 (20.8%) |
| Chronic pancreatitis | 43/53 (79.2%) |
| Alcoholic pancreatitis | 42/53 (79.2%) |
| Idiopathic pancreatitis | 7/53 (13.2%) |
| Traumatic pancreatitis | 4/53 (7.5%) |
| Ascites alone | 37/53 (69.8%) |
| Pleural effusion alone | 5/53 (9.4%) |
| Ascites with pleural effusion | 11/53 (20.8%) |
| Mean fluid amylase level IU/mL | 5617.21 ± 3311.6 |
| Variables | |||
|---|---|---|---|
| Ascites | 27 | 10 | 0.307 |
| Ascites with effusion | 7 | 4 | |
| Effusion | 5 | 0 | |
| Acute pancreatitis | 10 | 1 | 0.144 |
| Chronic pancreatitis | 29 | 13 | |
| Alcohol | 29 | 13 | 0.300 |
| Traumatic | 4 | 0 | |
| Idiopathic | 6 | 1 | |
| Body | 9 | 1 | 0.008 * |
| Genu | 4 | 0 | |
| Tail | 1 | 5 | |
| Pseudocyst | 22 | 6 | |
| Obscure | 3 | 2 | |
| Stent across the leak | 27 | 2 | 0.0001 * |
| Not crossed | 8 | 4 | |
| Sphincterotomy | 1 | 6 | |
| Stent placed empirically | 3 | 2 | |
| Odds Ratio | Standard Error | 95% Confidence Interval | p Value | |
|---|---|---|---|---|
| Etiology of pancreatitis | 0.3269185 | 0.4043291 | 0.0289523–3.691441 | 0.366 |
| Cause of pancreatitis | 0.4471668 | 0.2271196 | 0.1652485–1.210045 | 0.113 |
| Type of pancreatitis | 1.273519 | 1.946518 | 0.0636769–25.47003 | 0.874 |
| Leak site | 2.637887 | 1.21601 | 1.068735–6.510916 | 0.035 * |
| Stent aross the leak | 3.007118 | 1.163186 | 1.408953–6.418071 | 0.004 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Gaikwad, N.; Samarth, A.; Sawalakhe, N.; Sankalecha, T. Efficacy of Pancreatic Endotherapy In Pancreatic Ascites And Pleural Effusion. Med. Sci. 2017, 5, 6. https://doi.org/10.3390/medsci5020006
Gupta S, Gaikwad N, Samarth A, Sawalakhe N, Sankalecha T. Efficacy of Pancreatic Endotherapy In Pancreatic Ascites And Pleural Effusion. Medical Sciences. 2017; 5(2):6. https://doi.org/10.3390/medsci5020006
Chicago/Turabian StyleGupta, Sudhir, Nitin Gaikwad, Amol Samarth, Niraj Sawalakhe, and Tushar Sankalecha. 2017. "Efficacy of Pancreatic Endotherapy In Pancreatic Ascites And Pleural Effusion" Medical Sciences 5, no. 2: 6. https://doi.org/10.3390/medsci5020006




