cGAMP Promotes Germinal Center Formation and Production of IgA in Nasal-Associated Lymphoid Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Mice
2.3. Vaccination and ELISA
2.4. Cytometric Bead Array Assay
2.5. Flow Cytometric Analysis
2.6. Antigen-Specific T-Cell Responses
2.7. Real-Time Polymerase Chain Reaction
2.8. Statistical Analyses
3. Results
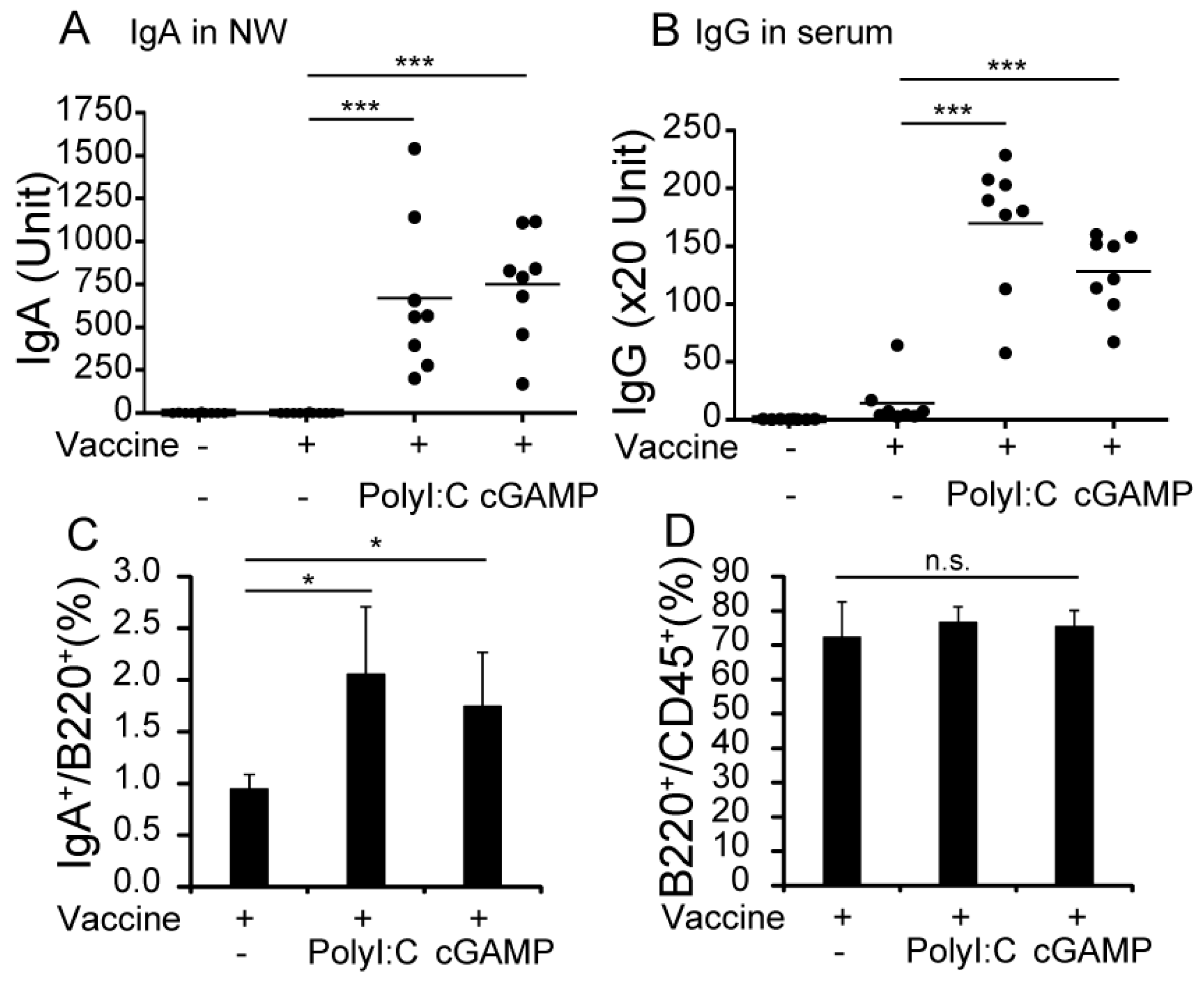
3.1. cGAMP Enhances Vaccine-Specific Ig Production
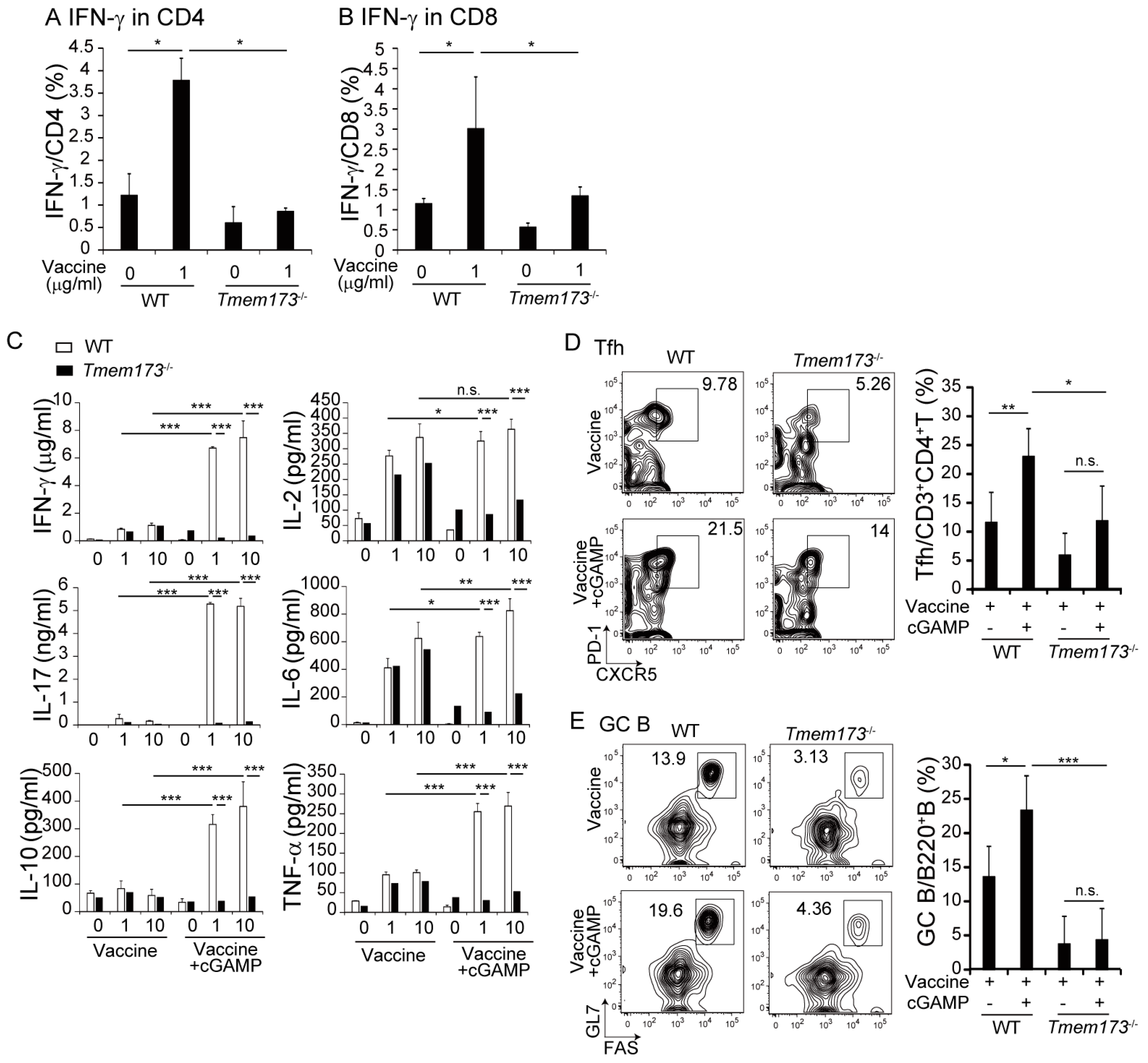
3.2. cGAMP Promotes Vaccine-Specific T-Cell Responses
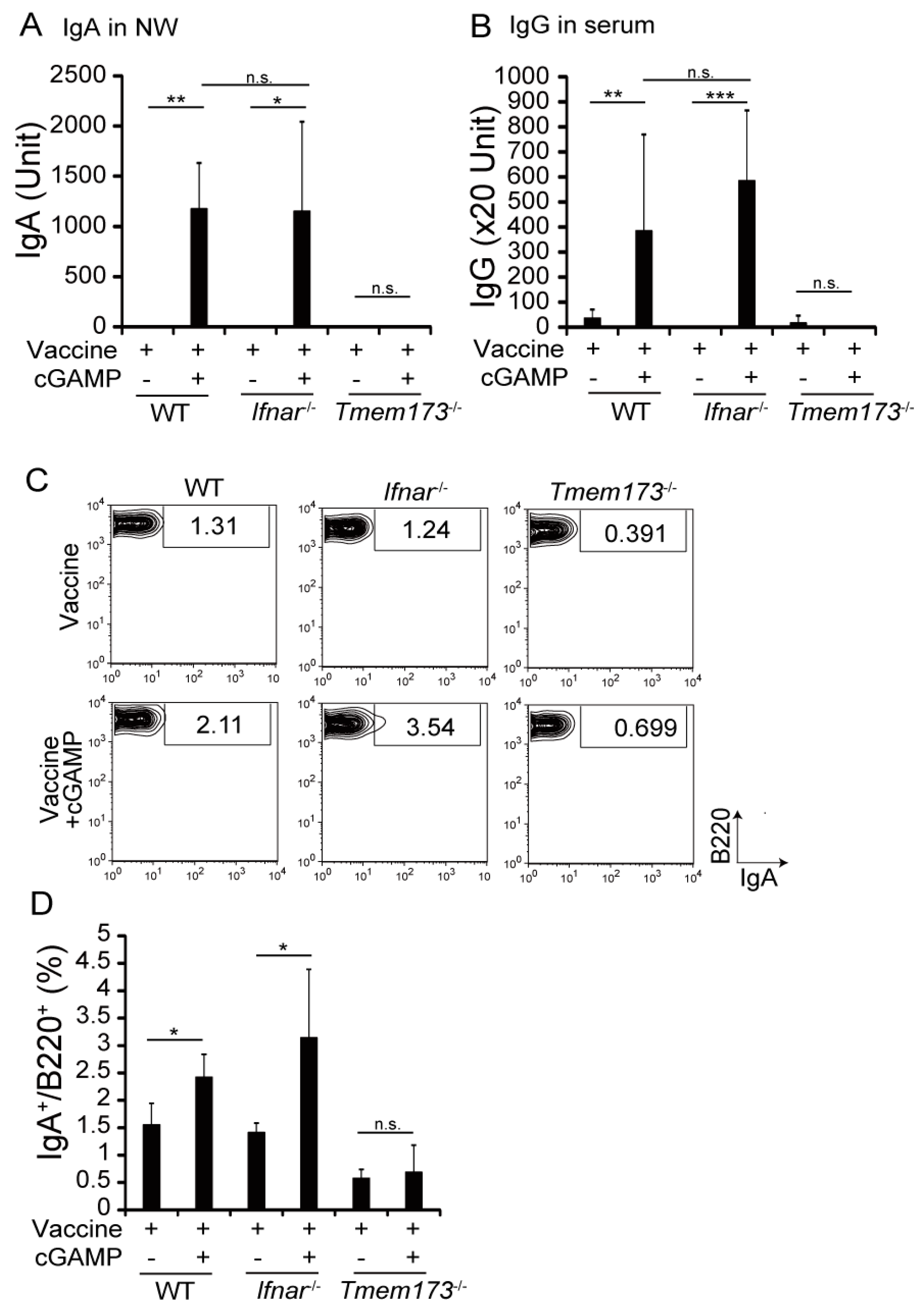
3.3. cGAMP Adjuvanticity is STING-Dependent
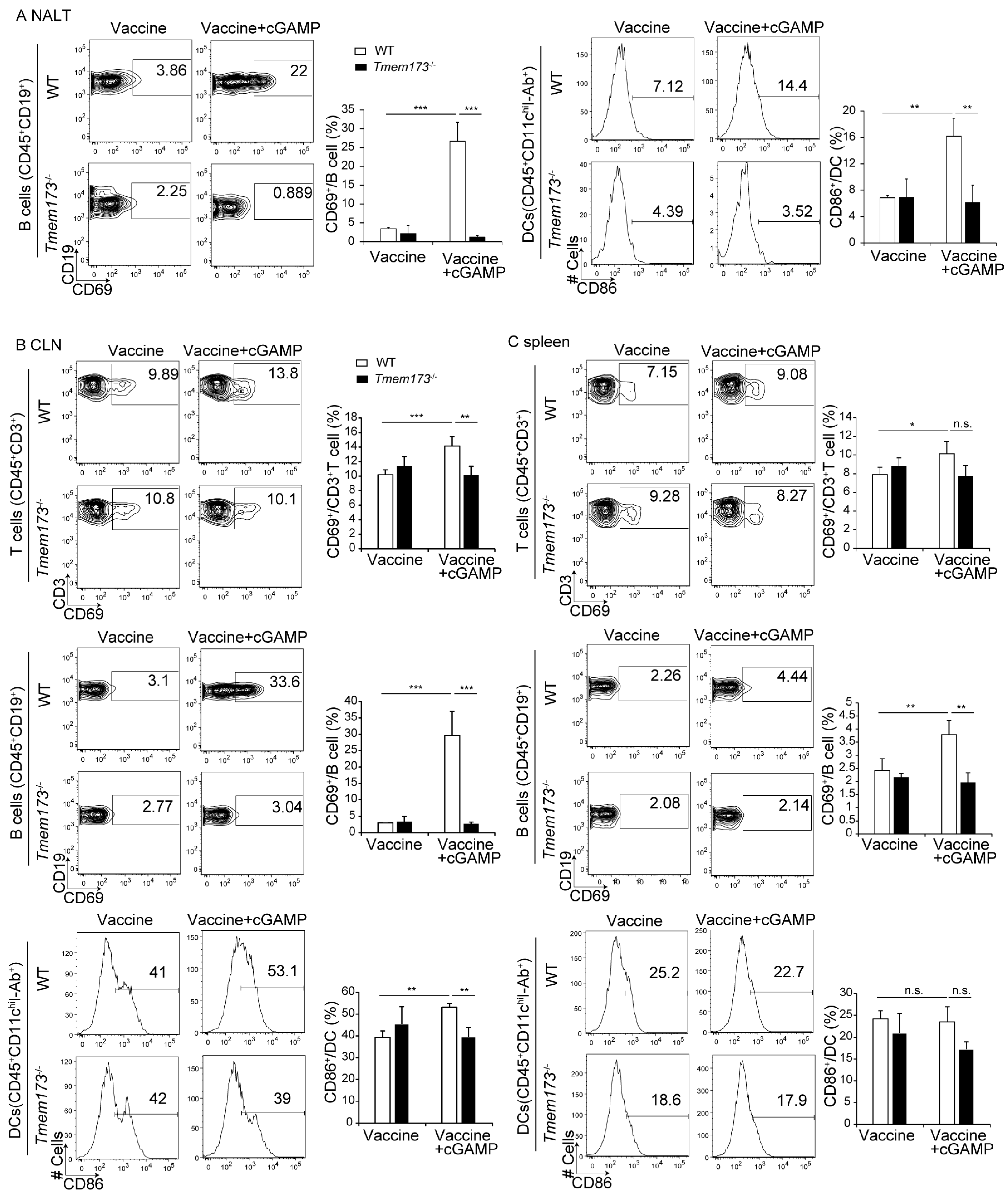
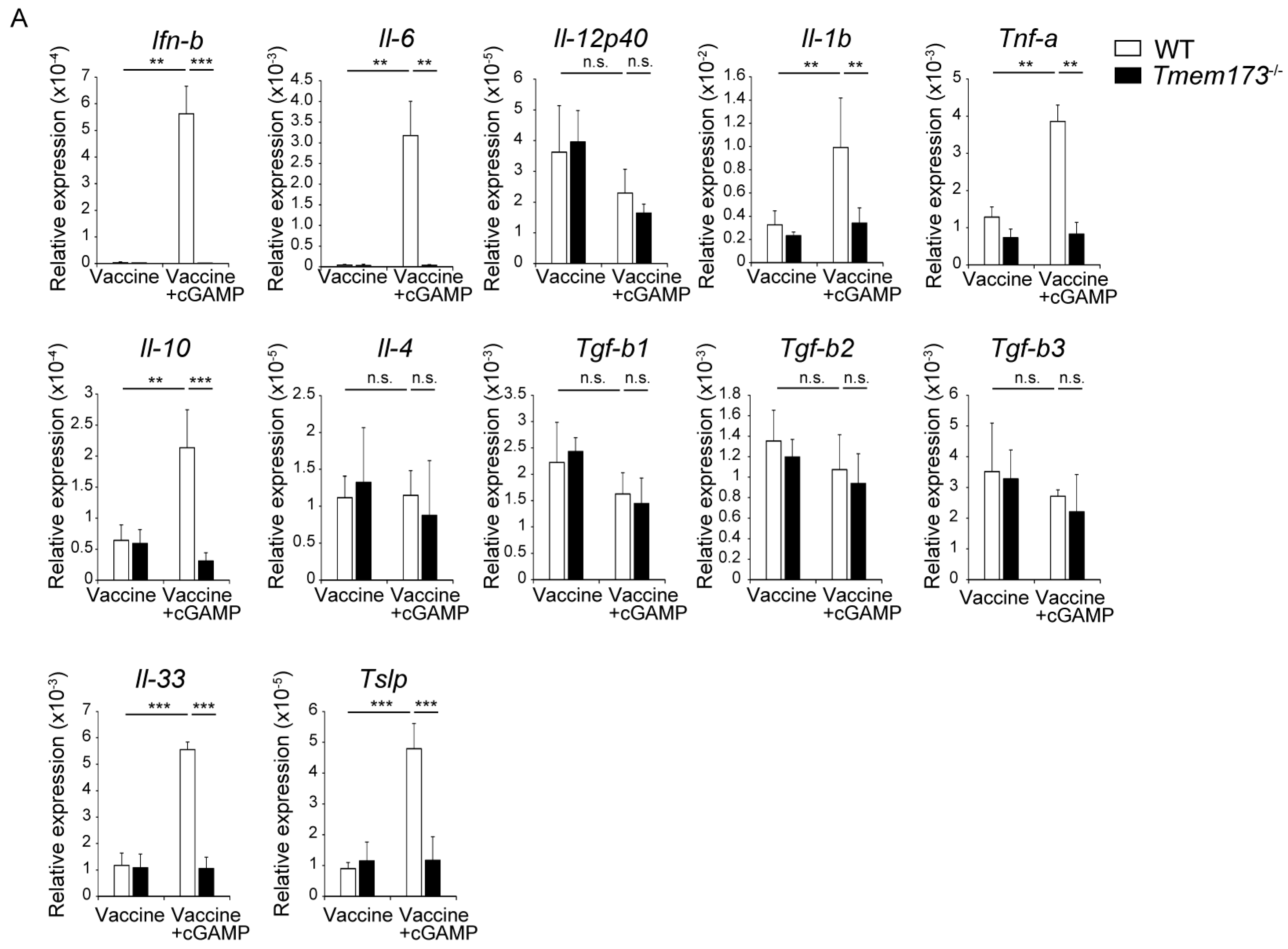
3.4. cGAMP Activates Immune Cells and Cytokine Expression in NALT via the STING Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Houser, K.; Subbarao, K. Influenza vaccines: Challenges and solutions. Cell Host Microbe 2015, 17, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Ainai, A.; Suzuki, T.; Kurata, T.; Hasegawa, H. Intranasal inactivated influenza vaccines: A reasonable approach to improve the efficacy of influenza vaccine? Jpn. J. Infect. Dis. 2016, 69, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Watanabe, I.; Ito, S.; Fujii, H.; Moriyama, M.; Tamura, S.; Takahashi, H.; Sawa, H.; Chiba, J.; Kurata, T.; et al. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J. Virol. 2005, 79, 2910–2919. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Ainai, A.; Tashiro, M.; Sata, T.; Hasegawa, H. PolyI:PolyC12U adjuvant-combined intranasal vaccine protects mice against highly pathogenic H5N1 influenza virus variants. Vaccine 2009, 27, 6276–6279. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Byun, Y.H.; Nguyen, C.T.; Kim, S.Y.; Seong, B.L.; Park, S.; Woo, G.J.; Yoon, Y.; Koh, J.T.; Fujihashi, K.; et al. Intranasal administration of a flagellin-adjuvanted inactivated influenza vaccine enhances mucosal immune responses to protect mice against lethal infection. Vaccine 2012, 30, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Blaauboer, S.M.; Mansouri, S.; Tucker, H.R.; Wang, H.L.; Gabrielle, V.D.; Jin, L. The mucosal adjuvant cyclic di-GMP enhances antigen uptake and selectively activates pinocytosis-efficient cells in vivo. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, H.; Fukuyama, S. NALT-versus PEYER’S-patch-mediated mucosal immunity. Nat. Rev. Immunol. 2004, 4, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Takaki, H.; Kure, S.; Oshiumi, H.; Sakoda, Y.; Suzuki, T.; Ainai, A.; Hasegawa, H.; Matsumoto, M.; Seya, T. Toll-like receptor 3 in nasal CD103+ dendritic cells is involved in immunoglobulin A production. Mucosal. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Blaauboer, S.M.; Gabrielle, V.D.; Jin, L. MPYS/STING-mediated TNF-α, not type I IFN, is essential for the mucosal adjuvant activity of (3′–5′)-cyclic-di-guanosine-monophosphate in vivo. J. Immunol. 2014, 192, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, H.; Shivalila, C.S.; Dawlaty, M.M.; Cheng, A.W.; Zhang, F.; Jaenisch, R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 2013, 153, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Takashima, K.; Takeda, Y.; Oshiumi, H.; Shime, H.; Okabe, M.; Ikawa, M.; Matsumoto, M.; Seya, T. STING in tumor and host cells cooperatively work for NK cell-mediated tumor growth retardation. Biochem. Biophys. Res. Commun. 2016, 478, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Awe, O.; Hufford, M.M.; Wu, H.; Pham, D.; Chang, H.C.; Jabeen, R.; Dent, A.L.; Kaplan, M.H. PU.1 expression in T follicular helper cells limits CD40L-dependent germinal center B cell development. J. Immunol. 2015, 195, 3705–3715. [Google Scholar] [CrossRef] [PubMed]
- Ebensen, T.; Schulze, K.; Riese, P.; Morr, M.; Guzmán, C.A. The bacterial second messenger cdiGMP exhibits promising activity as a mucosal adjuvant. Clin. Vaccine Immunol. 2007, 14, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Škrnjug, I.; Guzmán, C.A.; Rueckert, C.; Ruecker, C. Cyclic GMP-AMP displays mucosal adjuvant activity in mice. PLoS ONE 2014, 9, e110150. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, P.; Wu, M.X. Natural STING agonist as an “Ideal” Adjuvant for cutaneous vaccination. J. Investig. Dermatol. 2016, 136, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Madhun, A.S.; Haahein, L.R.; Nostbakken, J.K.; Ebense, T.; Chichester, J.; Yusibov, V.; Guzman, G.A.; Cox, R.J. Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional Th1 CD4+ cells and strong mucosal and systemic antibody responses in mice. Vaccine 2011, 29, 4973–4982. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Mariathasan, S.; Nahm, M.H.; Baranyay, F.; Peschon, J.J.; Chaplin, D.D. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science 1996, 271, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Le Hir, M.; Bluethmann, H.; Kosco-Vilbois, M.H.; Müller, M.; di Padova, F.; Moore, M.; Ryffel, B.; Eugster, H.P. Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor-1 signaling. J. Exp. Med. 1996, 183, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
- Dannappel, M.; Vlantis, K.; Kumari, S.; Polykratis, A.; Kim, C.; Wachsmuth, L.; Eftychi, C.; Lin, J.; Corona, T.; Hermance, N.; et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 2014, 513, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Aoshi, T.; Ozasa, K.; Kusakabe, T.; Momota, M.; Haseda, Y.; Kobari, S.; Kuroda, E.; Kobiyama, K.; Coban, C.; et al. RNA is an adjuvanticity mediator for the lipid-based mucosal adjuvant, endocine. Sci. Rep. 2016, 6, 29165. [Google Scholar] [CrossRef] [PubMed]
- Ebensen, T.; Libanova, R.; Schulze, K.; Yevsa, T.; Morr, M.; Guzmán, C.A. Bis-(3′,5′)-cyclic dimeric adenosine monophosphate: Strong Th1/Th2/Th17 promoting mucosal adjuvant. Vaccine 2011, 29, 5210–5220. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.M.; Forrest, G.; Wisniewski, T.; Porter, G.; Freed, D.C.; DeMartino, J.A.; Zaller, D.M.; Guo, Z.; Leone, J.; Fu, T.M.; et al. Evidence for cyclic diguanylate as a vaccine adjuvant with novel immunostimulatory activities. Cell. Immunol. 2012, 278, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A. The regulation of IgA class switching. Nat. Rev. Immunol. 2008, 8, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xing, F. Human TSLP-Educated DCs. Cell. Mol. Immunol. 2008, 5, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.Y.; Kita, H. IL-33: Biological properties, functions, and roles in airway disease. Immunol. Rev. 2017, 278, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Segawa, R.; Yamashita, S.; Mizuno, N.; Shiraki, M.; Hatayama, T.; Satou, N.; Hiratsuka, M.; Hide, M.; Hirasawa, N. Identification of a cell line producing high levels of TSLP: Advantages for screening of anti-allergic drugs. J. Immunol. Methods 2014, 402, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Ainai, A.; Ami, Y.; Nagata, N.; Iwata, N.; Kawaguchi, A.; Suzaki, Y.; Odagiri, T.; Tashiro, M.; Takahashi, H.; et al. Intranasal administration of adjuvant-combined vaccine protects monkeys from challenge with the highly pathogenic influenza A H5N1 virus. J. Med. Virol. 2010, 82, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Innate immunity to virus infection. Immunol. Rev. 2009, 227, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.D.; Diebold, S.S.; Slack, E.M.; Tomizawa, H.; Hemmi, H.; Kaisho, T.; Akira, S.; Reis e Sousa, C. Toll-like receptor expression in murine DC subsets: Lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 2003, 33, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Tatematsu, M.; Nishikawa, F.; Azuma, M.; Ishii, N.; Morii-Sakai, A.; Shime, H.; Seya, T. Defined TLR3-specific adjuvant that induces NK and CTL activation without significant cytokine production in vivo. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Kataoka, K.; Yamagishi, J.; Ogawa, S.; Seya, T.; Matsumoto, M. A TLR3-specific adjuvant relieves innate resistance to PD-L1 blockade without cytokine toxicity in tumor vaccine immunotherapy. Cell Rep. 2017, 19, 1874–1887. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takaki, H.; Takashima, K.; Oshiumi, H.; Ainai, A.; Suzuki, T.; Hasegawa, H.; Matsumoto, M.; Seya, T. cGAMP Promotes Germinal Center Formation and Production of IgA in Nasal-Associated Lymphoid Tissue. Med. Sci. 2017, 5, 35. https://doi.org/10.3390/medsci5040035
Takaki H, Takashima K, Oshiumi H, Ainai A, Suzuki T, Hasegawa H, Matsumoto M, Seya T. cGAMP Promotes Germinal Center Formation and Production of IgA in Nasal-Associated Lymphoid Tissue. Medical Sciences. 2017; 5(4):35. https://doi.org/10.3390/medsci5040035
Chicago/Turabian StyleTakaki, Hiromi, Ken Takashima, Hiroyuki Oshiumi, Akira Ainai, Tadaki Suzuki, Hideki Hasegawa, Misako Matsumoto, and Tsukasa Seya. 2017. "cGAMP Promotes Germinal Center Formation and Production of IgA in Nasal-Associated Lymphoid Tissue" Medical Sciences 5, no. 4: 35. https://doi.org/10.3390/medsci5040035





