Electrodermal Activity during Blood Pooling for Arterial Blood Gases Analysis in Sedated Adult Intensive Care Unit Patients
Abstract
:1. Introduction
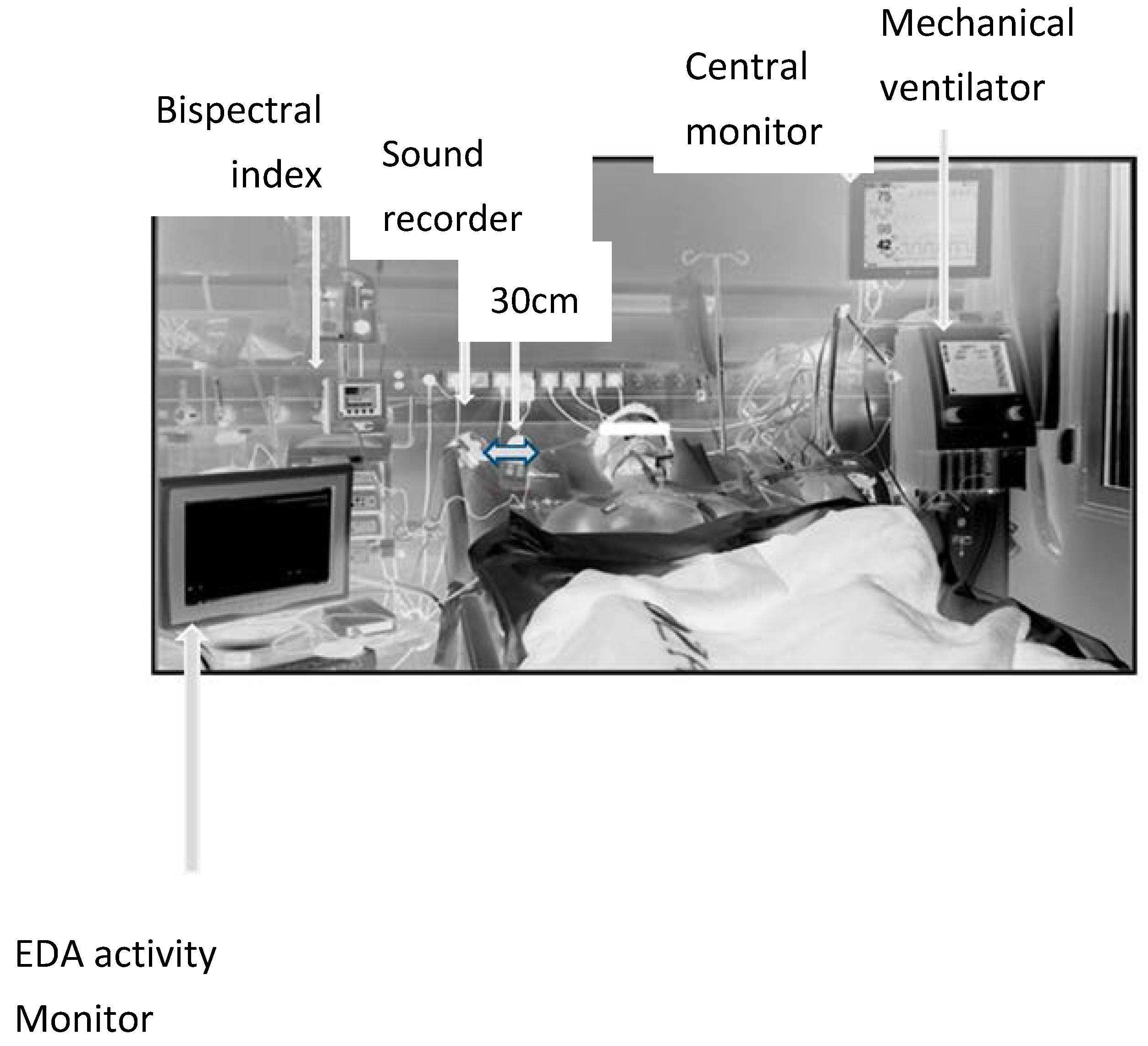
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A

| Group | Α (RSS 5–6), n = 9, 15 s Measurements | |||||
|---|---|---|---|---|---|---|
| Parameter→ Statistic | HR (bpm) | VPC (no) | STII | SAP (mmHg) | MAP (mmHg) | |
| B | (s) | 73.2 (18.9) | 0.4 (0.69) | 0.047 (0.75) | 131.4 (22.39) | 82.2 (13.2) |
| A | (s) | 73.8 (19.35) | 0.5 (0.85) | 0.048 (0.08) | 132.4 (22.91) | 82.8 (14.63) |
| p | 0.1114 + | 0.3466 + | 0.6811 + | 0.6618 + | 0.6477 + | |
| CI [95%] | [−1.53,0.19] | [−0.7,0.14] | [−0.007,0.14] | [−6.75,4.53] | [−3.9,2.57] | |
| Parameter→ statistic | DAP (mmHg) | RR (br/min) | ArHP (μSs) | ArSP (μSs) | NFSC (μSs) | |
| B | (s) | 56.7 (8.31) | 13.9 (3.84) | 0.006 (0.019) | 0.006 (0.019) | 0.027 (0.046) |
| A | (s) | 56.9 (9.02) | 13.9 (3.064) | 1.035 (1.463) | 0.095 (0.147) | 0.121 (0.102) |
| p | 0.7531 + | 0.8934 * | 0.0145 * | 0.1003 * | 0.0213 * | |
| CI [95%] | [−1.79,1.35] | [−10,10] | [−2.82,−0.09] | [−0.04,−0.09] | [−0.19,−0.01] | |
| Parameter→ statistic | AvRT | AvP (μSs) | AUC (μSs) | SC (μS) | ||
| B | (s) | 0 (0.006) | 0.007 (0.013) | 0.012 (0.025) | 6.985 (3.28) | |
| A | (s) | 0.03 (0.075) | 0.09 (0.082) | 1.035 (1.463) | 7.032 (3.31) | |
| p | 0.0975 * | 0.0145 * | 0.0141 * | 0.0273 * | ||
| CI [95%] | [−0.13,−1 × 10−3] | [−0.17,−0.03] | [−2.76,−0.9] | [−0.1,−0.002] | ||
| Group | Α (RSS 5–6), n = 9, 60 s Measurements | |||||
| Parameter→ statistic | HR (bpm) | VPC (no) | STII | SAP (mmHg) | MAP (mmHg) | |
| B | (s) | 87.41 (14.25) | 0.294 (0.68) | 0.037 (0.076) | 115.3 (21.8) | 70.76 (12.01) |
| A | (s) | 86.94 (14.04) | 0.235 (0.66) | 0.038 (0.077) | 115.4 (22.48) | 71.06 (12.37) |
| p | 0.0431 + | 0.3466 + | 0.6811 + | 0.3466 + | 0.3466 + | |
| CI [95%] | [−1.42,−0.03] | [−0.36,0.14] | [−7 × 10−3,−4 × 10−3] | [−6.97,2.75] | [−4.04,1.6] | |
| Parameter→ statistic | DAP (mmHg) | RR (br/min) | ArHP (μSs) | ArSP (μSs) | NFSC (μSs) | |
| B | (s) | 50.65 (8.57) | 12.59 (2.15) | 0.012 (0.035) | 0.022 (0.054) | 0.035 (0.075) |
| A | (s) | 50.59 (8.5) | 12.59 (2.15) | 0.161 (0.3) | 0.026 (0.04) | 0.142 (0.11) |
| p | 0.3466 + | 0.99 | 0.0142 * | 0.0224 * | 0.0207 * | |
| CI [95%] | [−1.84,0.72] | [−4,3] | [−61.23,−1.2] | [−3.68,−0.14] | [−0.08,−0.01] | |
| Parameter→ statistic | AvRT | AvP (μSs) | AUC (μSs) | SC (μS) | ||
| B | (s) | 0 | 0.004 (0.01) | 0.022 (0.054) | 7.83 (3.89) | |
| A | (s) | 6 × 10−4 (0.002) | 0.013 (0.008) | 0.171 (0.297) | 8.19 (3.59) | |
| p | 0.1814 * | 0.0224 * | 0.0142 * | 0.0514 + | ||
| CI [95%] | [−0.09,−0.01] | [−0.28,−0.02] | [−60.53,−1.2] | [−0.71,2.7 × 10−3] | ||
| Group | B (RSS 5–6), n = 17, 15 s Measurements | |||||
|---|---|---|---|---|---|---|
| Parameter→ statistic | HR (bpm) | VPC (no) | STII | SAP (mmHg) | MAP (mmHg) | |
| B | 87.4 (14.25) | 0.29 (0.68) | 0.037 (0.07) | 115.3 (21.8) | 70.7 (12) | |
| A | 86.94 (14) | 0.23 (0.66) | 0.38 (0.7) | 115.4 (22.5) | 71 (12.4) | |
| P | 0.587 * | 1 * | 0.728 * | 0.671 * | 0.269 * | |
| CI [95%] | [−1,4.9] | NA | [−0.01,0.01] | [–3,2] | [–3,1] | |
| Parameter→ statistic | DAP (mmHg) | RR (br/min) | ArHP (μSs) | ArSP (μSs) | NFSC (μSs) | |
| B | 50.65 (8.5) | 12.6 (2.1) | 0.012 (0.035) | 0.022 (0.054) | 0.035 (0.075) | |
| A | 50.6 (8.5) | 12.5 (2.1) | 0.161 (0.3) | 0.026 (0.04) | 0.142 (0.11) | |
| P | 0.999 * | NA | 0.162 * | 0.44 * | 0.0003 * | |
| CI [95%] | [–2,2] | NA | [−0.54,−0.029] | [−0.06,0.059] | [−0.135,−0.07] | |
| Parameter→ statistic | AvRT | AvP (μSs) | AUC (μSs) | SC (μS) | ||
| B | 0 | 0.004 (0.01) | 0.022 (0.054) | 7.83 (3.89) | ||
| A | 6 × 10−4 (0.02) | 0.013 (0.08) | 0.171 (0.297) | 8.14 (3.59) | ||
| P | NA | 0.006 * | 0.023 * | 0.013 * | ||
| CI [95%] | NA | [−0.01,−0.009] | [−0.5,−0.02] | [−0.05,−4e−3] | ||
| Group | B (RSS 5–6), n = 17, 60 s Measurements | |||||
| Parameter→ statistic | HR (bpm) | VPC (no) | STII | SAP (mmHg) | MAP (mmHg) | |
| B | 87.4 (14.2) | 0.29 (0.68) | 0.037 (0.07) | 115.3 (21.8) | 70.7 (12) | |
| A | 87 (14) | 0.26 (0.68) | 0.038 (0.07) | 115.6 (22.3) | 71.12 (12.3) | |
| P | 0.821 * | NA | 0.727 * | 0.44 * | 0.169 * | |
| CI [95%] | [−1,3.5] | NA | [−0.019,0.01] | [–3,2] | [–3,1] | |
| Parameter→ statistic | DAP (mmHg) | RR (br/min) | ArHP (μSs) | ArSP (μSs) | NFSC (μSs) | |
| B | 50.65 (8.7) | 12.6 (2) | 0.338 (0.76) | 0.148 (0.231) | 0.048 (0.07) | |
| A | 50.6 (8) | 12.6 (2) | 1.74 (2.41) | 0.159 (0.187) | 0.1 (0.098) | |
| P | 0.9 * | NA | 0.0041 * | 0.593 | 0.0006 * | |
| CI [95%] | [–2,2] | NA | [−2.2,−0.46] | [−0.104,0.07] | [−0.08,−0.03] | |
| Parameter→ statistic | AvRT | AvP (μSs) | AUC (μSs) | SC (μS) | ||
| B | 0 | 0.008 (0.008) | 0.412 (0.752) | 7.837 (3.89) | ||
| A | 0 | 0.011 (0.006) | 1.748 (3.74) | 7.86 (3.88) | ||
| P | NA | 0.096 * | 0.0058 * | 0.013 * | ||
| CI [95%] | NA | [−1 × 10−2,−2 × 10−5] | [−2.2,−0.4] | [−0.05,−6 × 10−3] | ||
References
- Bucsein, W. Physiology of Electrodermal system. In Electrodermal Activity, 2nd ed.; Bucsein, W., Ed.; Springer Science + Business Media LLC: Berlin, Germany, 2012; pp. 17–33. [Google Scholar]
- Paulus, J.; Roquilly, A.; Beloeil, H.; Théraud, J.; Asehnoune, K.; Lejus, C. Pupillary reflex measurement predicts insufficient analgesia before endotracheal suctioning in critically ill patients. Crit. Care 2013, 17, R161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslanidis, Τ. Electrodermal activity: Applications in Perioperative Care. Int. J. Med. Res. Health Sci. 2014, 3, 687–695. [Google Scholar]
- Günther, A.C.; Bottai, M.; Schandl, A.R.; Storm, H.; Rossi, P.; Sackey, P.V. Palmar skin conductance variability and the relation to stimulation, pain and the motor activity assessment scale in intensive care unit patients. Crit. Care 2013, 17, R51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gjerstad, A.C.; Wagner, K.; Henrichsen, T.; Storm, H. Skin Conductance versus the Modified COMFORT Sedation Score as a Measured of Discomfort in Artificially Ventilated Children. Pediatrics 2008, 122, e848–e853. [Google Scholar] [CrossRef] [PubMed]
- Karpe, J.; Misiołek, A.; Daszkiewicz, A.; Misiołek, H. Objective assessment of pain-related stress in mechanically ventilated newborns based on skin conductance fluctuations. Anaesthesiol. Intensive Ther. 2013, 45, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Sabourdin, N.; Arnaout, M.; Louvet, N.; Guye, M.L.; Piana, F.; Constant, I. Pain monitoring in anesthetized children: First assessment of skin conductance and analgesia-nociception index at different infusion rates of remifentanil. Paediatr. Anaesth. 2013, 23, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Czaplik, M.; Hübner, C.; Köny, M.; Kaliciak, J.; Kezze, F.; Leonhardt, S.; Rossaint, R. Acute pain therapy in postanesthesia care unit directed by skin conductance: A randomized controlled trial. PLoS ONE 2012, 7, e41758. [Google Scholar] [CrossRef] [PubMed]
- Ledowski, T.; Bromilow, J.; Wu, J.; Paech, M.J.; Storm, H.; Schug, S.A. The assessment of postoperative pain by monitoring skin conductance: Results of a prospective study. Anaesthesia 2007, 62, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Med Storm Monitor User Manual v1.0 English MA001-25; Part Number 4001; Med Storm Innovation AS: Oslo, Norway, 2010.
- Günther, A.C.; Schandl, A.R.; Berhardsson, J.; Bjärtå, A.; Wällgren, M.; Sundin, O.; Alvarsson, J.; Bottai, M.; Martling, C.R.; Sackey, P.V. Pain rather than induced emotions and ICU sound increases skin conductance variability in healthy volunteers. Acta Anaesthesiol. Scand. 2016, 60, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Doberenz, S.; Roth, W.T.; Maslowski, N.I.; Wollburg, E.; Kim, S. Methodological Considerations in Ambulatory Skin Conductance Monitoring. Int. J. Psychophysiol. 2011, 80, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Putz-Maidl, C.; MacAndrew, S.N.; Leske, J.S. Noise in ICU: Sound levels can be harmful. Nurs. Crit. Care 2014, 9, 29–35. [Google Scholar] [CrossRef]
- Goldstein, J.M.; Jerram, M.; Poldrack, R.; Ahern, T.; Kennedy, D.N.; Seidman, L.J.; Makris, N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J. Neurosci. 2005, 25, 9309–9316. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, E.; Moya-Albiol, L.; González-Bono, E.; Salvador, A.; Ricarte, J.; Gómez-Amor, J. Gender differences in cardiovascular and electrodermal responses to public speaking task: The role of anxiety and mood states. Int. J. Psychophysiol. 2001, 42, 253–264. [Google Scholar] [CrossRef]
- Tobaldini, E.; Costantino, G.; Solbiati, M.; Cogliati, C.; Kara, T.; Nobili, L.; Montano, N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci. Biobehav. Rev. 2017, 74, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Aslanidis, T. Perspectives of Autonomic nervous system perioperative monitoring-focus on selected tools. Int. Arch. Med. 2015, 8, 1–9. [Google Scholar]
- Storm, H.; Myre, K.; Rostrup, M.; Stokland, O.; Lien, M.D.; Raeder, J.C. Skin conductance correlates with perioperative stress. Acta Anaesthesiol. Scand. 2002, 46, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.O.; Storm, H.; Boglino-Hörlin, A.; Le Guen, M.; Gayat, E.; Fischler, M. Skin conductance as a pain assessment tool during chest tube removal: An observational study. Eur. J. Pain 2017, 21, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, V.; Reinsberger, C.; Scirica, B.; O’Brien, M.H.; Avery, K.R.; Henderson, G.; Lee, J.W. Continuous electrodermal activity as a potential novel neurophysiological biomarker of prognosis after cardiac arrest—A pilot study. Resuscitation 2015, 93, 128–135. [Google Scholar] [CrossRef] [PubMed]
| Group A | Group B | Group A | Group B | ||
|---|---|---|---|---|---|
| (n) measure | 10 | 15 | APACHE II | 15.4 (1.55) | 19.6 (1.66) |
| Sex | Male = 10, Female = 0 | Male = 9, Female = 6 | SOFA | 6.3 (0.9) | 7.9 (0.4) |
| Age (years) | 66.5 (14.8) | 63.8 (10.9) | GOSE | 6.4 (0.9) | 5.2 (0.8) |
| Weight (kg) | 90.6 (15.1) | 89.95 (12.6) | t (°C) | 37.2 (0.3) | 37.1 (0.4) |
| ΒMI (kg/m2) | 28 (1.65) | 30.3 (0.85) | PaO2/FiO2 | 294 (69.3) | 230 (81.8) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslanidis, T.; Grosomanidis, V.; Karakoulas, K.; Chatzisotiriou, A. Electrodermal Activity during Blood Pooling for Arterial Blood Gases Analysis in Sedated Adult Intensive Care Unit Patients. Med. Sci. 2018, 6, 20. https://doi.org/10.3390/medsci6010020
Aslanidis T, Grosomanidis V, Karakoulas K, Chatzisotiriou A. Electrodermal Activity during Blood Pooling for Arterial Blood Gases Analysis in Sedated Adult Intensive Care Unit Patients. Medical Sciences. 2018; 6(1):20. https://doi.org/10.3390/medsci6010020
Chicago/Turabian StyleAslanidis, Theodoros, Vasilios Grosomanidis, Konstantinos Karakoulas, and Athanasios Chatzisotiriou. 2018. "Electrodermal Activity during Blood Pooling for Arterial Blood Gases Analysis in Sedated Adult Intensive Care Unit Patients" Medical Sciences 6, no. 1: 20. https://doi.org/10.3390/medsci6010020






