Risk Factors Including Age, Stage and Anatomic Location that Impact the Outcomes of Patients with Synovial Sarcoma
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Immunohistochemical Staining Characteristics
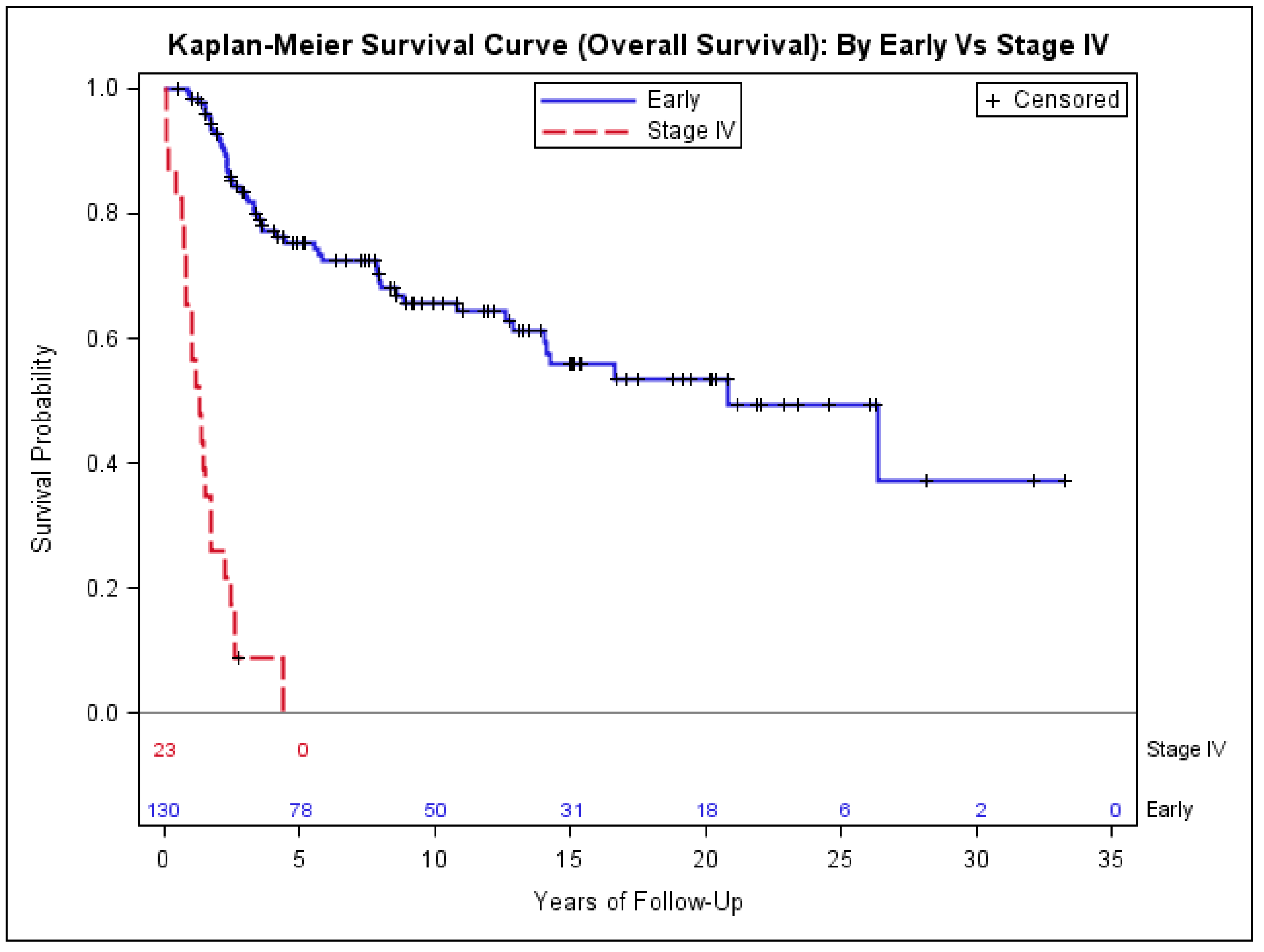
3.2. Risk Factors Associated with Stage IV Disease at Presentation
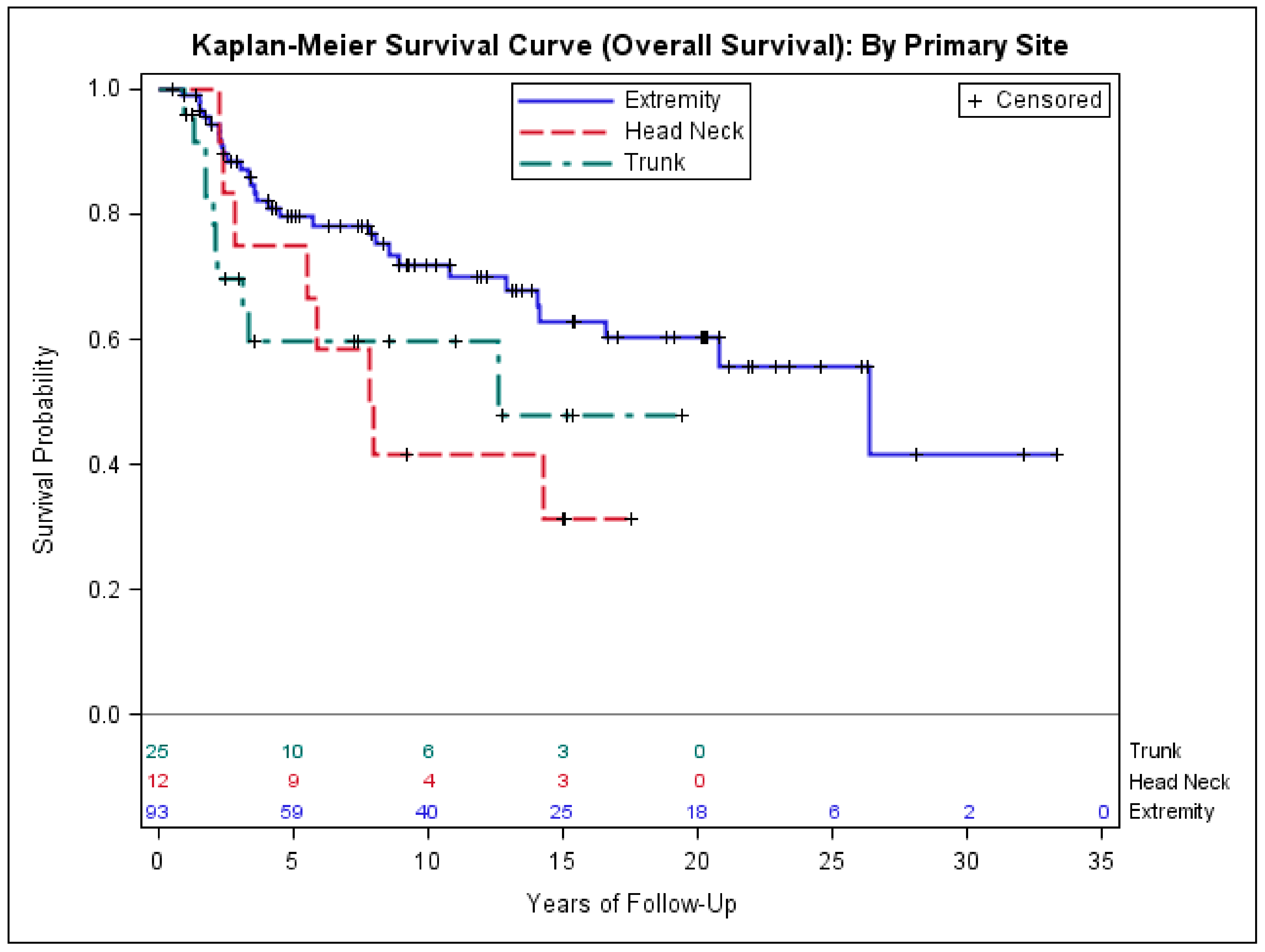
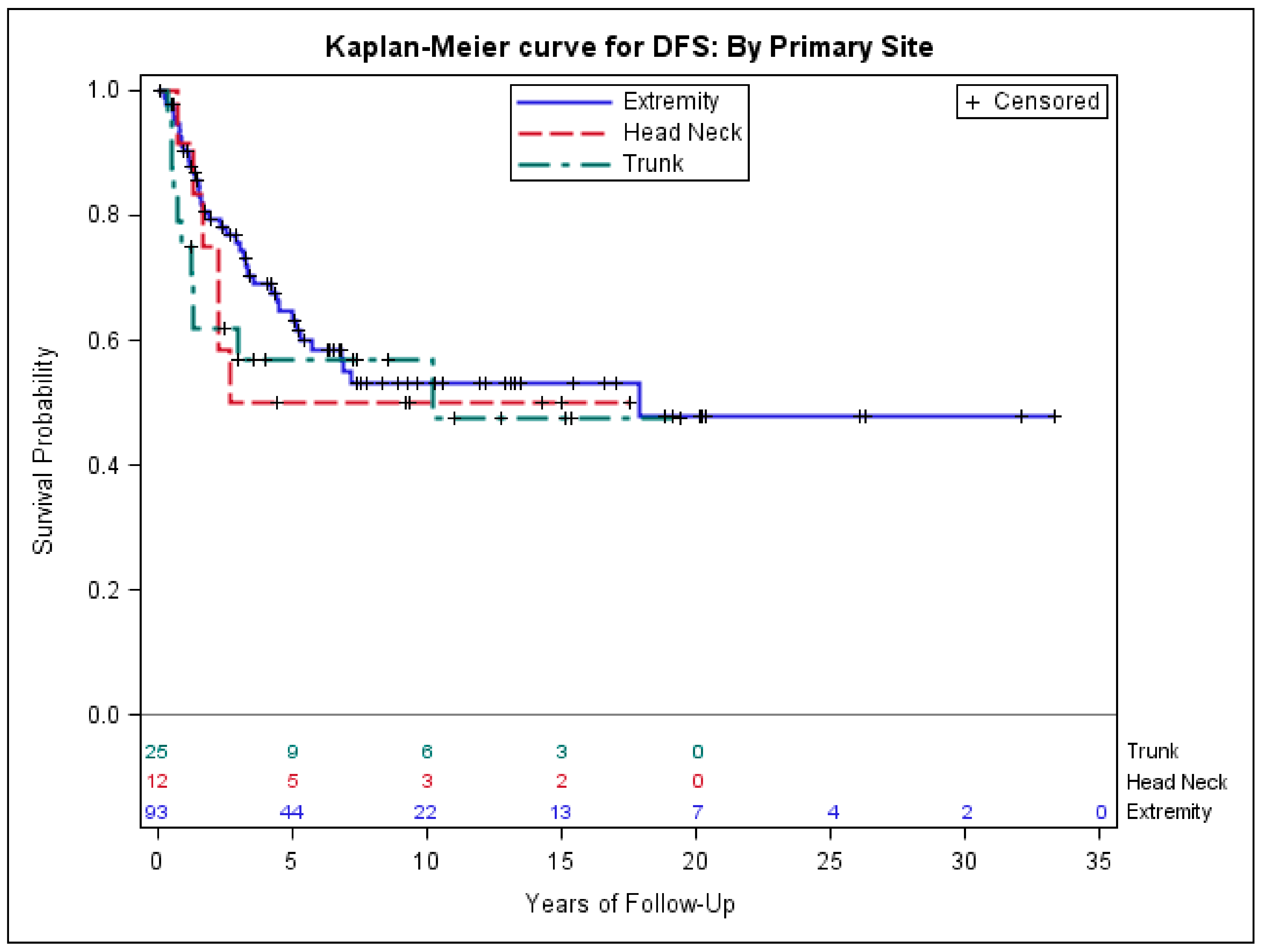
3.3. Risk Factors Associated with Poor Outcomes in Early-Stage Synovial Sarcoma
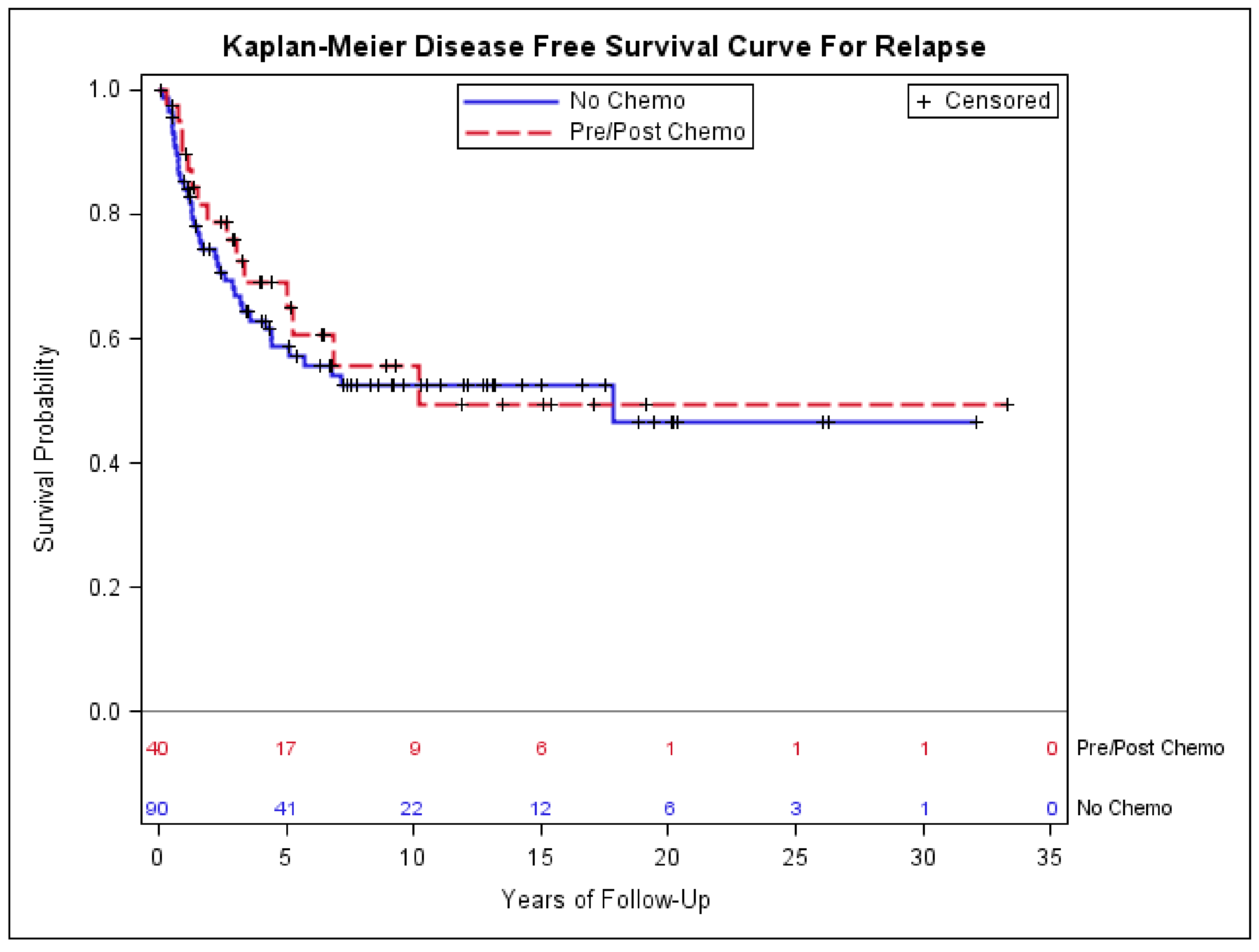
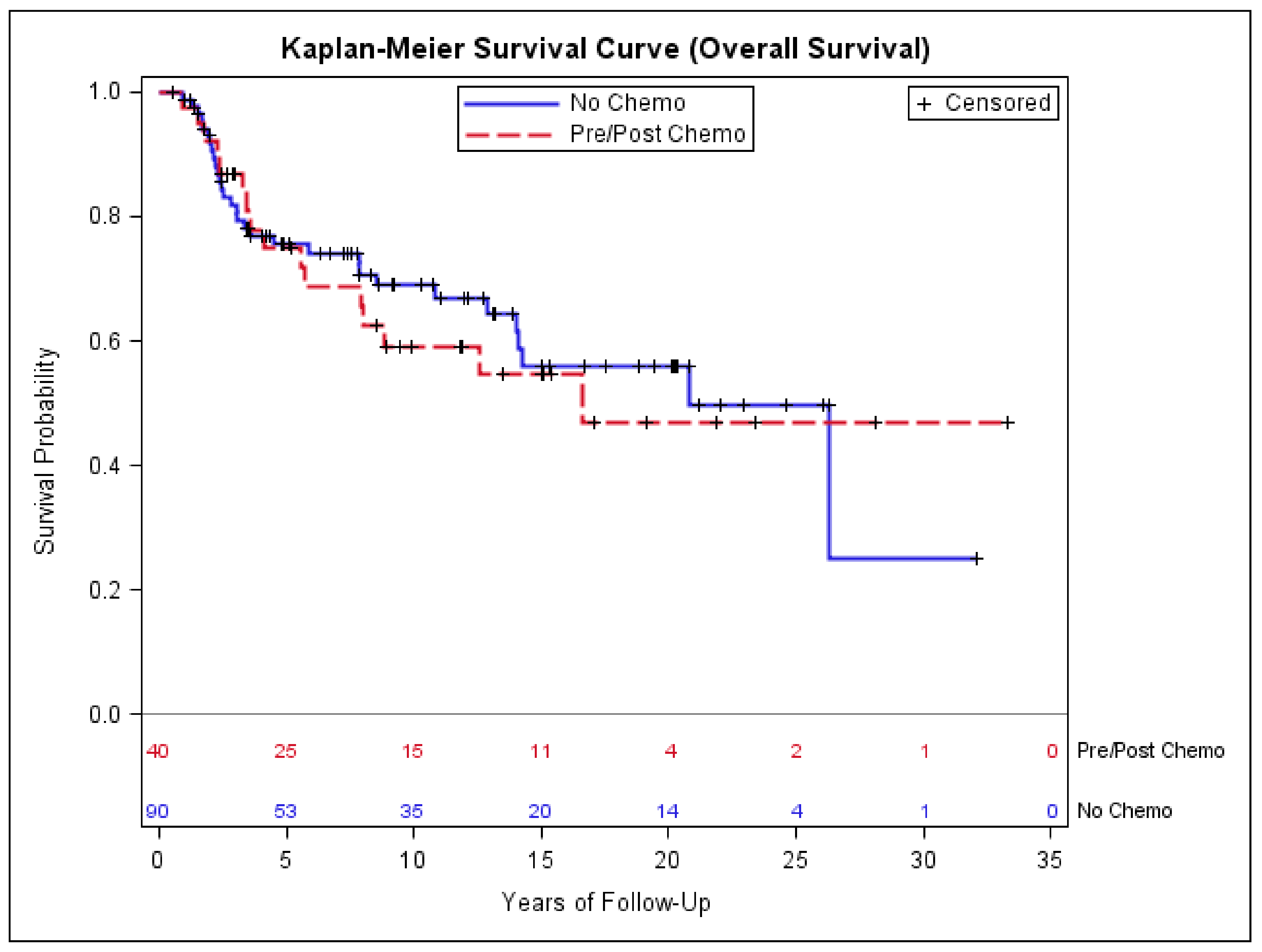
3.4. Impact of Chemotherapy on Outcomes of Patients with Early-Stage Synovial Sarcoma
3.5. Metastatectomy and Overall Survival of Patients with Lung Metastasis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fisher, C. Synovial sarcoma. Ann. Diagn. Pathol. 1998, 2, 401–421. [Google Scholar] [CrossRef]
- Clark, J.; Rocques, P.J.; Crew, A.J.; Gill, S.; Shipley, J.; Chan, A.M.; Gusterson, B.A.; Cooper, C.S. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat. Genet. 1994, 7, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Kawai, A.; Woodruff, J.; Healey, J.H.; Brennan, M.F.; Antonescu, C.R.; Ladanyi, M. SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N. Engl. J. Med. 1998, 338, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Amary, M.F.; Berisha, F.; Bernardi Fdel, C.; Herbert, A.; James, M.; Reis-Filho, J.S.; Fisher, C.; Nicholson, A.G.; Tirabosco, R.; Diss, T.C.; et al. Detection of SS18-SSX fusion transcripts in formalin-fixed paraffin-embedded neoplasms: Analysis of conventional RT-PCR, qRT-PCR and dual color FISH as diagnostic tools for synovial sarcoma. Mod. Pathol. 2007, 20, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Cagle, L.A.; Mirra, J.M.; Storm, F.K.; Roe, D.J.; Eilber, F.R. Histologic features relating to prognosis in synovial sarcoma. Cancer 1987, 59, 1810–1814. [Google Scholar] [CrossRef]
- Crew, A.J.; Clark, J.; Fisher, C.; Gill, S.; Grimer, R.; Chand, A.; Shipley, J.; Gusterson, B.A.; Cooper, C.S. Fusion of SYT to two genes, SSX1 and SSX2, encoding proteins with homology to the Kruppel-associated box in human synovial sarcoma. EMBO J. 1995, 14, 2333–2340. [Google Scholar] [PubMed]
- Skytting, B.; Nilsson, G.; Brodin, B.; Xie, Y.; Lundeberg, J.; Uhlen, M.; Larsson, O. A novel fusion gene, SYT-SSX4, in synovial sarcoma. J. Natl. Cancer Inst. 1999, 91, 974–975. [Google Scholar] [CrossRef] [PubMed]
- Reeves, B.R.; Smith, S.; Fisher, C.; Warren, W.; Knight, J.; Martin, C.; Chan, A.M.; Gusterson, B.A.; Westbury, G.; Cooper, C.S. Characterization of the translocation between chromosomes X and 18 in human synovial sarcomas. Oncogene 1989, 4, 373–378. [Google Scholar] [PubMed]
- Kadoch, C.; Crabtree, G.R. Reversible disruption of mSWI/SNF (BAF) complexes by the SS18-SSX oncogenic fusion in synovial sarcoma. Cell 2013, 153, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Stegmaier, S.; Leuschner, I.; Poremba, C.; Ladenstein, R.; Kazanowska, B.; Ljungman, G.; Scheer, M.; Blank, B.; Bielack, S.; Klingebiel, T.; et al. The prognostic impact of SYT-SSX fusion type and histological grade in pediatric patients with synovial sarcoma treated according to the CWS (Cooperative Weichteilsarkom Studie) trials. Pediatr. Blood Cancer 2016, 64, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Canter, R.J.; Qin, L.X.; Maki, R.G.; Brennan, M.F.; Ladanyi, M.; Singer, S. A synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clin. Cancer Res. 2008, 14, 8191–8197. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.B.; Barrott, J.J.; Xie, M.; Haldar, M.; Jin, H.; Zhu, J.F.; Monument, M.J.; Mosbruger, T.L.; Langer, E.M.; Randall, R.L.; et al. The impact of chromosomal translocation locus and fusion oncogene coding sequence in synovial sarcomagenesis. Oncogene 2016, 35, 5021–5032. [Google Scholar] [CrossRef] [PubMed]
- Okcu, M.F.; Munsell, M.; Treuner, J.; Mattke, A.; Pappo, A.; Cain, A.; Ferrari, A.; Casanova, M.; Ozkan, A.; Raney, B. Synovial sarcoma of childhood and adolescence: A multicenter, multivariate analysis of outcome. J. Clin. Oncol. 2003, 21, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Conrad, E.U., 3rd; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; et al. Soft Tissue Sarcoma, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2016, 14, 758–786. [Google Scholar] [CrossRef]
- Campbell, C.; Gallagher, J.; Dickinson, I. Synovial sarcoma—Towards a simplified approach to prognosis. ANZ J. Surg. 2004, 74, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Guillou, L.; Coindre, J.; Gallagher, G.; Terrier, P.; Gebhard, S.; de Saint Aubain Somerhausen, N.; Michels, J.; Jundt, G.; Vince, D.R.; Collin, F.; et al. Detection of the synovial sarcoma translocation t(X;18) (SYT;SSX) in paraffin-embedded tissues using reverse transcriptase-polymerase chain reaction: A reliable and powerful diagnostic tool for pathologists. A molecular analysis of 221 mesenchymal tumors fixed in different fixatives. Hum. Pathol. 2001, 32, 105–112. [Google Scholar] [PubMed]
- Deshmukh, R.; Mankin, H.J.; Singer, S. Synovial sarcoma: The importance of size and location for survival. Clin. Orthop. Relat. Res. 2004, 155–161. [Google Scholar] [CrossRef]
- Spillane, A.J.; A’Hern, R.; Judson, I.R.; Fisher, C.; Thomas, J.M. Synovial sarcoma: A clinicopathologic, staging, and prognostic assessment. J. Clin. Oncol. 2000, 18, 3794–3803. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.H.; Hefti, F.; Speth, B.M.; Jundt, G.; Guillou, L.; Exner, U.G.; von Hochstetter, A.R.; Cserhati, M.D.; Fuchs, B.; Mouhsine, E.; et al. Synovial sarcomas usually metastasize after > 5 years: A multicenter retrospective analysis with minimum follow-up of 10 years for survivors. Ann. Oncol. 2011, 22, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, R.S. Evidence for using adjuvant chemotherapy as standard treatment of soft tissue sarcoma. Semin. Radiat. Oncol. 1999, 9, 349–351. [Google Scholar] [CrossRef]
- Italiano, A.; Penel, N.; Robin, Y.M.; Bui, B.; Le Cesne, A.; Piperno-Neumann, S.; Tubiana-Hulin, M.; Bompas, E.; Chevreau, C.; Isambert, N.; et al. Neo/adjuvant chemotherapy does not improve outcome in resected primary synovial sarcoma: A study of the French Sarcoma Group. Ann. Oncol. 2009, 20, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Baheti, A.D.; Tirumani, S.H.; Sewatkar, R.; Shinagare, A.B.; Hornick, J.L.; Ramaiya, N.H.; Jagannathan, J.P. Imaging features of primary and metastatic extremity synovial sarcoma: A single institute experience of 78 patients. Br. J. Radiol. 2015, 88. [Google Scholar] [CrossRef] [PubMed]
- Bakri, A.; Shinagare, A.B.; Krajewski, K.M.; Howard, S.A.; Jagannathan, J.P.; Hornick, J.L.; Ramaiya, N.H. Synovial sarcoma: Imaging features of common and uncommon primary sites, metastatic patterns, and treatment response. AJR Am. J. Roentgenol. 2012, 199, W208–W215. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, T.; Mezzelani, A.; Riva, C.; Fabbri, A.; Dal Bo, L.; Sampietro, G.; Perego, P.; Casali, P.; Zunino, F.; Sozzi, G.; et al. Analysis of SYT-SSX fusion transcripts and bcl-2 expression and phosphorylation status in synovial sarcoma. Lab. Investig. J. Tech. Methods Pathol. 2000, 80, 805–813. [Google Scholar] [CrossRef]
- Hirakawa, N.; Naka, T.; Yamamoto, I.; Fukuda, T.; Tsuneyoshi, M. Overexpression of BCL-2 protein in synovial sarcoma: A comparative study of other soft tissue spindle cell sarcomas and an additional analysis by fluorescence in situ hybridization. Hum. Pathol. 1996, 27, 1060–1065. [Google Scholar] [CrossRef]
- Rocchi, A.; Manara, M.C.; Sciandra, M.; Zambelli, D.; Nardi, F.; Nicoletti, G.; Garofalo, C.; Meschini, S.; Astolfi, A.; Colombo, M.P.; et al. CD99 inhibits neural differentiation of human Ewing sarcoma cells and thereby contributes to oncogenesis. J. Clin. Investig. 2010, 120, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Choong, P.; Pritchard, D.; Sim, F.; Rock, M.; Nascimento, A. Long-term survival in high-grade soft-tissue sarcoma—Prognostic factors in synovial sarcoma. Int. J. Oncol. 1995, 7, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, W.G.; Gold, J.S.; Dyall, R.; Wolchok, J.D.; Hoos, A.; Bowne, W.B.; Srinivasan, R.; Houghton, A.N.; Lewis, J.J. Immunization with DNA coding for gp100 results in CD4 T-cell independent antitumor immunity. Surgery 2000, 128, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Brecht, I.B.; Ferrari, A.; Int-Veen, C.; Schuck, A.; Mattke, A.C.; Casanova, M.; Bisogno, G.; Carli, M.; Koscielniak, E.; Treuner, J. Grossly-resected synovial sarcoma treated by the German and Italian Pediatric Soft Tissue Sarcoma Cooperative Groups: Discussion on the role of adjuvant therapies. Pediatr. Blood Cancer 2006, 46, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; De Salvo, G.L.; Brennan, B.; van Noesel, M.M.; de Paoli, A.; Casanova, M.; Francotte, N.; Kelsey, A.; Alaggio, R.; Oberlin, O.; et al. Synovial sarcoma in children and adolescents: The European Pediatric Soft Tissue Sarcoma Study Group prospective trial (EpSSG NRSTS 2005). Ann. Oncol. 2015, 26, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; de Salvo, G.L.; Oberlin, O.; Casanova, M.; de Paoli, A.; Rey, A.; Minard, V.; Orbach, D.; Carli, M.; Brennan, B.; et al. Synovial sarcoma in children and adolescents: A critical reappraisal of staging investigations in relation to the rate of metastatic involvement at diagnosis. Eur. J. Cancer 2012, 48, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Gronchi, A.; Casanova, M.; Meazza, C.; Gandola, L.; Collini, P.; Lozza, L.; Bertulli, R.; Olmi, P.; Casali, P.G. Synovial sarcoma: A retrospective analysis of 271 patients of all ages treated at a single institution. Cancer 2004, 101, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Stanelle, E.J.; Christison-Lagay, E.R.; Healey, J.H.; Singer, S.; Meyers, P.A.; La Quaglia, M.P. Pediatric and adolescent synovial sarcoma: Multivariate analysis of prognostic factors and survival outcomes. Ann. Surg. Oncol. 2013, 20, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bergh, P.; Meis-Kindblom, J.M.; Gherlinzoni, F.; Berlin, O.; Bacchini, P.; Bertoni, F.; Gunterberg, B.; Kindblom, L.G. Synovial sarcoma: Identification of low and high risk groups. Cancer 1999, 85, 2596–2607. [Google Scholar] [CrossRef]
- Spurrell, E.L.; Fisher, C.; Thomas, J.M.; Judson, I.R. Prognostic factors in advanced synovial sarcoma: An analysis of 104 patients treated at the Royal Marsden Hospital. Ann. Oncol. 2005, 16, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Bisogno, G.; Alaggio, R.; Cecchetto, G.; Collini, P.; Rosolen, A.; Meazza, C.; Indolfi, P.; Garaventa, A.; de Sio, L.; et al. Synovial sarcoma of children and adolescents: The prognostic role of axial sites. Eur. J. Cancer 2008, 44, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, S.; Gherlinzoni, F.; de Paoli, A.; Bonetti, M.; Azzarelli, A.; Comandone, A.; Olmi, P.; Buonadonna, A.; Pignatti, G.; Barbieri, E.; et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: Results of the Italian randomized cooperative trial. J. Clin. Oncol. 2001, 19, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, H.M.; Bramwell, V.H.; Mouridsen, H.T.; Somers, R.; Vendrik, C.P.; Santoro, A.; Buesa, J.; Wagener, T.; van Oosterom, A.T.; van Unnik, J.A.; et al. Cyvadic in advanced soft tissue sarcoma: A randomized study comparing two schedules. A study of the EORTC Soft Tissue and Bone Sarcoma Group. Cancer 1984, 53, 1825–1832. [Google Scholar] [CrossRef]
- Woll, P.J.; Reichardt, P.; Le Cesne, A.; Bonvalot, S.; Azzarelli, A.; Hoekstra, H.J.; Leahy, M.; van Coevorden, F.; Verweij, J.; Hogendoorn, P.C.; et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): A multicentre randomised controlled trial. Lancet Oncol. 2012, 13, 1045–1054. [Google Scholar] [CrossRef]
- Pervaiz, N.; Colterjohn, N.; Farrokhyar, F.; Tozer, R.; Figueredo, A.; Ghert, M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008, 113, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, S.M.-A. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: Meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 1997, 350, 1647–1654. [Google Scholar]
- Gronchi, A.; Stacchiotti, S.; Verderio, P.; Ferrari, S.; Martin Broto, J.; Lopez-Pousa, A.; Llombart-Bosch, A.; Dei Tos, A.P.; Collini, P.; Jurado, J.C.; et al. Short, full-dose adjuvant chemotherapy (CT) in high-risk adult soft tissue sarcomas (STS): Long-term follow-up of a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. Ann. Oncol. 2016, 27, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Wang, C.; Shi, Y. Adjuvant chemotherapy decreases and postpones distant metastasis in extremity stage IIB/III synovial sarcoma patients. J. Surg. Oncol. 2012, 106, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Eilber, F.C.; Brennan, M.F.; Eilber, F.R.; Eckardt, J.J.; Grobmyer, S.R.; Riedel, E.; Forscher, C.; Maki, R.G.; Singer, S. Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma. Ann. Surg. 2007, 246, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kang, M.C.; Lee, H.W.; Park, J.H.; Baek, H.J.; Cho, S.J.; Jeon, D.G. Pulmonary metastasectomy in adult patients with synovial sarcoma: A single-center experience. Korean J. Thorac. Cardiovasc. Surg. 2016, 49, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Burt, B.M.; Ocejo, S.; Mery, C.M.; Dasilva, M.; Bueno, R.; Sugarbaker, D.J.; Jaklitsch, M.T. Repeated and aggressive pulmonary resections for leiomyosarcoma metastases extends survival. Ann. Thorac. Surg. 2011, 92, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Pak, Y.; Kraybill, W.; Kane, J.M., 3rd. Factors associated with actual long-term survival following soft tissue sarcoma pulmonary metastasectomy. Eur. J. Surg. Oncol. 2009, 35, 356–361. [Google Scholar] [CrossRef] [PubMed]
| Positive | Negative | % Positive | |
|---|---|---|---|
| BCL2 (N = 26) | 26 | 0 | 100 |
| CD99 (N = 32) | 30 | 2 | 94 |
| Pan-cytokeratin (N = 35) | 28 | 7 | 80 |
| EMA (N = 21) | 13 | 8 | 62 |
| S100 (N = 39) | 13 | 26 | 33 |
| CD34 (N = 33) | 1 | 32 | 3 |
| SMA (N = 38) | 0 | 38 | 0 |
| Desmin (N = 37) | 0 | 37 | 0 |
| Ki67 (N = 24) | 24 | 0 | 100 |
| Stage IV (N = 23 (%)) | Early-Stage (N = 130 (%)) | p-Value | |
|---|---|---|---|
| Median age (IQR) | 50 (32) | 36.5 (25) | 0.02 * |
| Sex | |||
| Female | 11 (48) | 49 (38) | 0.36 ** |
| Race | |||
| Asian | 4 (17) | 17 (13) | 0.87 ** |
| Black | 1 (4) | 11 (8) | |
| Hispanic | 5 (22) | 25 (19) | |
| White | 13 (57) | 77 (60) | |
| Tumor size (centimeter) | |||
| <5.0 | 1 (4) | 57 (44) | <0.0001 ** |
| >5.0 | 16 (70) | 69 (53) | |
| Unknown | 6 (26) | 4 (3) | |
| Primary Site | |||
| EXTREMITY | 13 (57) | 93 (72) | 0.10 ** |
| HEAD_NECK | 1 (4) | 12 (9) | |
| TRUNK | 9 (39) | 25 (19) | |
| Histologic type | |||
| Biphasic | 5 (22) | 50 (38) | 0.18 ** |
| Monophasic | 13 (56) | 67 (52) | |
| Poorly Differentiated | 3 (13) | 7 (5) | |
| Unknown | 2 (9) | 6 (5) | |
| Characteristic | DFS | Sarcoma Mortality | All-Cause Mortality | |||
|---|---|---|---|---|---|---|
| HR * (CI) | p-Value | HR * (CI) | p-Value | HR * (CI) | p-Value | |
| Tumor size >5.0 cm vs. <5.0 cm | 2.9 (1.5–5.5) | 0.002 | 3.4 (1.5–7.5) | 0.003 | 2.8 (1.4–5.8) | 0.003 |
| Primary Site | ||||||
| Head and neck vs. extremity | 1.2 (0.5–2.9) | 0.759 | 2.8 (1.0–7.5) | 0.049 | 2.5 (1.0–5.9) | 0.04 |
| Trunk vs. extremity | 1.6 (0.8–3.3) | 0.220 | 3.2 (1.3–7.7) | 0.012 | 2.4 (1.1–5.5) | 0.03 |
| Histology: biphasic vs. monophasic | 0.6 (0.3–1.2) | 0.139 | 0.8 (0.4–1.5) | 0.438 | 0.7 (0.4–1.3) | 0.2 |
| Chemotherapy Given (N = 40) | Chemotherapy not Given (N = 90) | p-Value | |
|---|---|---|---|
| Median Age (IQR) | 36 (29.5) | 38.5 (25) | 0.39 |
| Sex (%) | |||
| Female | 12 (30.0) | 37 (41.1) | 0.23 |
| Race (%) | |||
| Asian | 6 (15.0) | 11 (12.2) | 0.59 |
| Black | 3 (7.5) | 8 (8.9) | |
| Hispanic | 5 (12.5) | 20 (22.2) | |
| White | 26 (65.0) | 51 (56.7) | |
| Tumor size (centimeter) | |||
| <5.0 | 12 (30.0) | 45 (50.0) | 0.07 |
| >=5.0 | 27 (67.5) | 42 (46.7) | |
| Unknown | 1 (2.5) | 3 (3.3) | |
| Primary Site | |||
| EXTREMITY | 30 (75.0) | 63 (70.0) | 0.83 |
| HEAD_NECK | 3 (7.5) | 9 (10.0) | |
| TRUNK | 7 (17.5) | 18 (20.0) | |
| Histology | |||
| Biphasic | 16 (40.0) | 34 (37.8) | 0.80 |
| Monophasic | 20 (50.0) | 47 (52.5) | |
| Poorly Differentiated | 3 (7.5) | 4 (4.4) | |
| Unknown | 1 (2.5) | 5 (5.6) | |
| Type of Chemotherapy | Frequency (N = 40 (%)) |
|---|---|
| AIM | 30 (75) |
| Adriamycin/cyclophosphomide | 5 (12.5) |
| Adriamycin only | 1 (2.5) |
| MAID | 1(2.5) |
| MAIV | 1 (2.5) |
| VID | 1 (2.5) |
| Dactinomycin/vincristine | 1 (2.5) |
| Number of Cycles | Frequency (N = 40 (%)) |
| 1 | 1 (2.5) |
| 2 | 4 (10) |
| 3 | 14 (35.0) |
| 4 | 12 (30.0) |
| 5 | 3 (7.5) |
| 6 | 6 (15) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, M.; Merchant, M. Risk Factors Including Age, Stage and Anatomic Location that Impact the Outcomes of Patients with Synovial Sarcoma. Med. Sci. 2018, 6, 21. https://doi.org/10.3390/medsci6010021
Pan M, Merchant M. Risk Factors Including Age, Stage and Anatomic Location that Impact the Outcomes of Patients with Synovial Sarcoma. Medical Sciences. 2018; 6(1):21. https://doi.org/10.3390/medsci6010021
Chicago/Turabian StylePan, Minggui, and Maqdooda Merchant. 2018. "Risk Factors Including Age, Stage and Anatomic Location that Impact the Outcomes of Patients with Synovial Sarcoma" Medical Sciences 6, no. 1: 21. https://doi.org/10.3390/medsci6010021





